A novel purification process for dodecanedioic acid by molecular distillation
Jiang Yu,Xigang Yuan,Aiwu Zeng*
State Key Laboratory of Chemical Engineering,Collaborative Innovation Center of Chemical Science and Engineering(Tianjin),School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
Keywords:
A B S T R A C T
1.Introduction
Dodecanedioic acid(DC12),a typical long-chain α,ω-dicarboxylic acid,usually serves as a monomer of polyamides or some special nylons such as nylon 612 and nylon1212[1-3],which present better characteristics than nylon 66,nylon 11 and nylon 12[4].It is also widely used as a cross-linker for acrylic powder coating[5]and the additive in high quality dye[6]as well as lubricants[7].In recent researches,DC12is of interest as potential alternate fuel substrates in parenteral nutrition[8].
With the high value and growing trend of consumer preference,there exist keen interests in developing appropriate routes for the production of DC12.To date,microbial oxidation of n-alkanes[9]or fatty acids[10]toDC12has reached a commercial scale since its implifies production steps and reduces overall cost compared with chemical synthesis[11].However,it suffers problems in re fining high purity DC12from the fermentation broth because of complex components:cells,mycoproteins,metal ions,colored materials,alkanes and especially monobasic acid whose physical properties is so close to diacid that the elimination encounters some drawbacks in conventional separation processes such as solvent extraction and crystallization[12,13].Li et al.[14]obtained 99%DC12for a feed composition of 96%by falling film crystallization with a time-consuming process.Generally,crystallization is unfavorable due to poor yield,abundant employment of chemicals and a mass of waste production.For solvent extraction[15,16],adverse distribution coefficient and environmental contamination with the employment of hazardous solvent reduce its practical value.Technologically,these processes are complex and need additional steps to obtain commercial grade dodecanedioic acid.
Molecular distillation,generally considered as the most appropriate method to separate heat sensitive and low-volatile compounds[17,18],is characterized by low pressure,relatively low temperature in evaporating space,short residence time of liquid and small distance between evaporator and condenser[19].It has been reported that molecular distillation exhibits outstanding performance for separation or purification of high boiling point mixtures such as octacosanol from transesterified rice bran wax[20],triacylglycerol from free fatty acids mixtures[21]and carotenoids from palm oil[22].
The present study develops a new purification procedure with a recrystallization followed by molecular distillation from a crude product refined from fermentation broth.The application of an alternative refrigerant and relatively high operating pressure for molecular distillation will reduce the cost and complexity of the operation.The purity,yield and distilling ratio of residue to distillate are monitored with varying conditions(distilling temperature,pressure and feed flow rate).In order to increase the purity of DC12,a purification procedure with three-pass molecular distillation is employed.The effect of re-crystallization step on the performance of molecular distillation is explored by comparison studies.
2.Materials and Method
2.1.Materials and reagents
A crude product containing 91.40%of DC12was provided by a Biotechnology Co.(Zibo,China).All solvents or chemicals used were of analytical grade or gas chromatography(GC)grade.Ethanol,methanol,cyclohexane,acetic acid and sulfuric acid were purchased from Guangfu Chemical Co.(Tianjin,China).
2.2.Molecular distillation equipment
The experiments were performed using a laboratory centrifugal short-path distillation unit Lab3 of Myers Company,USA.The main part of the still consists of a conical evaporating rotator,vacuum equipment as well as feed,distillate and residue receivers.The rotator is enclosed in a glass bell jar providing a condensing surface.The evaporating rotator is heated by electric power with a controller inside to maintain a constant evaporating surface temperature.The scheme of the centrifugal evaporator is shown in Fig.1[23].The evaporator diameter is 7.6 cm and the angle of conic is 82.5°.
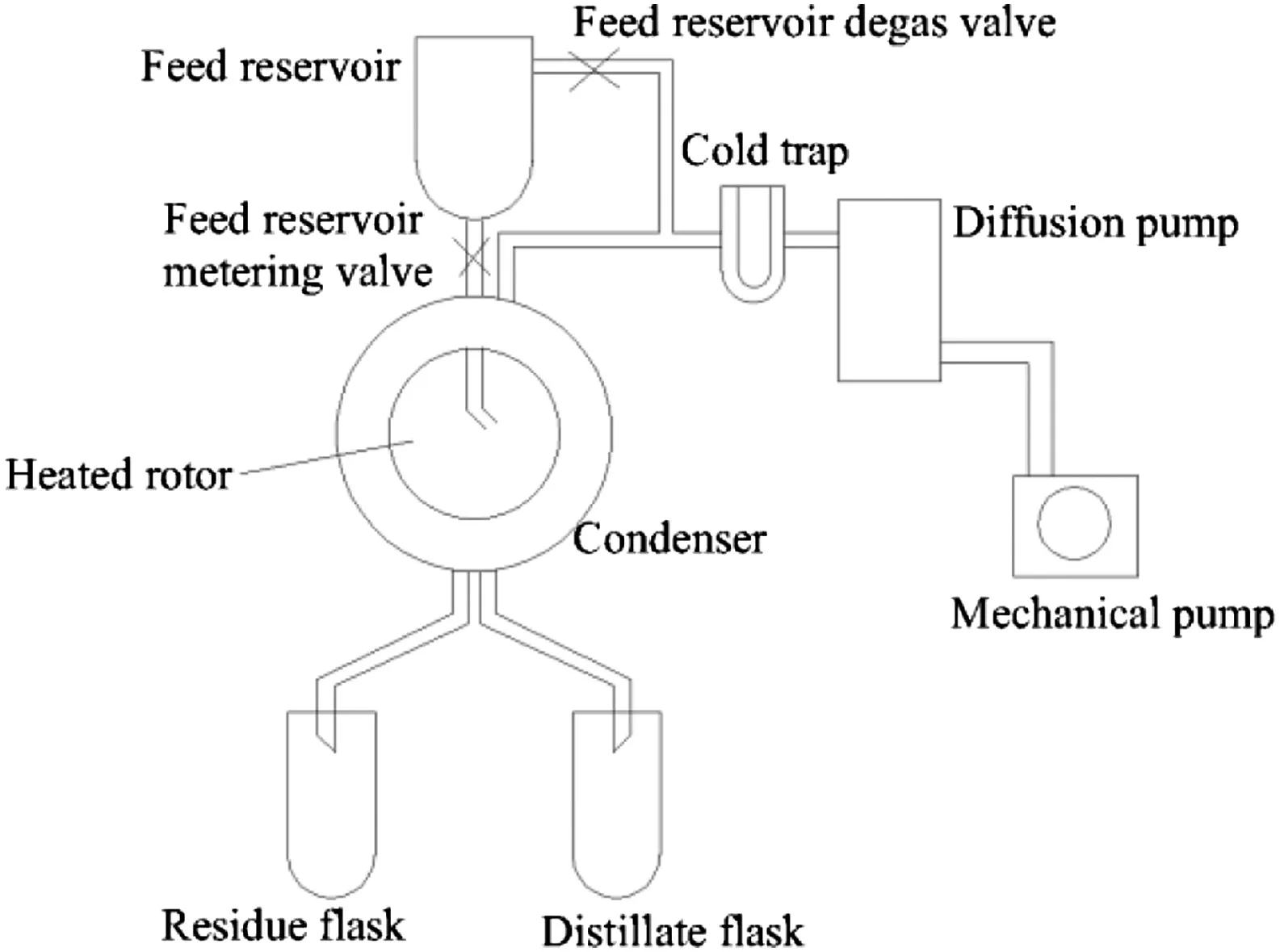
Fig.1.Scheme of Lab3 short-path molecular distillation unit.
2.3.Analytical determinations
The concentration of DC12in raw material and product was determined by GC using the modular equipment Agilent 7890A equipped with a flame-ionization on a DB-FFAP 30 m × 0.25 mm × 0.25 μm i.d.column(Agilent,USA).Saturated C8-C30 fatty acid methyl esters provide standards for making fatty acid assignments.
Procedures for DB-FFAP analysis were designed as follows.The gas flow rates of N2,H2and air were50,35and350ml·min-1,respectively.The injected volume was 1 μl with split ratio 50:1.The operating temperature was set and changed:injector 300 °C,detector 320 °C and initial oven temperature 150°C,rested for 5 min,then increased with a rate of 10 °C·min-1to 250 °C.
2.4.Purification process using centrifugal molecular distillation
The process was proposed by adding a re-crystallization of samples before molecular distillation.Acetic acid,as the solvent,was used in the procedure.The molecular distillation was further applied to purify the extracts.To examine the effect of pretreatment,experiments with or without the re-crystallization were carried out.
The process for molecular distillation is laid out through the following steps.The material is heated and melted to a homogeneous mixture liquid before being pumped to the feed reservoir.When the system is stable(with static pressure and temperature),the mixture is fed to the center of evaporator after degassing.Once on the hot spinning surface,a thin film forms where the lower molecular weight materials evaporate.The range of operation variables such as pressure,temperature,and feed flow rate were set to 5-105 Pa,150-198°C and 200-1000 ml·h-1,respectively.For all distilling process,the temperature of the condenser maintained at 60°C.It is worthy of special attention that the condenser should not be too cold for avoiding solidification of the distillate stream.The refrigerant in the cold trap is low temperature(-20°C)ethanol instead of conventional expensive reagents(liquid nitrogen or dry ice)as the melting point of aliphatic acid sample is high and the vapor in the cold trap is easier to be trapped.Due to the difference of vapor pressure and the mean free path of molecule,it was expected that lighter components will evaporate into the distillate stream and DC12preferentially concentrates in the residue stream.However,depending on the temperature and feed flow rate,DC12may also appear in the distillate.
2.5.Statistical analysis
The mean value of triplicate determinations of samples by GC was reported.Standard deviations for all the GC data were less than±0.5%.
3.Results and Discussion
Experiences have shown that distilling temperature,pressure and feed flow rate are the three major matters affecting the purification in centrifugal molecular distillation unit.Consequently,in this paper,most work put emphasis on the evaluation of the factors through a series of experiments.
3.1.Influence of distilling temperature
The distilling temperature ranged from 150 to 198°C.Fig.2 presents the relation between distilling temperature and purity.The purity of DC12in the residue displays a remarkable increase with temperature from 150 to 180°C,mainly because of the enlarged evaporation rate and the elimination of lighter cuts as temperature grows.At 180°C and 30 Pa,for example,the purity of DC12reaches 97.55%.However,the uptrend of purity slows down over 180°C.At higher temperature(198°C),the purity even reduces.According to the definition of the mean free path(MFP)of evaporating molecules,where k is the Boltzmann constant and d is the effective molecular diameter][24],MFP is augmented by an increase in temperature.Once the distilling temperature is high enough,the MFP of DC12becomes long and molecules at the liquid film surface acquire suf ficient energy,leaving and then reaching the condenser.Consequently,DC12in the residue will reduce,which is extremely obvious at relatively high temperature(198°C).
The yield of DC12is calculated by

where,W is the mass of the residue,XWis the purity of DC12in the residue,F is the mass of the feed and XFis the purity of DC12in the feed material.
Fig.2(b)shows that the yield of DC12presents a reverse tendency with the purity over the temperature range studied.Since materials including DC12evaporated into distillated streams at a higher temperature,the yield exhibits a remarkable decline over 180°C.
Fig.2(c)shows a decline in the yield of residue and the split mass ratio,which is defined as the residue stream to the part in distillate W/D instead of convention al D/W since the residue stream is the expected product here.W and D mean the residue and distillate stream,respectively.The split mass ratio of W/D is also a significant parameter for the yield of product.Though the target here is to eliminate lighter cuts as much as possible and obtain higher purity of DC12,the yield should hold to an accepted level.Different ratios of materials are distillated depending on the heating temperature.When the temperature exceeds 180°C,similar to the yield,the split mass ratio of W/D reduces sharply,against the output of the residue.At lower temperature(150°C),the mass ratio of residue to distillate is about 1.7 times as that at 198°C.Too more fraction in the distillate will result in a massive loss of product.Unfortunately,maximized split ratio is at the sacrifice of purity.It is feasible and favorable to choose a distilling temperature of 180°C.

Fig.2.Effects of distilling temperature on:purity of DC12in the residue(a);yield of DC12(b)and split mass ratio of W/D(c).
3.2.Influence of distilling pressure
In these experiments,the temperature was chosen at low(150°C and 168 °C)and high(180 °C and 198 °C)levels.The pressure ranged from 5 to 105 Pa,unlike that in typical molecular distillation where distilling pressure is usually around 0.1 Pa[25],since maintaining extremely high vacuum in industry is complicated and costive[26].Relatively high pressure is also in favor of the recovery of DC12.The purity reduces pressure increases[Fig.3(a)],for example,from 97.93%to 94.31%at 180°C.This can be explained by that at higher pressure,the collisions between evaporating molecules themselves as well as the evaporating and noncondensable molecules turn out to be rather frequent,hindering evaporating molecules from being captured at the condensing surface[25].On the other hand,an increase of pressure reduces the MFP,which makes it difficult for the lighter cuts to cross the evaporation chamber.This phenomenon tends to be obvious in high pressure range(>80 Pa).

Fig.3.Effects of distilling pressure on:purity of DC12in the residue(a);yield of DC12(b)and split mass ratio of W/D(c).
Though high pressure is not beneficial to the purity,it helps to improve the recovery and reduce cost.The yield of DC12presents a rapid augment when pressure goes from 5 Pa to 30 Pa,as shown in Fig.3(b).The yield increases continuously and reaches a turning point at 30 Pa.When the pressure is higher,most of the heavier components could not reach the condenser and DC12mainly exists in the residue.Meanwhile,the MFP of lighter cuts gets small so that more and more light impurities remain in the residue,considerably declining the purity[Fig.3(a)]with a little enlargement of the yield[Fig.3(b)].
Similarly,as more materials remain in the residue,the split mass ratio increases rapidly with pressure[Fig.3(c)].The split mass ratio is about 0.55 at 5 Pa while it is 1.02 with pressure maintaining at 30 Pa.Given the poor split ratio under high vacuum condition,the pressure in this paper is set at a relatively high level.A pressure of 30 Pa is regarded as the best as it gives better purification efficiency.
3.3.Influence of feed rate
Once fed to the center of the rotor,the feed fluid forms a uniform and thin liquid film immediately owing to the centrifugal force.Film thickness affects the mass and heat transfers significantly since the transfer resistance caused by diffusion is rather significant within the film.Feed rate usually exerts a direct influence on film thickness and residence time[27],subsequently on the distillation efficiency as well as purity and yield.Fig.4(a)relates the purity with feed rate.The purity of DC12in the residue increases as flow rate increases from 200 ml·h-1to 700 ml·h-1in high distilling temperature range(180 °C and 198 °C),and then decreases continuously,with the highest purity up to 97.55% around a rate of 700ml·h-1.This can be illustrated by the following reasons.At small flow rate(less than 700ml·h-1),as distilling temperature increases,more volatile components are distillated,improving the purity.With feed rate increasingly growing,the residence time of the flow becomes so small that the light impurities in the liquid film could not evaporate in time and inevitably remain in the residue.Consequently,the purity of DC12declines.

Fig.4.Effects of feed rate on:purity of DC12in the residue(a)and yield of DC12(b).
Fig.4(b)shows an increase of yield with feed rate at the same distilling temperature.Considering a distilling temperature of 150°C,the yield is 28.81%at a rate of 200 ml·h-1,54.64%at 700 ml·h-1and 65.73%at 1000 ml·h-1.The viscosity of DC12is quite high,unfavorable to fluid flow.This is are as on for setting the feed flow rate in this work in a relatively large range(200 ml·h-1to 1000 ml·h-1).Moreover,the melting point of DC12is 128.4°C[28],which makes it liable to solidify at low temperature.When the experiments finished and the evaporator temperature reduced to the room temperature,we found some white solid powders in the residue stream adhere to the evaporator surface,pipes and residue collector.There is about total 5-10 ml waste product(feed stock ranged from 50 to 150 ml)during each distillation.According to the computational formula,the loss in the still reduces the yield.The effect s especially obvious for the low flow rate(200-400ml·h-1),where the yield increases very slowly.
3.4.Effect of pretreatment on the purification process
To investigate how re-crystallization contributes to the purification,a comparison study between serial distillation experiments with or without re-crystallization is designed with respect to distilling temperature.Other parameters are maintained under the optimized condition:pressure 30Pa and feed rate 700ml·h-1.The purity and yield of DC12in two different operations are compared in Fig.5.Both the purity and yield present similar trend but with remarkable differences between two operations.Considering the distilling temperature of 180°C,the yield with the re-crystallization pretreatment is 53.18%,a little lower than that in direct distillation(53.8%),with about 0.91%-2.13%of DC12lost in the extraction procedure.With the analysis on the components in the feed,the contribution of the re-crystallization step is mainly to remove color material as well as the highly viscous and thick impurities.It is these matters that deteriorate the flow characteristic,hindering heat from transferring to more volatile component and blocking the evaporation of lighter impurities[25].If the color material is not removed completely,it will contaminate the color of final product,as shown by the practical pictures of real feed materials presented in Fig.6.This improvement in color would greatly enhance the value of DC12.Obviously,the separation process will suffer a limitation without the pretreatment.Although the elimination by re-crystallization seems in significant to promote the recovery,it can ameliorate the flow,avoid solidification and utilize the effective evaporation area as much as possible.In the novel process,the purity can be up to 97.55%[Fig.5(a)]while it is 93.07%in the comparative trial.This difference can be more evident through the multi-pass distillation.

Fig.5.Effects of pretreatment on the purification process:purity of DC12(a),yield of DC12(b);(I)distillation with pretreatment,(II)distillation without pretreatment.
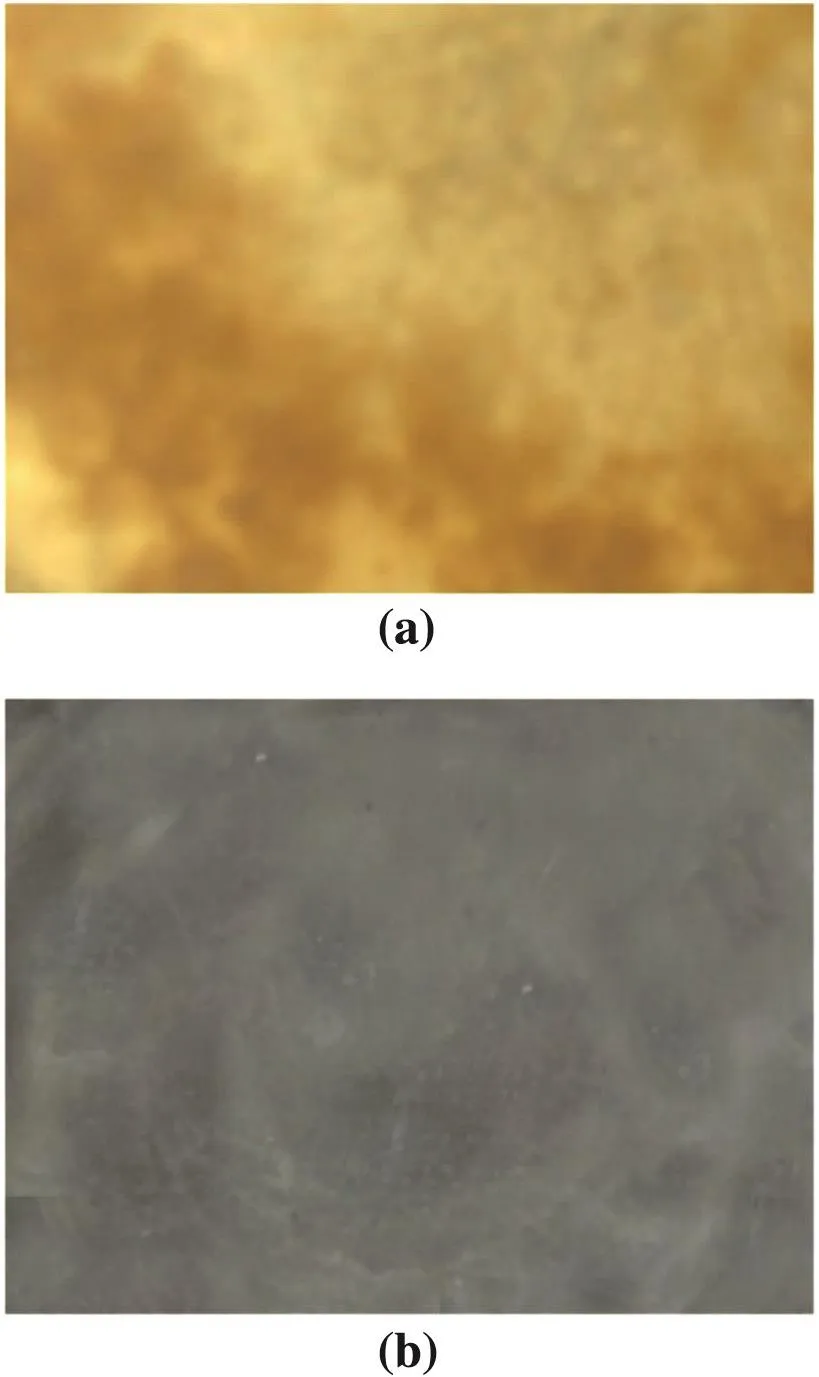
Fig.6.The picture of feed material;without the pretreatment(a),with the pretreatment(b).
3.5.Multiple-pass distillation
With the finite evaporation area of Lab-3 molecular distillation unit,single-pass distillation is sometimes insufficient for anticipated separation especially for materials that possess close molecule weight and volatility[29].In this situation,the so-called multiple-pass operation is usually applied with the distillation repeated in one still or the tandem of apparatuses.To verify the efficiency of multiple-pass distillation,experiments at 180 °C and a flow rate of 700 ml·h-1were conducted.The results are shown in Table 1.As the residue fraction was redistilled under similar conditions,the second and third stage distillation gave lower purification yields of 49.23%and 46.36%,which is not desired.However,owing to almost complete elimination of impurities such as solvent,color materials and monobasic acid,the DC12concentration can reach a higher level.When the third stage is accomplished,the fin al purity of DC12in the residue increases to 99.22%.
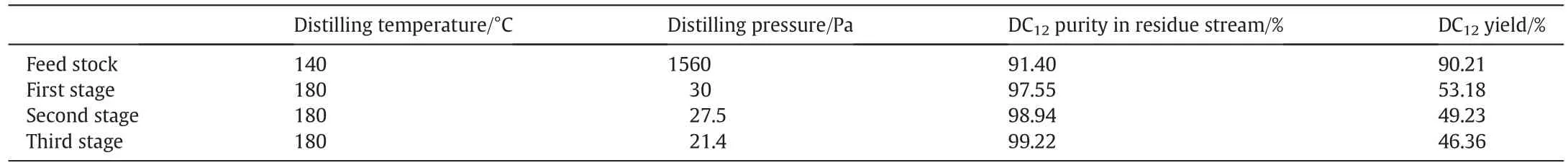
Table 1 Multiple-pass distillation of the residue
4.Conclusions
This study demonstrates that dodecanedioic acid(DC12)can be effectively purified with a centrifugal molecular distillation unit.With a relatively high pressure of 30 Pa and a low-cost refrigerant,the method still achieves a sufficient elimination of impurities.The DC12purity and yield reach as high as 97.55%and 53.18%,respectively,under the optimum conditions.Furthermore,the purity of DC12in the residue can be improved to 99.22%through a three-pass distillation.It indicates that the application of molecular distillation to re fine DC12from the crude product is worthy of special attention.
 Chinese Journal of Chemical Engineering2015年3期
Chinese Journal of Chemical Engineering2015年3期
- Chinese Journal of Chemical Engineering的其它文章
- Micromixing characteristics in a gas-liquid-solid stirred tank with settling particles☆
- An experimental study of drag reduction by nanofluids in slug two-phase flow of air and water through horizontal pipes☆
- Effect of surfactant type on interfacial area and liquid mass transfer for CO2absorption in a bubble column☆
- Effects of bubbly flow on bending moment acting on the shaft of a gas sparged vessel stirred by a Rushton turbine☆
- Enhanced heat transfer in a heat exchanger square-duct with discrete V- finned tape inserts☆
- Purification and separation of durene by static melt crystallization☆
