Dechlorination of pentachlorophenol by grinding at low rotation speed in short time
Zhi Xu,Xiaoyu Zhang,Qingzhi Fei,*
1Department of Environmental and Chemical Engineering,Dalian Jiaotong University,Dalian 116028,China
2Institute of Environmental Engineering,Dalian Jiaotong University,Dalian 116028,China
Keywords:
A B S T R A C T
1.Introduction
Pentachlorophenol(PCP)is a typical hazardous chlorinated organic compound,which is easily ionized and insoluble in water.It has a wide range of application,such as fungicide,phytocide,insecticide and wood preservative.Although the production of PCP has been prohibited since the 1980s,the environmental problems caused by PCP last for a long time since PCP may transform to polychlorinated dibenzo-pdioxin(PCDD)and polychlorinated dibenzofuran(PCDF),which present stronger toxicity in burning or high temperature pyrolysis.New method is needed for treating PCP with safe and hazardous-free degradation.There are two main types of hazard-free treatment for PCP,thermal and athermal disposal technologies.As thermal disposal technologies,rotary kiln pyrolysis/gasification[1]and meltingsolidification[2,3]are not suitable to use for a long time,because burning at a high temperature requires more energy and it is difficult to control,while low temperature treatment[4]may cause secondary pollution due to incomplete combustion.On the other hand,athermal disposal technology,including alkali catalytic dechlorination[5],ultraviolet degradation[6,7]and bioremediation technique[8,9],requires a great quantity of additives and takes a long time to dispose,which fails to serve the increasing needs of today's society.
Mechanochemical(MC)degradation method was first introduced for hazard-free treatment of POPs in the 1990s which initiated the development of innovative remediation and decontamination processes,and this versatile technique is designated as“dehalogenation by mechanochemical reaction”(DMCR)[10].DMCR is easily performed in ball mills,which are readily available in different sizes and constructions.The pollutants are eliminated directly,no matter how complex their structures may be and how strong the pollutants may be bound adsorptively to particular compounds.
However,as far as we know,MC approaches for destruction of hazardous polyhalogenated pollutants have not been scaled up to an industrial scope or even commercialized.Two possible causes will be discussed as follows.To start with the rotation speed of a grinding device is relatively high(700 r·min-1or more)[11-13].With that large amounts of energy are needed in MC reactions.Secondly,the long reaction time which ranges from hours(≥6 h)[13,14]to several days,is a disadvantage compared to other common well-established destruction techniques such as incineration.In this study,readily available reagents,CaO and SiO2,are used to increase the efficiency of dechlorination and the optimal proportion is determined.The dechlorination will increase with hydrogen donor in the reaction,while hydrogen donors are usually liquid,such as alcohol,aldehyde or amine.A new solid hydrogen donor,carbamide,is used in the dechlorination reaction in this study.The optimal ball diameter is also determined.Uncertain intermediates and degradation products will be characterized by HPLC,FTIR and XRD,to guarantee the degradation of PCP.
2.Experimental
Planetary ball mills(ND7-1L,Nanjing Nantian Tianzun Instrument Corporation,China),each equipped with a 300 ml steel grinding jar(containing 200 g steel balls),were used for bench-scale study.The grinding jar was also loaded with 5g of mixture containing PCP(98.5%),CaO(98.0%,Kemiou Chemical Reagent Co.,Tianjin),SiO2(99.8%,Tianli Chemical Reagent Co.,Tianjin)and CO(NH2)2(99.0%,Kang Pu Hui Wei Technology Co.,Ltd,Beijing)in different molar ratios.The milling process was operated for 5 h with a constant rotation speed about 300 r·min-1.After cooling the grinded mixture at 0 °C for 1 h,1 g of finely powdered mixture was transferred into a flask and mixed with 100 ml water.The chloridion concentration was detected by titration[15]and the dechlorination rate is calculated by

where[Cl-]and[Cl]stand for the chloride ion detected by titration and the total amount of chlorine in PCP,respectively.2 ml carbinol was added into a 0.5 g mixture,then the organic phase was separated and injected into a HPLC line(Dalian Elite Analytical Instruments Co.,model P230,DAD230 detector,Hypersil ODS2 column 250 mm×4.6mm,mobile phase UPW,chromatography methanol),the other mixture was characterized by FTIR(Bruker TENSOR 27 spectrophotometer in the wavelength range of 7800-350 cm-1using KBr pellets)and XRD(Empyrean diffractometer).
3.Results and Discussion
3.1.Dechlorination reaction in different molar ratios of PCP:CaO
Fig.1 shows the dechlorination with different molar ratios of CaO.The reductive dechlorination ratio of PCP increases from 3.0%to 22.3%until the molar ratio reaches 1:60.The color of the grinded mixture gets deeper as the dechlorination proceeds,with the appearance of amorphous carbon.As the ratio increases further,dechlorination ratio decreases quickly from 9.4%to 5.0%.Excessive CaO may decrease the grinding probability of PCP to reduce the efficiency of dechlorination.

Fig.1.Reductive dechlorination of PCP with different molar ratios of CaO.
Thus the optimal proportion of PCP:CaO is 1:60.
The FTIR spectra of PCP with different molar ratios of CaO are shown in Fig.2.After the treatment,they are similar to that of CaO due to excessive CaO.The band with a peak at 700 cm-1attributed to C-Cl stretching vibration is weakened with the increase of CaO.Then C-Cl is attacked by a free electron and broken.It is consistent with Fig.1.The band with a peak at1500cm-1attributed to benzene rings appears,with the intensity weakened gradually.The result demonstrates that benzene rings decompose in the grinding process and resolve in low chlorinated hydrocarbon.Other characteristic peaks of pure PCP as signed to 3500cm-1and ascribed to symmetrical stretching vibration and asymmetrical stretching vibration of hydroxyl completely disappear after treatment,demonstrating that the hydroxyl of benzene rings is destroyed.Another reason for lower peak intensity of pure PCP could be excessive CaO.

Fig.2.FTIR spectra of PCP with different molar ratios of CaO.
For further clarifying whether PCP transforms completely,the chromatograms of PCP with different molar ratios of CaO are made,and the results are shown in Fig.3.The chromatograms illustrate that PCP is not broken completely.The specific peak of PCP still appears after 5 h milling,which is consistent with Fig.1.However,there is a slight difference among these chromatograms.The peak width increases with the increase of CaO ratio,and the same concept also applies to dechlorination.Moreover,the peak time is shorter.By combining with Fig.1,it could state that PCP transforms gradually in the milling process.

Fig.3.Chromatograms of pure PCP and PCP with different molar ratios of CaO.
According to Figs.1-3,the comparison of alkali metal efficiencies and their own oxides in reductive dechlorination is arranged as:Mg>CaO>MgO≫Zn>Al[10,12,14].The results show that alkali environment and free electron are the most important factors in dechlorination.Moreover,the reductive dechlorination of PCP could be promoted obviously by adding CaO.We can infer two functions of CaO.First,CaO can maintain alkali environment and provide electrons as alkali metals,so that radicals are easily generated to attack chlorine of benzene ring,
Secondly,the reaction of Cl with CaO makes CaO as a chlorine receptor,promoting reductive dechlorination.
3.2.Dechlorination reaction in different molar ratios of SiO2or CO(NH2)2
The dechlorination result with different molar ratios of SiO2/mixture(PCP:CaO=1:60)is shown in Fig.4.The increase in molar ratio of PCP:CaO:SiO2from 1:60:1 to 1:60:60 enhances the dechlorination rate significantly.The molar ratio of 1:60:60 presents the highest destruction efficiency of 58.4%after 5 h grinding.There are two reasons to explain the effect of silica.(1)Silica acts as the medium during the grinding process,increasing contact area of PCP and CaO and grinding of PCP.(2)Silica changes energy distribution of PCP.Hydrogen bond is formed by O__Si__O contacting with PCP and changes PCP bond energy distribution,so that chlorine is easily separated from benzene ring.As the ratio increases to1:60:80,dechlorination descends slightly,since excessive SiO2reduces the impact possibility.

Fig.4.Reductive dechlorination of PCP with different molar ratios of SiO2.
Fig.5 shows the FTIR spectra of PCP with different molar ratios of SiO2.The main characteristic peaks of silica are assigned as follows:1100 cm-1and 750 cm-1are attributed to Si__O__Si asymmetrical stretching vibration.After grinding with SiO2added,the characteristic peaks of pure PCP at 3500 cm-1,1400 cm-1and 700 cm-1disappear completely.Thus silica could promote reactions with further breakage of C__Cl.
According to Fig.6,it is inferred that PCP is destroyed completely with the disappearance of specific peak.With the increase of SiO2ratio,some undetermined compositions are transformed by PCP appearance gradually and are changed as milling proceeds.

Fig.5.FTIR spectra of PCP with different molar ratios of SiO2.
Fig.7 displays X-ray diffraction patterns of pure CaO,SiO2,PCP,and mixtures with different molar ratios.In the reaction with PCP and CaO only,the X-ray scattering pattern of the ground powder is similar to that of pure CaO.Thus the crystal structure of PCP is destroyed with excessive CaO.With excessive SiO2,the crystal structure of CaO and SiO2changes in a different extent.The peak intensity of pure CaO at 2θ angles around 13°,33°and 47°weakens evidently,and that of pure SiO2at 2θ angles around 78°also decreases.These results indicate that silica acts as medium and participates in the reductive dechlorination same as CaO.In the X-ray scattering pattern of 1:60:60,a new sharp peak appears at 2θ angle around 20°.According to the form of crystal,the peak represents Ca(OH)2generated in the grinding process because water vapor is absorbed by CaO.The result also con firms the disappearance of all absorption peaks of PCP.
The dechlorination of PCP with different charge ratios is shown in Fig.8.The dechlorination rate with silica increases faster than that with urea.Although the dechlorination rate is improved with the increase of charge ratio,the dechlorination with urea is even lower than that with CaO only,just 21.1%.For understanding the inhibition better,a quadratic linear fitting is shown in Fig.9.The dechlorination rate by adding urea decreases gradually,while that by adding silica increases rapidly with the increase of charge ratio.The inhibition of urea may be related to p-π conjugate formed by C═O and two charges of N,which have strong electron-withdrawing ability to release H+,not H atom.And electropositive H+would destroy alkaline environment and restrain degradation reaction.Therefore,urea cannot be used as a hydrogen donor.
Silica surface has a complicated amorphous structure,a lot of tetrahedrons irregularly arranging with the silicon atom as the center and oxygen atoms as vertices.In the tetrahedron holes,many hydroxyls

Fig.6.HPLC of PCP with different molar ratios of SiO2.

Fig.7.X-ray scattering patterns of pure CaO,SiO2,PCP,and mixture with different molar ratios.

Fig.8.Comparison of reductive dechlorination of PCP with different molar ratios of SiO2 and CO(NH2)2.
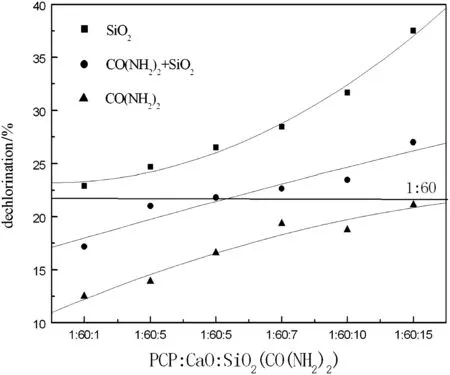
Fig.9.Quadratic linear fitting of reductive dechlorination of PCP with different molar ratios of SiO2and CO(NH2)2.
[16,17]attach to silicon atom with different distances and do not equally contribute to the chemical reaction[18].When contacting with wet air,the surface silicon atom will“react”with water,keeping oxygen tetrahedron coordination and satisfying silicon atom valence.In other words,hydroxyls form at the silica surface.In general,there are three kinds of hydroxyl:isolated free hydroxyl,united hydroxyl,and twin hydroxyl.The isolated and twin hydroxyls cannot form hydrogen[19,20].On account of the characteristics of silica,in a high-speed milling process,surface hydroxyl has some effect on the energy distribution of pentachlorophenol and stable structure,making chlorine to be easily off from the benzene ring.
3.3.Dechlorination reaction with grinding balls in different diameters
Fig.10 shows the dechlorination of PCP with grinding balls in different diameters in the presence of CaO.The mixture in 10 mm balls presents higher degradation than that in 5 mm balls.The result clarifies that mass addition could promote the reaction.The weight of 5 mm grinding balls is too light to provide enough energy for reductive dechlorination.On the other hand,the lowest degradation with mixing balls of 5 and 10 mm implies that 5 mm balls may fill the space among 10 mm balls in the grinding process,decreasing the space for dechlorination and limiting the motion of balls.Fig.11 compares the dechlorination of PCP in 10,15 and 20mm grinding balls in the presence of CaO and SiO2.The degradation effect of 15mm grinding balls is better than that of 10 mm and much better than that of 10 and 15 mm mixing balls.With 20 mm grinding balls,dechlorination decreases greatly.The results indicate that there is an optimal diameter for reductive degradation.Larger balls present less space and smaller impact area,so the grinding process could not provide enough energy in the dechlorination.

Fig.10.Reductive dechlorination of PCP with grinding balls in different diameters with CaO.
The definition of collision energy is[21,22]


Fig.11.Reductive dechlorination of PCP with grinding balls in different diameters with CaO and SiO2.
where Ewis the specific impact energy of balls,Vjis the relative velocity between two colliding balls or a ball colliding against the mill wall,m is the mass of grinding medium,n is the number of collisions in 1 s,and w is the mass of the sample.Larger grinding ball generates higher collision energy and promotes the reaction,but their movement is inhibited in a limited space.Wei[14]proposed that shear force would be generated more easily between balls in different sizes so that some powder dropped from the ball surface to participate in the reaction.The impact energy during grinding could avoid cold welding.On the contrary,Figs.10 and 11 in this experiment show that dechlorination with uniform size balls is higher than that with balls in different sizes.The reason is due to different rotation speeds.In this experiment the rotation speed was 300 r·min-1,lower than others,so the shear force could not provide enough collision energy for reaction.Grinding balls in different sizes fill each other at such a low rotation speed,so that their movement is restrained and the effect of dechlorination decreases.
4.Conclusions
The results demonstrate that(1)the optimal molar ratio of PCP and CaO is 1:60,excessive CaO makes PCP grinding inadequately and inhibits reductive degradation;(2)the reaction is promoted with SiO2added in the grinding process,with the optimal molar ratio of PCP,CaO and SiO2at 1:60:60,while urea restrains the dechlorination so that it is not a substitute for hydrogen donor;(3)at low rotation speed of 300 r·min-1,the reductive degradation with 15 mm grinding balls is better than 5,10 mm and mixing sizes,so dechlorination with equal size balls is more efficient.For increasing collision energy,two parameters should be considered,the mass of the grinding balls and filling rate of the grinding jar.
Experiments in this study con firm that proper proportion of different materials and appropriate size of grinding balls increase the
efficiency of dechlorination at low rotation speed in short time.There are still some rooms for improvement.Grinding method will be developed to an industrial scale with appropriate materials and optimized operation parameters.
 Chinese Journal of Chemical Engineering2015年3期
Chinese Journal of Chemical Engineering2015年3期
- Chinese Journal of Chemical Engineering的其它文章
- Micromixing characteristics in a gas-liquid-solid stirred tank with settling particles☆
- An experimental study of drag reduction by nanofluids in slug two-phase flow of air and water through horizontal pipes☆
- Effect of surfactant type on interfacial area and liquid mass transfer for CO2absorption in a bubble column☆
- Effects of bubbly flow on bending moment acting on the shaft of a gas sparged vessel stirred by a Rushton turbine☆
- Enhanced heat transfer in a heat exchanger square-duct with discrete V- finned tape inserts☆
- A novel purification process for dodecanedioic acid by molecular distillation
