Densities and viscosities of binary mixtures of n-decane+1-pentanol,+1-hexanol,+1-heptanol at temperatures from 293.15 to 363.15 K and atmospheric pressure☆
Alejandro Estrada-Baltazar*,Micael Gerardo Bravo-SanchezGustavo Arturo Iglesias-SilvaJuan Francisco Javier AlvaradoEdgar Omar Castrejon-GonzalezMariana Ramos-Estrada
1Department of Chemical Engineering,Instituto Tecnológico de Celaya,Celaya,Guanajuato 38010,Mexico
2Faculty of Chemical Engineering,Universidad Michoacana de San Nicolás de Hidalgo,Morelia,Michoacan 58060,Mexico
Keywords:
A B S T R A C T
1.Introduction
A complete knowledge of thermodynamic and transport properties of liquid mixtures is essential for numerous industrial processes,in particular for chemical engineering industries.These properties are necessary in the modeling,design,construction,and optimization of process equipment such as condensers,heat transfer equipment,and distillation columns.
There has been a systematic study of the excess thermodynamic and transport properties for 1-alcohol+n-alkane mixtures.These properties have been extensively analyzed from both of experimental and theoretical points of view.From the experimental point of view,the analysis is important because it provides information about composition related changes,hydrogen bonding,and effects of size and structure of the molecules.This information allows the understanding of the molecular structure and the ruling intermolecular interactions.This knowledge can be used to develop models and theories about the mixtures behavior.In the last years there have been several efforts to develop models for excess properties such as enthalpy[1-6]and heat capacity[2,7-10].Also,it is worth to mention the association models[1,4,11-13]and the group contribution methods[14-17]for the molar volume.
For1-alcohol+n-alkane there are numerous reports on experimental data for excess molar volume or density[18-30]and viscosity[31-39].Different thermodynamic properties for the binary mixtures with n-decane and 1-pentanol,1-hexanol,or 1-heptanol have been reported in the literature.These properties are the excess enthalpy[40]and the heat capacity[41].Density and excess volume data have been reported by different authors.The excess volumes for n-decane+1-pentanol have been reported by Yun et al.[42],Al-Dujaili and Awwad[28]and Kaur et al.[43]at 298.15 K.Densities at other temperatures are measured by Al-Dujaili and Awwad[28]at 288.15,308.15 and 318.15 K.Experimental densities for the mixture of n-decane+1-hexanol are measured at 298.15 K by Treszczanowicz and Benson[24],Kaur et al.[43]and Dubey and Sharma[26].Dubey and Sharma[26]reported densities for this mixture at 303.15 and 308.15 K.To the best of our knowledge,density measurements for the binary n-decane+1-heptanol do not exist in the literature.
In recent years there has been an increasing interest on the viscosities of liquid mixtures.For example,the study of the viscosity of nalkane+1-alcohol mixtures,together with other thermodynamic properties,will allow us to know the molecular interactions and structure of these liquid mixtures.Some of these systems exhibit a peculiar behavior[31,34,44-46]which cannot be explained in terms of traditional approaches that have been observed experimentally.Experimental viscosities have been only measured for the mixture n-decane+1-hexanol at 298.15,303.15,and 308.15 K[26].
In this work we report density and viscosity data for the binary mixtures of n-decane with 1-pentanol,1-hexanol and 1-heptanol at atmospheric pressure.The molar composition covered the entire range with increments in mole fraction of 0.1 at temperatures from 293.15 to363.15K.Excess molar volumes,partial molar volumes,excess partial molar volumes and the corresponding values at in finite dilution are calculated from experimental density values.Also,viscosity deviations and the excess Gibbs energy of activation of viscous flow a recalculated from the viscosity measurements.
2.Experimental
2.1.Materials
n-Decane,1-pentanol,and 1-hexanol are from Sigma-Aldrich with a molar percentage purity of>99%for the three substances.1-Heptanol is obtained from Fluka with a purity of>99%.The purity of each chemical is checked indirectly by measuring the density and viscosity,and comparing with literature values.Table 1 depicts this comparison.High purity chemical substances are used as received from the manufacturer.The experimental techniques to remove impurities reported by the producers are the same as those we used in our laboratory.
2.2.Apparatus and procedure
Density and viscosity are measured in an automatic densimeterviscometer Stabinger(SVM)from Anton-Paar model 3000.This equipment enables to measure dynamic viscosity and density simultaneously with two separated cell for the density and the viscosity.Both cells are filled up with 2.5 ml of sample.The SVM 3000 viscometer employs a Peltier element for a fast and efficient thermal stabilization of the sample.The temperature uncertainty is 0.02 K in a range from 288.15 K to 378.15K and the repeatability is 0.005K as reported by the manufacturer.
The density measurement is based upon the oscillation of a U tube.The principle behind this technique is the change of the natural frequency of the oscillating U tube when it is filled with different liquids or gases.The oscillator is excited harmonically with an electromagnetic force and the oscillation direction is perpendicular to the plane of the glass tube.The frequency of the oscillator is influenced only by the fluid inside the vibrating tube.The ends of the tube are secured to a block to prevent from external vibrations.
On the free section of the tube two magnets are mounted and covered by two bobbins(mounted on the viscometer).The tube vibrates transversally and the second magnet moves in and out from the output bobbin.This movement induces a current with a frequency equal to the vibration of the tube.The oscilloscope of the equipment detects this signal and measures the resonance frequency of the tube.The Stabinger viscometer is programmed with internal software to calculate directly the fluid density.The density uncertainty is 0.0005g·cm-3for an interval form 0.65to1.5g·cm-3and its repeatability is 0.0002g·cm-3.Both values are reported by the fabricant.The manufacturer has calibrated the apparatus with ultrapure water and air.We have reported the principle of measurement in our previous work[47]together with measured water densities to test the calibration.
The viscometer is a rotating cylinder built according to the modified Couette principle.There is an external tube in fast rotation and one slower internal measuring rotor.The measurement of viscosity from rotating viscometers is based upon the measurement of the torque and the speed of rotation.Inside the SVM3000,one rotating magnet induces a current field with an exact stop momentum dependent of the rotation speed.The torque of the induced current is measured with a resolution of 50 pN·m.The cell for measurement is very small and contains one tube rotating at constant speed.In the measurement,the rotor with the magnet is placed floating on the sample.The rotor floats freely with no need of bearing.The absence of bearing means that there is no friction.Therefore,the equipment is quite insensitive from externalvibrations.In a relatively short time the rotor reaches a stable velocity.This is determined by the equilibrium between the breaking effect of the induced current and the propulsive forces from the shear stress of the sample.The dynamic viscosity is calculated from the speed of the rotor.The measurement range for the dynamic viscosity goes from 0.2 mPa·s to 20 Pa·s,and the fabricant reports the repeatability of 0.1%and an uncertainty of 0.35%.Kinematic viscosity of the samples was determined at following ASTM method D7042.Viscosity indices were calculated according to ASTM standard method D2270-93.Hence,the apparatus was only adjusted and calibrated by the manufacturer.We have verified the viscosity uncertainty with two reference flu ids from Cannon Instrument Comp.,S60 and N100, finding that the deviations are lower than 1%[48].
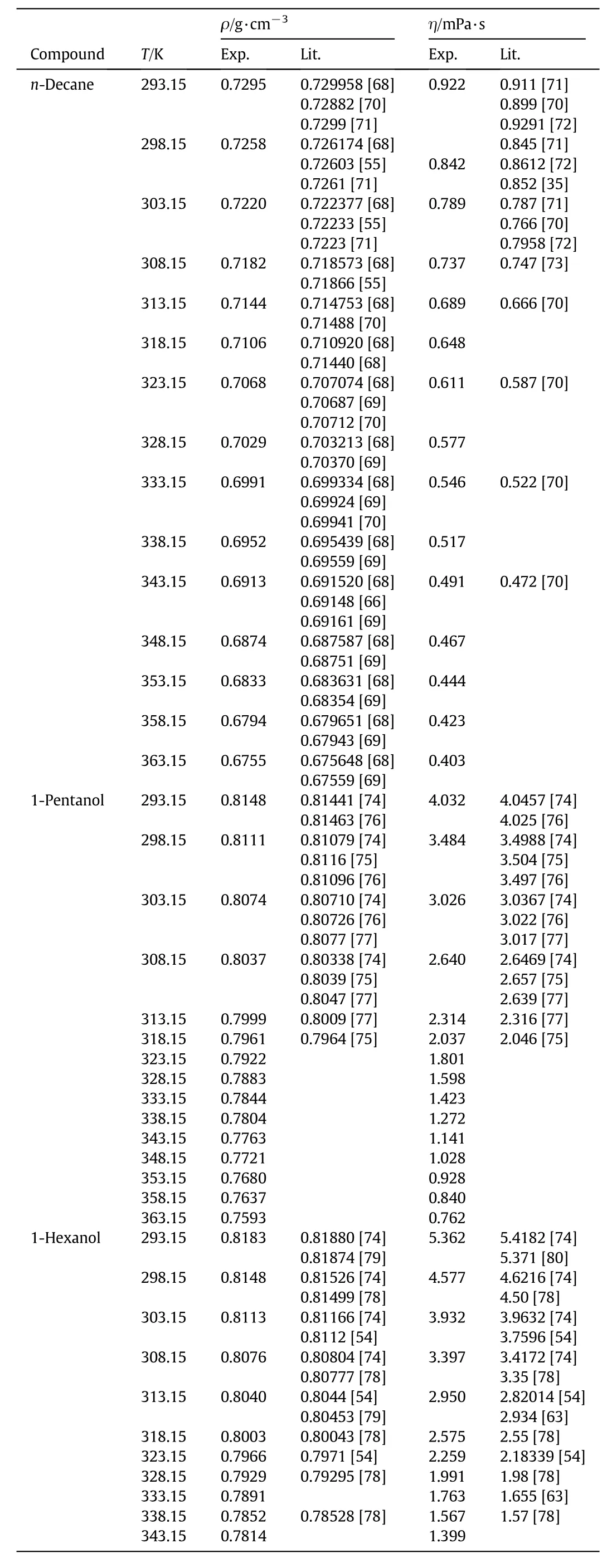
Table 1 Experimental density and viscosity values of pure liquids with literature values
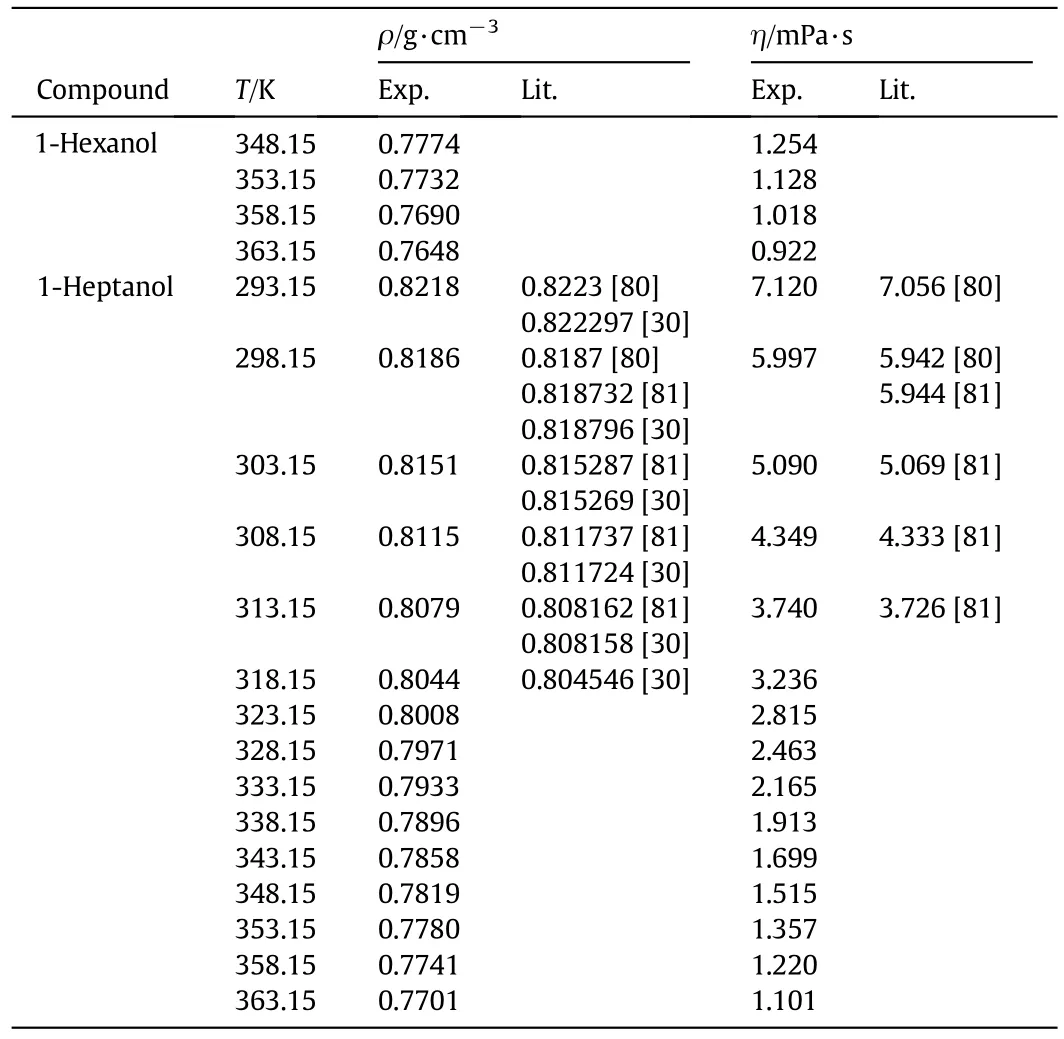
Table 1(continued)
The complete set of mixtures is gravimetrically prepared using an Ohaus analytical scale(model Voyager,AS120S).This scale has a precision of±0.1 mg.The total uncertainty for the molar fraction is estimated to be 0.2%.
3.Results and Discussion
Densities for the binary mixtures of n-decane with 1-pentanol,1-hexanol or 1-heptanol at different compositions,atmospheric pressure and temperature from 293.15 to 363.15 K have been measured and reported in Tables 2-4,respectively.The dynamic and kinematic viscosities are also reported in these tables.
The density and viscosity measurements of these mixtures have been reported in the literature.Only one report is found for the mixture n-decane+1-hexanol[26].Our measurements and those reported by Dubey and Sharma[26]agree within 0.15%and 1.20%for the density and viscosity,respectively.
The excess molar volume is calculated using:
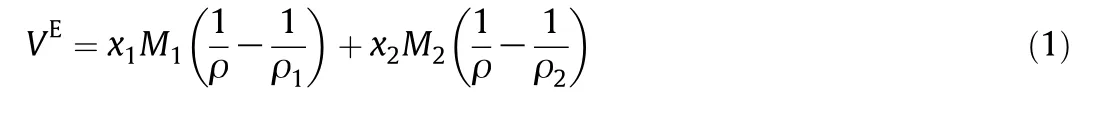
where M1and M2are the molar masses,ρ1and ρ2are the densities of components 1 and 2,respectively and ρ is the density of the mixture.Also,the excess molar volumes are shown in Tables 2-4.Al-Dujaili and Awwad[28],Kaur et al.[43]and Yun et al.[42]reported the excess molar volumes for then-decane+1-pentanol mixture while Dubey and Sharma[26],Kaur,et al.[43]and Treszczanowicz and Benson[24]did it for the n-decane+1-hexanol mixture.The average deviation of our observed molar volumes in comparison with those of Dubey and Sharma[26],Kaur et al.[43]and Treszczanowicz and Benson[24]is 0.0051,0.0098and0.0124cm3·mol-1,respectively.The maximum deviation observed between these authors and our experimental data is 0.0119,0.0289 and 0.0465 cm3·mol-1,respectively.For the binary system of ndecane+1-pentanol Awwad Al-Dujaili[28]showed an average deviation of excess molar volumes cm3·mol-1of 0.0060 and a maximum deviation of0.0109 cm3·mol-1,while0.0134 cm3·mol-1and 0.0283 cm3·mol-1by Kaur et al.[43],and 0.0178 cm3·mol-1and 0.0382 cm3·mol-1by Yun et al.[42].
It is observed that the largest deviations obtained for both systems were presented by Treszczanowicz and Benson[24],Kaur et al.[43]and Yun et al.[42]here a dilatometer was used to obtain the excess molar volume,while the smallest deviations were observed by Al-Dujaili and Awwad[28]and Dubey and Sharma[26]where a densimeter was used to measure the density and hence obtain molar excess volume.Therefore,the observed deviations are due to the different techniques used to obtain the volume of excess.
Viscosity deviations are calculated from the experimental viscosity measurements using:

where xiand ηiare the molar fraction and the dynamic viscosity of pure component i,respectively and η is the dynamic viscosity of the mixture.Dubey and Sharma[26]reported viscosity deviations for the ndecane+1-hexanol system at 298.15.Fig.1 shows the comparison of their data with our experimental measurements.The agreement between the two data is within 2.51%.To the best of our knowledge these are the only reported viscosity values in the literature for the systems treated here.
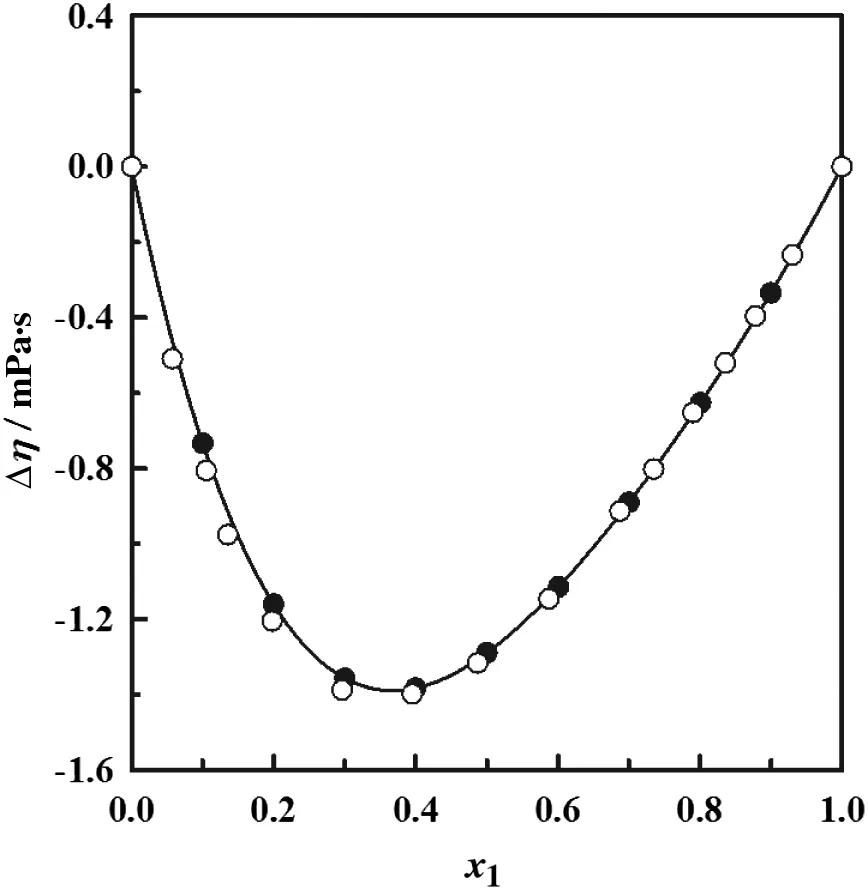
Fig.1.Deviation of dynamic viscosity(Δη)for the n-decane(1)+1-hexanol(2)as a function of mole fraction at T=298.15K.●this work;○ DubeySharma[26];the solid line corresponds to the Eq.(4).
The excess Gibbs energy of activation of viscous flow(ΔG*E)is also calculated using as the absolute reaction rate theory[49]:
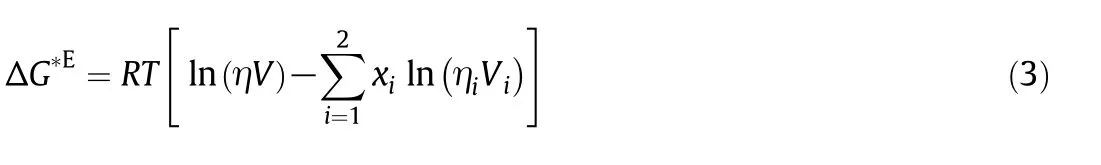

Table 2 Density,viscosity,kinematic viscosity,viscosity deviations and excess molar volumes for the binary mixture of n-decane(1)+1-pentanol(2)
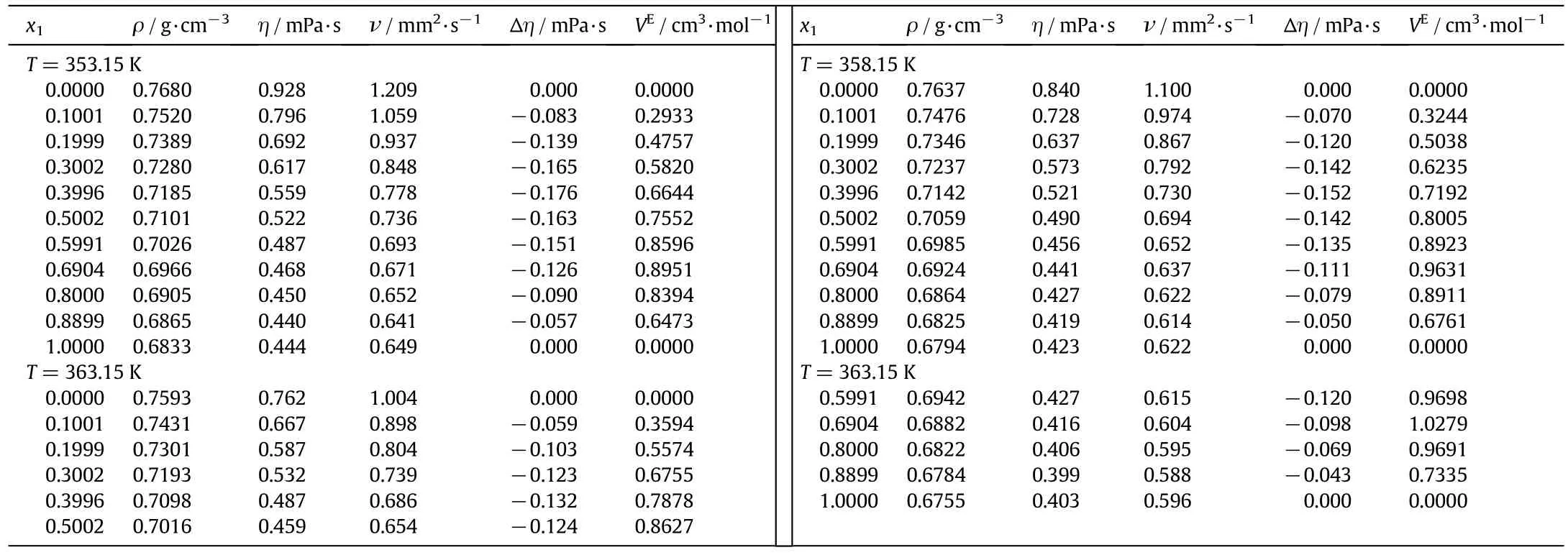
Table 2(continued)
where R is the universal gas constant,T the absolute temperature,V the molar volume of the mixture and Vithe molar volume of the pure components.Calculated viscosity deviations(Δη)and excess Gibbs energy of activation are shown in Tables 2-4and Tables1S-3S,respectively.Excess Gibbs energy of activation of viscous flow has not been reported in the literature for these mixtures.
The dependency of VE,Δη and ΔG*Eon composition is also expressed using a Redlich-Kister equation[50]:

The standard deviation is calculated by:

Thermodynamic parameters for the viscous flow have been studied using the Eyring equation[52,53]:

where h is the Planck constant,A is the Avogadro's number and ΔG*is the Gibbs energy of activation of viscous flow.


Fig.2.Molar Gibbs energy of activation for viscous flow dimensionless[ΔG*/RT=ln(ηV/hN)]as a function of reciprocal temperature at a fixed molar fraction of n-decane(x1)for binary mixtures of n-decane.● 1-pentanol(x1=0.5002);○ 1-hexanol(x1=0.5002);▼ 1-heptanol(x1=0.5008).
The ethanol,1-propanol and 1-butanol,as any other alcohols,tend to strongly associate their molecules through hydrogen bonding.If,in addition,a non-polar solvent is added,as for example n-decane,then a larger molar volume of the mixture would be expected because the theory predicts perturbation and breaking of the hydrogen bonds in the alcohols[41,54,55].The experimental data in Tables 2-4 and Fig.3 show that for all the mixtures,VEis positive in the whole composition range and also an increase of VEwith temperature.This is due to the increase of the kinetic energy of the molecules which diminishes themolecular interactions and finally generates a volume expansion,i.e.the VEincreases.Dubey et al.[35]observed that for the n-decane+1-butanol there is a positive deviation of VEwith the same temperature effect as mentioned here.Dubey et al.[35]suggested that the increase of the VEvalues with the temperature is due to an increase in degrouping of the alcohol molecules at higher temperatures.

Table 3 Density,viscosity,kinematic viscosity,viscosity deviations and excess molar volumes for the binary mixture of n-decane(1)+1-hexanol(2)

Table 3(continued)
From Tables 2-4,it can be observed that the positive deviation extent follows the order:1-pentanol>1-hexanol>1-heptanol.All mixtures display a parabolic functionality of their VEcurves with respect to composition without unusual behavior as seen in Fig.3.However,the excess molar volume of the n-decane+1-heptanol mixture shows a small deviation from the parabolic path at the rich n-decane composition.It is also observed that for this mixture,the VEcurve approaches zero at the lowest temperature.From the macroscopic point of view,the positive value of VEfor all these mixtures indicates a volume expansion during the mixing dominating the physical interaction.
Also,a number of authors have pointed out that the VEbehavior of the 1-alcohol+n-alkane mixtures have been described as a balance of a positive and negative contribution[24,25,56-60].The positive contribution is due mainly to a partial destruction of the hydrogen bridge bonding structure of the 1-alcohols and also to the fact that the physical contribution from the dispersion interactions between two different molecules are weaker than those between two similar ones.The result is an expansion of the mixture.On the other hand,the negative contributions are of structural(packing)nature.These contributions emerge from the interstitial embedding of the n-alkanes molecules inside the hydrogen bonding structures of the 1-alcohols,from the free volume changes,etc.For short chain alcohols+large linear chain n-alkanes mixtures the positive contributions dominate over the negative ones.Our results indicate that the positive contributions dominate over the negative ones.It is agreed with the findings of Al-Dujaili and Awwad[28]and Dubey and Sharma[26]for n-decane+1-pentanol and n-decane+1-hexanol,mixtures,respectively.
In addition,the calculated excess thermodynamic properties from viscosity data and the viscosity deviations(Δη)support the absence of specific interactions between the different molecules in the mixtures,as seen in Fig.4.For all the mixtures treated here,the value ofΔη is negative in the whole composition and temperature intervals.This is an indication of dispersion interactions[33,35,58-60].The absolute value of Δη decreases as the temperature increases for all the systems.
Oh, good father, cried the young man, you will not forsake10 me? Stay with me, I pray you, and lead me to the king! If you wish it, I will, said the hermit, on condition that you will give me half of anything you get
VEis positive and Δη is negative due to the perturbation or breaking of the associative bonds of the molecules.Another important aspect is that,as the molecular chain of the alcohol increases,the absolute value of Δη is also increased.An opposite effect can be observed for the VEproperty.Both effects are due to the structural interactions emerging because the changes in the interstitial embedding of the molecules.These changes are due to the differences in shape and size of the alcohol molecules as observed by Treszczanowicz and Benson[56,57]for 1-alcohol+n-heptane and+n-octane mixtures.
The ΔG*Edisplays a negative deviation of ideality for the whole composition and temperature intervals,as shown in the Supplementary data Tables 1S-3S.Then,the dispersion forces dominate[33,35],in principle,due to the breaking of hydrogen bonds.Also,this deviation indicates that the flow of the mixture is facilitated in comparison to the observed behavior of pure liquids.This mixture behavior is classical of associative systems[39,61].Our results agree with the fin dings of Fort and Moore[62],the viscosity deviation(Δη)and the excess Gibbs energy of activation of viscous flow tend both to be more negative as the interaction forces decrease.The actual negative deviation tendency in Δη and ΔG*Eexplains the weak interactions between different molecules.
The entropy of activation of viscous flow,ΔS*,for the three mixtures shows a gradual decreasing as the molar composition of n-decane increases.The curves for the three systems follow similar paths.These curves start at 10.66,11.40,and 12.72 J·mol-1·K-1for the 1-pentanol,1-hexanol,and 1-heptanol,respectively.The zero value entropy corresponds to x1≈0.266,x1≈0.216 and x1≈0.304,for the mixtures of n-decane with ethanol,1-propanol and 1-butanol,respectively.The negative value of the entropy of activation for the pure ndecane leads us to infer that this liquid is ordered in their transition state of flow[63]as shown in Fig.5,whereas the positive values of entropy for pure 1-pentanol,1-hexanol and 1-heptanol indicate less order in their transition state of flow[63].This is similar to the behavior observed by Chowdhury et al.[54].
The Gibbs energy of activation at T=298.15K for the above systems is depicted in Fig.6.For the mixture of n-decane+1-pentanol the Gibbs energy of activation initially diminishes as the molar fraction of ndecane increases reaching a minimum at x1≈0.87.This indicates that the flow of the mixture is easier at mole fractions lower than 0.87.For the n-decane+1-hexanol and n-decane+1-heptanol mixtures,the Gibbs energy of activation diminishes as the molar fraction of ndecane augments.There are no minimum points along the whole composition range.This indicates that the flow of the mixture becomes progressively easier as the n-decane composition is increased as observed by Chowdhury et al.[54].
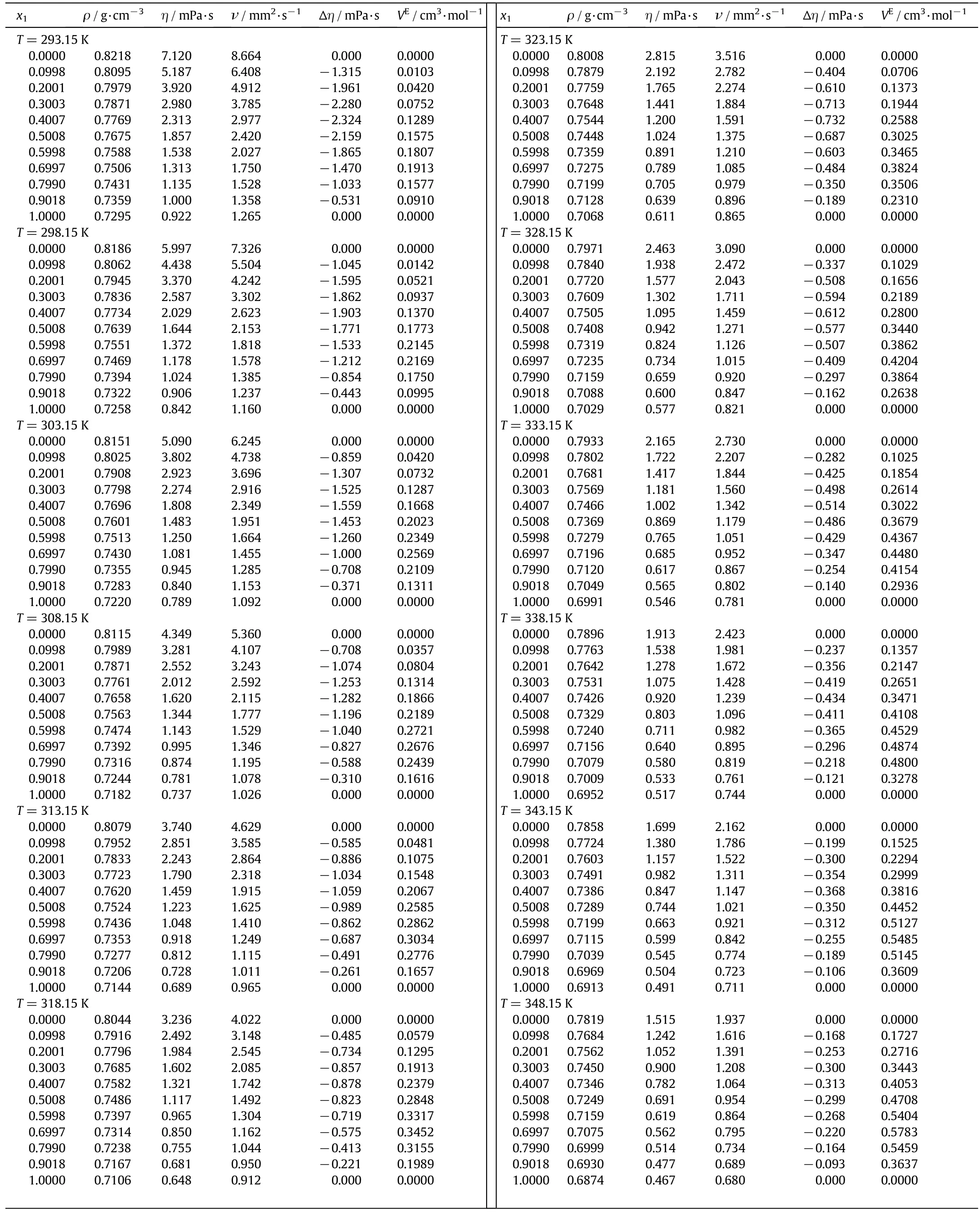
Table 4 Density,viscosity,kinematic viscosity,viscosity deviations and excess molar volumes for the binary mixture of n-decane(1)+1-heptanol(2)

Table 4(continued)
The values of ΔH*are always positive and they tend to diminish as the molar composition of n-decane increases.The curves for the n-decane with 1-pentanol,1-hexanol or 1-heptanol have the same behavior.This suggests the formation of an activated complex which is necessary to facilitate the viscous flow in the n-decane rich region as compared to that in the alcohol rich region.The magnitude of the molar enthalpy of activation ΔH*is greater than TΔS*.Therefore,it can be concluded that the energetic contribution,corresponding to the molar enthalpy of activation of viscous flow,is more important than the entropic contribution.Also,the shape of the curves of(ΔG*,ΔH*,TΔS*)as a function of composition indicates that the same type of interactions can be applied to the binary mixtures of n-decane with 1-pentanol,1-hexanol,or 1-heptanol.
For the whole composition range,the partial molar volumes of these mixturesare also calculated using:
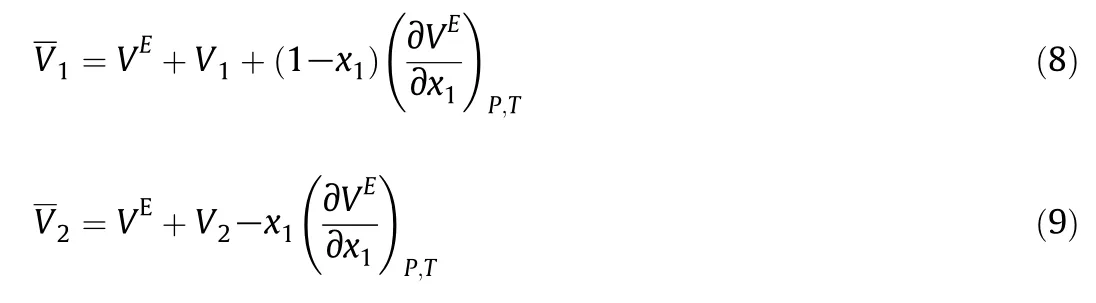
where V1and V2are the molar volumes of pure components 1 and2,respectively.The final equations are:

The calculated partial molar volumes are shown in the Tables 7S-9S.On the other hand,the excess partial molar volumesare also be obtained from:
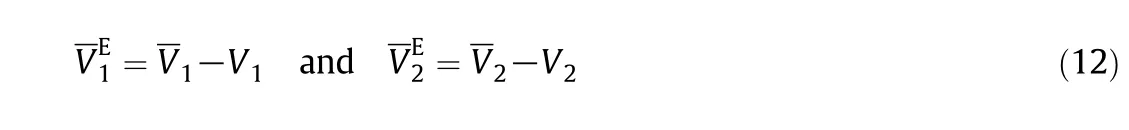
Tables 7S-9S show the calculated values.Then partial molar volume and excess partial molar volumes at in finite dilution can be calculated from Eqs.(11)-(12):
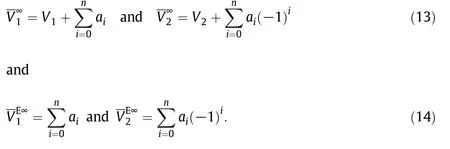
Values of these properties are reported in Supplementary Table 10S at different temperatures.
The positive tendency exhibited byobtained from the experimental studies,is complemented with the partial molar volumes obtained from Eqs.(13)and(14).It is observed that the partial molar volume at infinite dilution of each componentis slightly larger than the corresponding molar volume of the pure ones(V1and V2).The set of excess partial molar volumes at infinite dilutionare positive with the exception of the n-decane+1-heptanol at 293.15 K which is negative,see Table 10S.The observed difference diminishes as the alcohol chain increases but augments with temperature.Further,the excess partial molar volumesare positive for the three mixtures in the whole ranges of composition and temperature,as shown in Tables 7S to 9S.This[58,64-66]also indicates that the interactions between different types of molecules are as weaker as those of molecules of the same nature(see the values ofThese values are small compared to the molar volume of the pure components.The excess partial molar volumes follow the tendency of 1-pentanol1-hexanol1-heptanol,respectively.The positive values ofindicate the presence of interactions of the kind solute-solute/solvent solvent between the molecules in the mixture,whereas the negative values show the presence of important interactions of the solute-solvent type among molecules of different nature[67].
4.Conclusions
In this work density and viscosity data are reported for three binary mixtures of n-decane with 1-pentanol,1-hexanol or 1-heptanol as thesecond component.Data are taken at the temperature range from 293.15 to 363.15 K in intervals of 5 K covering the composition range at atmospheric pressure.The literature data for density and viscosity are available at some temperatures but only for the binary mixture of n-decane+1-hexanol.We extend the temperature interval and take data for two additional binary mixtures,particularly viscosity data.Our experimental results agree with the literature values within 0.15%and 1.20%for the density and viscosity,respectively.Agreement with molar excess volumes and viscosity deviation and literature values is within 0.0086 cm3·mol-1and 2.21%,respectively.For the whole composition and temperature ranges,the excess molar volume(VE)and the viscosity deviation(Δη)displayed negative and positive values respectively.The excess Gibbs energy of activation of viscous flow(ΔG*

Table 5 Coefficients aiof the fitting Eq.(4)and corresponding standard deviation of Eq.(5)
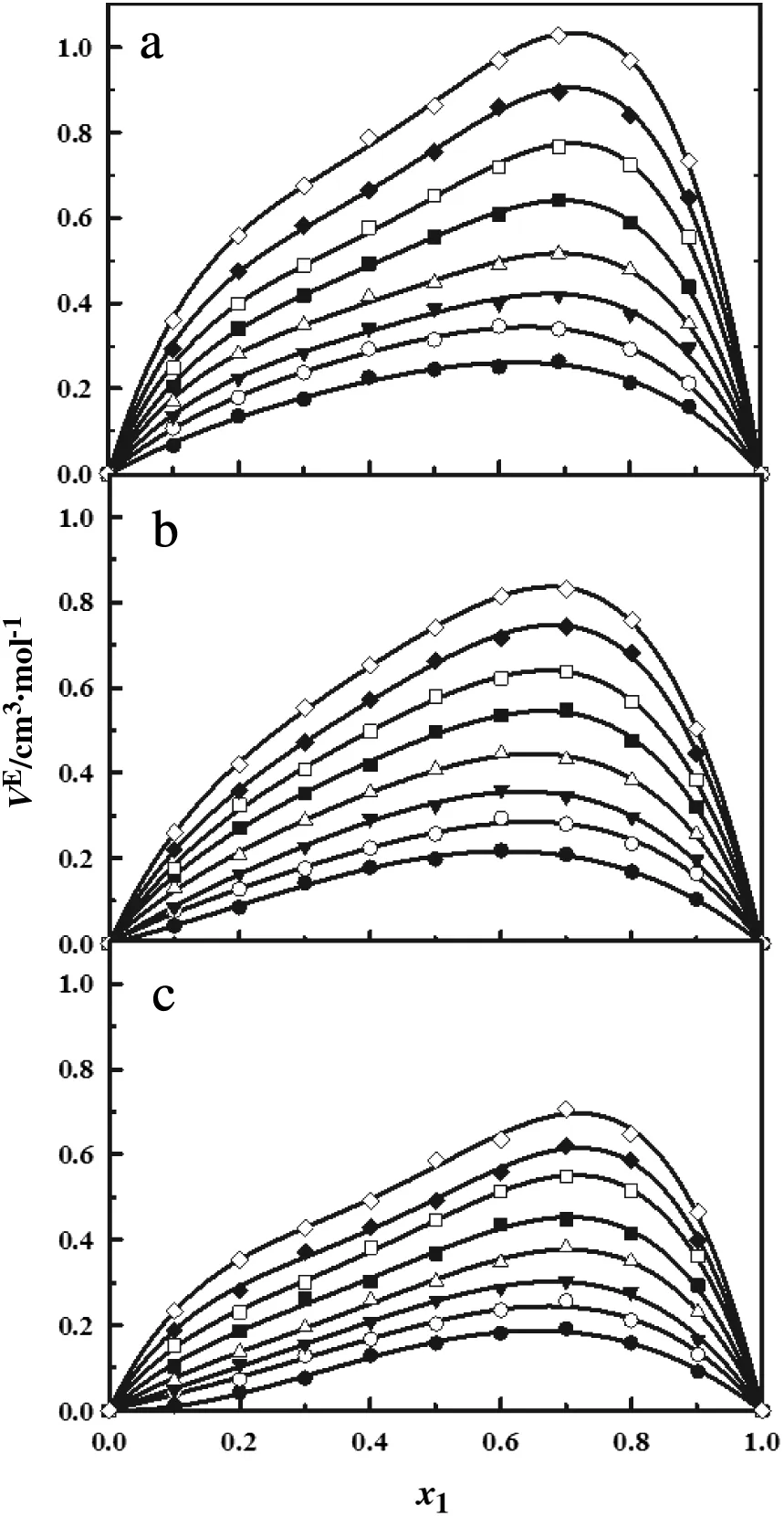
Fig.3.Excess molar volume for the n-decane(1)+1-pentanol(2)(a),+1-hexanol(2)(b)and+1-heptanol(2)(c)mixture as a function of the mole fraction.●293.15 K;○ 303.15 K;▼313.15 K;△ 323.15 K;■ 333.15 K;□343.15 K;◆353.15 K;363.15 K.The solid line corresponds to the Eq.(4).
E)are negative for the three mixtures.In general,the physical or dispersive interactions are the dominant basically due to the breaking of the hydrogen bonds.Weak interactions between the n-decane and the alcohols are observed.These results are additionally complemented with the partial molar and the excess partial molar volumes obtained from Redlich-Kister polynomials.These new measurements will facilitate the understanding of the interactive behavior between polar and non-polar substances.Fig.6.Molar Gibbs energy of activation for viscous flow(ΔG*)as a function of the mole fraction of the n-decane(x1)at T=298.15 K,to the binary mixture.●n-decane(1)+1-pentanol(2);○n-decane(1)+1-hexanol(2);▼n-decane(1)+1-heptanol(2).

Fig.4.Deviation of dynamic viscosity for the n-decane(1)+1-pentanol(2)(a),+1-hexanol(2)(b)and+1-heptanol(2)(c)mixture as a function of the mole fraction.●293.15 K;○303.15 K;▼313.15 K;△323.15 K;■333.15 K;□343.15 K;◆353.15 K;363.15 K.The solid line corresponds to the Eq.(4).
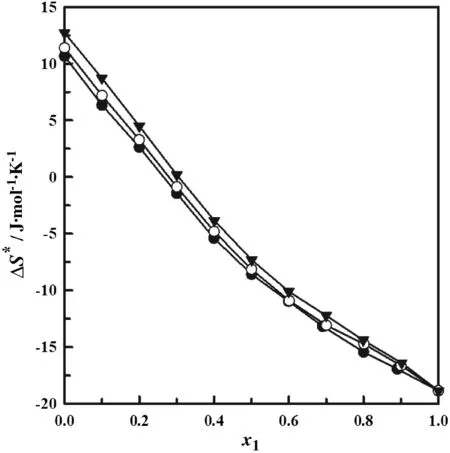
Fig.5.Entropic of activation forviscous flow(ΔS*)as a function of the molefractionofthe n-decane(x1)for binary mixtures.● n-decane(1)+1-pentanol(2);○ n-decane(1)+1-hexanol(2);▼n-decane(1)+1-heptanol(2).
Nomenclature
A Avogadro's number
aicoefficients of the Redlich-Kister polynomial
ΔG* Gibbs energy of activation of viscous flow,J·mol-1
ΔG*
Eexcess Gibbs energy of activation,J·mol-1
ΔH* enthalpy of activation of viscous flow,J·mol-1
h Planck constant
Mimolar mass of species i
N number of experimental data
n number of estimated parameters
R universal gas constant
ΔS* entropy of activation of viscous flow,J·mol-1
T absolute temperature,K
V molar volume mixture,cm3·mol-1
VEexcess volume,cm3·mol-1
Vimolar volume of species i,cm3·mol-1

ximole fraction of species i,mol·mol-1
YEdependent variable of Eq.(4)
Δη dynamic viscosity deviations,mPa·s
η dynamic viscosity of the mixture,mPa·s
ηidynamic viscosity of pure component i,mPa·s
ρ densities of the mixture,g·cm-3
ρidensities of species i,g·cm-3
σ Standard deviation
Subscripts
1,2 component
exp experimental data
cal value calculate of Eq.(4)
P pressure
Appendix A.Supplementary Data Tables
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2013.10.001.
 Chinese Journal of Chemical Engineering2015年3期
Chinese Journal of Chemical Engineering2015年3期
- Chinese Journal of Chemical Engineering的其它文章
- Micromixing characteristics in a gas-liquid-solid stirred tank with settling particles☆
- An experimental study of drag reduction by nanofluids in slug two-phase flow of air and water through horizontal pipes☆
- Effect of surfactant type on interfacial area and liquid mass transfer for CO2absorption in a bubble column☆
- Effects of bubbly flow on bending moment acting on the shaft of a gas sparged vessel stirred by a Rushton turbine☆
- Enhanced heat transfer in a heat exchanger square-duct with discrete V- finned tape inserts☆
- A novel purification process for dodecanedioic acid by molecular distillation
