Physicochemical properties of aqueous solutions of sodium glycinate in the non-precipitation regime from 298.15 to 343.15 K
Muhammad Shuaib Shaikh,Azmi Mohd Shariff*,Mohd Azmi Bustam,Ghulam Murshid
Research Center for CO2Capture(RCCO2C),Department of Chemical Engineering,Universiti Teknologi PETRONAS,31750 Tronoh,Perak,Malaysia
Keywords:
A B S T R A C T
1.Introduction
At present,the global warming is a well-known issue of the worldwide concern.The gases that contributed in global warming include carbon dioxide,methane,nitrous oxide and water vapors.Among these,carbon dioxide is a major gas causing an increase in global temperature to 6°C.The emissions of carbon dioxide are caused by different anthropogenic activities,including combustion of fossil fuels,power plants fired by coal,oil re fineries,sweetening processes of natural gas,automobiles,deforestation and various industries[1-6].The straight effects of global warming are the loss of lives,resources and infrastructure destruction,which finally affect negatively the social,economic and environmental agents[1,7-10].
There are so many options for capturing CO2from various gas streams,which include absorption through chemical solvent,physical absorption,separation by membrane,cryogenic process and microbial/algal systems[7].Among all technological options,the most commonly used one is chemical absorption,indicating 70%of all the techniques used for CO2removal[6].Various types of solvents have been used since long time to capture CO2from various gas streams.The most widely used solvents in industrial applications are alkanoamines,like monoethanolamine(MEA),diethanolamine(DEA)and methyldiethanolamine(MDEA)[12-14].Originally,the use of various conventional solvents such as MEA,DEA,and MDEA has been preferred for several benefits.One of the bene fits is the alcohol group,which minimizes drastically the vapor pressure of amine,resulting in almost no contamination of the treated gas.They also allow tunable amine reactivity[16].After extensive investigations,it was found that,these alkanoamines solvents have several limitations,such as the shorter lifetime due to oxidative degradation of amine.They can cause corrosion of pipelines and equipment,which reduces the life of equipment,unscheduled maintenance,loss of production and increasing management expenses to repair the corroded system[15,17,18].During regeneration,energy cost is also high in combination with the loss of liquid due to evaporation of the solvent due to high volatility of solvent[19,22].Besides, flooding and entrainment of the absorption liquid limit the process and the liquid gas stream cannot be controlled independently[11].
Recently,amino acid salt solutions in an aqueous form have been recommended as an effective substitute to alkanoamine solvents,as they react with CO2in the same manner like alkanoamines.The interest is increased toward their use due to various positive characteristics such as higher resistance to degradation,low volatility and smaller amount of oxidative degradation products[11,16,20,21].These amino acids can be made non-volatile by adding salt functionality,which lowers the liquid loss and consumption of energy associated with the process[11].Amino acids have also been investigated together with the amines like MEA as a solvent blend for CO2absorption for enhancing the properties of amino acids[23].The potential benefits shown by the amino acids have motivated us to investigate further the properties of amino acids as potential application in bulk removal of CO2from natural gas.
For proper selection of solvent based on physical properties,it is essential to study the main physicochemical properties like density,viscosity and refractive index of the solvent with close interval of the
parameters like temperature and concentration[27-29].Density and viscosity data are very important to determine the rate modeling and reaction rate constants,which are required to design and optimize acid gas contact or.Whereas,the refractive index data is helpful to determine the composition of solvents.Molar refractions can also be calculated from refractive index data,which can be further used to determine the molecular interactions of the solvent mixtures[30-32].Physical property data on density,viscosity and surface tension of aqueous sodium glycinate as potential solvent for CO2capture have been previously reported[24,25].The studies were conducted at high solvent concentrations as their interest is to achieve high CO2removal.However,this solvent was precipitated at concentrations higher than 2.0 mol·L-1[21],which can result in operational issues in the absorption system such as a blockage of equipment and flow lines.It is also identified that the concentration range below 2.0 mol·L-1is a non-precipitating region.Therefore,for the removal of CO2without precipitation,physicochemical analysis of aqueous sodium glycinate at lower concentrations needs to be studied.In this work,physicochemical properties of aqueous sodium glycinate solutions from 0.0096 to 0.1769 mass fraction have been studied and reported.
2.Materials and Methods
The chemicals,i.e.,glycine(≥99%pure)and sodium hydroxide(≥99%pure),were obtained from MerckSdn.Bhd,Malaysia.The further description of materials used in this study is given in Table 1.Sodium glycinate was prepared in an aqueous form by taking an equimolar quantity of glycine and sodium hydroxide.The chemical structure is shown in Fig.1.The different mass fractions as 0.0096,0.0474,0.0928,0.1365,and 0.1769 of sodium glycinate were prepared for this study.The concentration values were accurate with the uncertainty of±0.001.The wide range of temperature of 298.15,303.15,308.15,313.15,318.15,323.15,328.15,333.15,338.15,and343.15K was used to measure all three properties of aqueous sodium glycinate solutions.Before the analysis of samples,all apparatus were calibrated with the water of Millipore quality.The calibration data obtained in this work was compared with literature and reported with percentage average absolute deviation.The aqueous solutions of sodium glycinate of the same concentration as published in the literature[24,25,37]were prepared and analyzed for validation of the methods and results.
Digital Anton Par Density meter(DMA-4500 M)was used to measure the densities.The equipment was calibrated repeatedly after completing each measurement by standard water of Millipore quality.The data reported was average of three readings with uncertainty of±0.00003 g∙cm-3and ±0.01 K respectively.Coefficients of thermal expansion for all the concentrations were calculated from the correlated parameters of the densities using Eq.(5).
Digital Anton Par micro viscometer(rolling ball type)model(Lovis-2000 M/ME)was used to measure the viscosities in mPa·s.The equipment was calibrated continually with Millipore quality water after the end of each measurement.Before each measurement,the sample was kept inside the viscometer until the set temperature reached at equilibrium condition,and the temperature accuracy was±0.02 K.Each experiment was repeated three times and the data reported was the average with the uncertainty of±0.002 mPa·s.
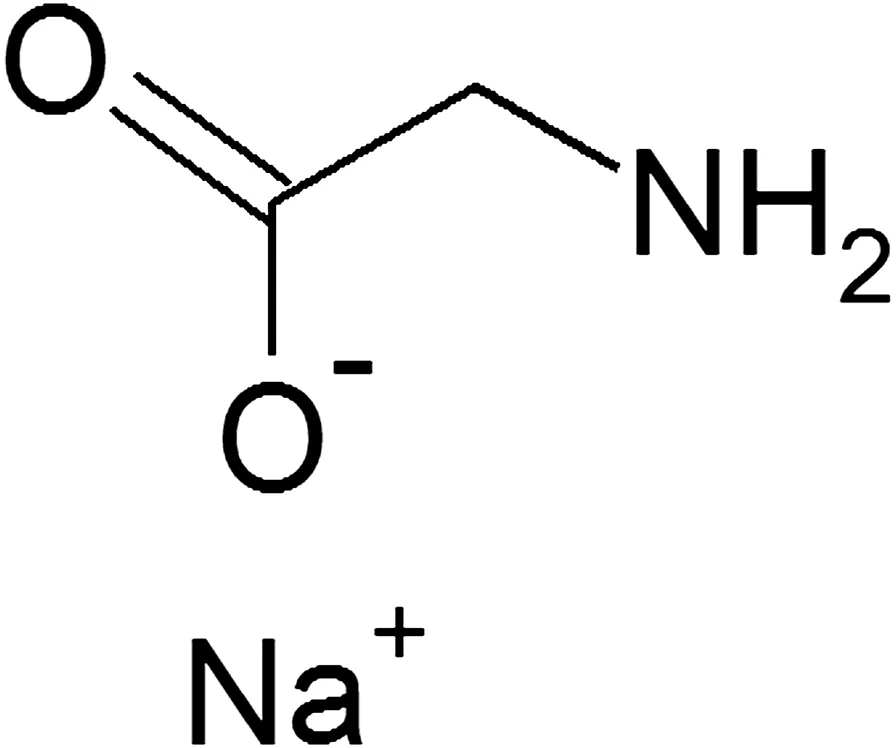
Fig.1.Chemical structure of sodium glycinate.
Digital Anton Par refractometer(Abbemat,model WR)was used to measure the refractive index,nDof aqueous sodium glycinate solutions.The refractive indices were measured with the temperature accuracy of±0.03 K.At the end of each experiment,the equipment was calibrated by standard water of Millipore quality.The reported data was the average of three measurements with the uncertainty of±0.00005 nD.
3.Results and Discussion
The experimentally measured data for calibration is presented in Table 2 with percent average absolute deviation(AAD)of 0.0276 for density,0.0411for viscosity and 0.0071for refractive index respectively.The percent average absolute deviation(AAD)was calculated by the following(Eq.(1))[27].

The calibration of the equipment was conducted using Millipore water,and the calibration results were compared with the literature values.The deviation of the results indicated the proper calibration of the equipment.Furthermore,to confirm that the appropriate methodology was followed,the literature data were also compared with the experimental ones on sodium glycinate for density and viscosity of the same concentration as published in[24,25,37].The comparison for the refractive index of aqueous sodium glycinate solution was not conducted since the literature data were not available.From Table 3,it was observed that the literature data are in good agreement with the reported data for density and viscosity of the aqueous sodium glycinate.The percent average absolute deviations(AAD)for density and viscosity of sodium glycinate solutions are for 10%(by mass),0.0357 and 0.0113,20%(by mass),0.0104 and 0.0206 and 30%(by mass),0.0108 and 0.0105,respectively.
The experimentally measured values of the physicochemical properties,including densities and viscosities of aqueous sodium glycinate solutions of different mass fractions of 0.0096,0.0474,0.0928,0.1365,and 0.1769 at different temperatures of 298.15,303.15,308.15,313.15,318.15,323.15,328.15,333.15,338.15 and 343.15 K are presented in Tables 4 and 5 respectively.The temperature dependence of density and viscosity of aqueous solution of sodium glycinate is shown in Figs.2 and 3,respectively.The experimental results of the density measurement show that with increasing temperature,the density values decrease,while the density increases with increasing concentration.The similar trends have been reported by various researchers[23-25].It is observed that the viscosity of the sodium glycinate increases with increasing concentration of sodium glycinate due to the reason thatsodium glycinate is an ionic compound.It has the potential to enhance the ionic strength of the solution that can results in the viscosity increase of the solution.The effect of temperature on the viscosity shows that,the viscosity decreases with increasing temperature.The decreasing trend of the measured density and viscosity with rising temperature is due to the reason that when temperature of solution mixtures increases,the intermolecular forces of attraction between them decrease,therefore,the molecules of liquid mixtures occupy wider space thus reducing the density and viscosity[23-25].The experimental results of density and viscosity are correlated by the least square method as a function of temperature by using Eqs.(2)and(3),respectively,and coefficients are reported in Tables 6,and 7 with AADs.The average absolute deviations for both properties are calculated using Eq.(4)[38].

Table 1 Description of materials used

Table 2 Calibration of apparatus
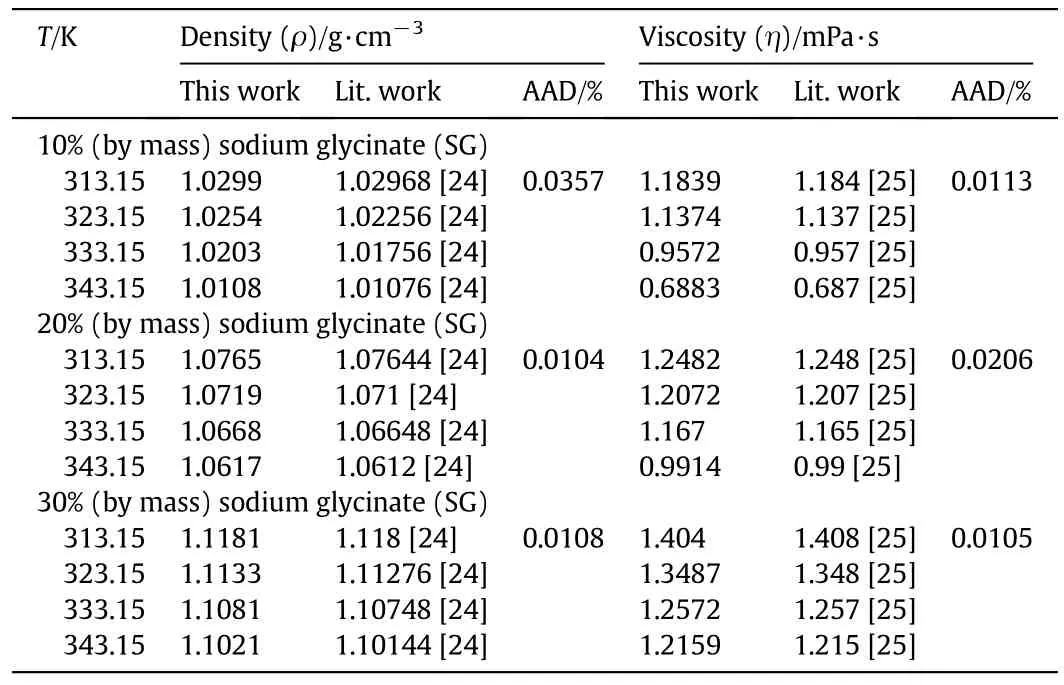
Table 3 Comparison of experimental and literature data
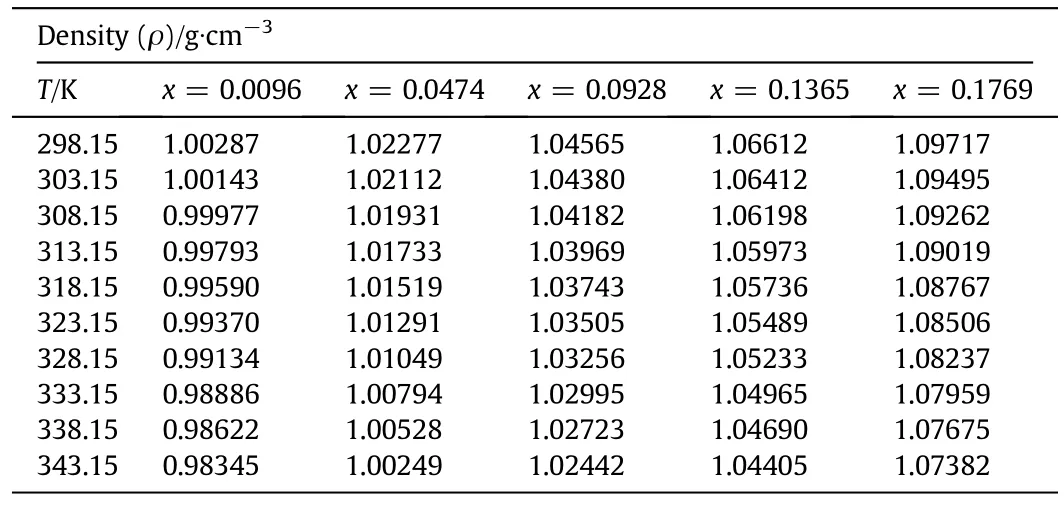
Table 4 Densities(ρ,g·cm-3)of different mass fractions(x)of aqueous sodium glycinate(SG)solutions
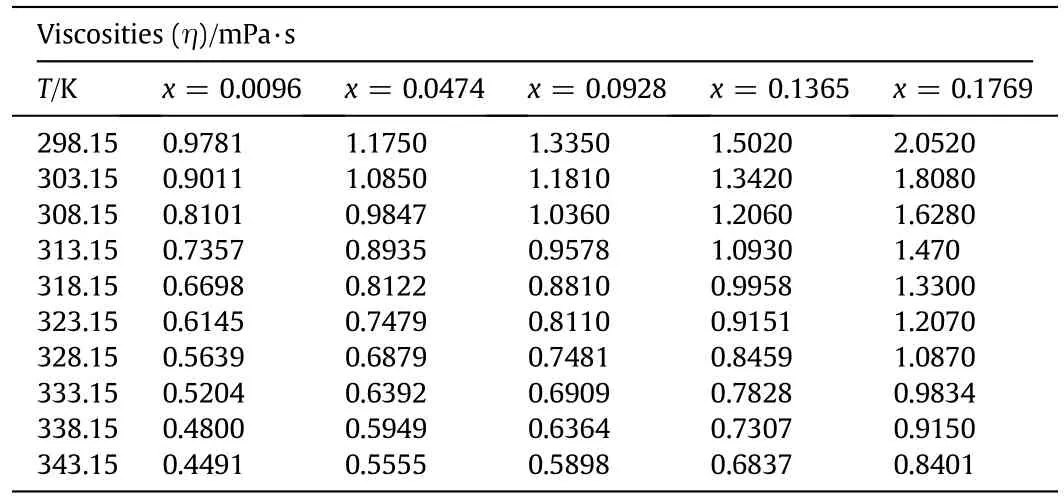
Table 5 Viscosities(η,mPa·s)of different mass fractions(x)of aqueous sodium glycinate(SG)solutions

Fig.2.Densities of different mass fractions of aqueoussodiumglycinate(SG)solutions as a function of temperature:◊0.0096;Δ 0.0474;□0.0928;○ 0.1365;× 0.1769.
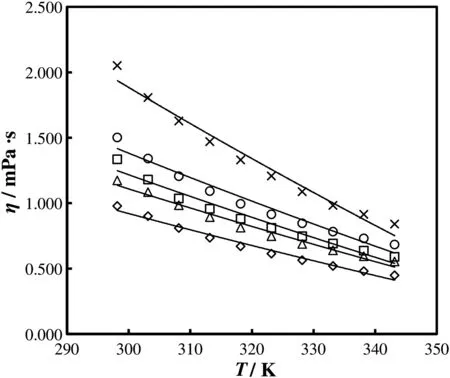
Fig.3.Viscosities of different mass fractions of aqueoussodiumglycinate(SG)solutions as a function of temperature:◊0.0096;Δ 0.0474;□ 0.0928;○ 0.1365;× 0.1769.

where(ρ,g·cm-3)is the density,(η,mPa·s)denotes viscosity,and A1and A2in Eq.(2)and B1and B2in Eq.(3)are correlation parameters.InEq.(4),yexpandycalrepresent the experimental and calculated values of the measured properties,and n is the number of data points.

Table 6 Correlation Eq.(2)parameters and AAD for densities(ρ,g·cm-3)of different mass fractions of aqueous sodium glycinate(SG)solutions
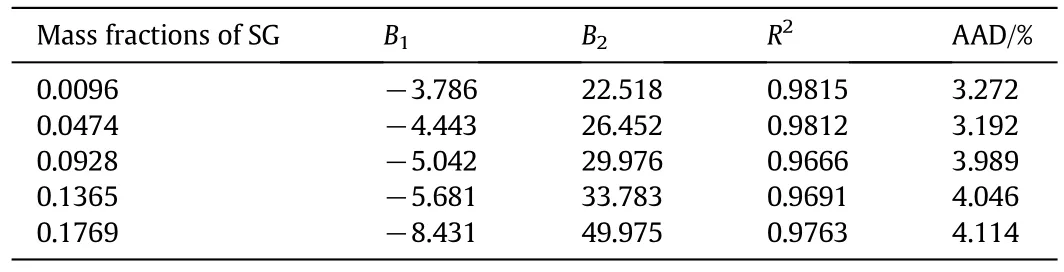
Table 7 Correlation Eq.(3)parameters and AAD for viscosities(η,mPa·s)of different mass fractions of aqueous sodium glycinate(SG)solutions
Thermal expansion coefficients for various concentrations are calculated from the correlation parameters of the densities using Eq.(5)obtained from density correlation[35].
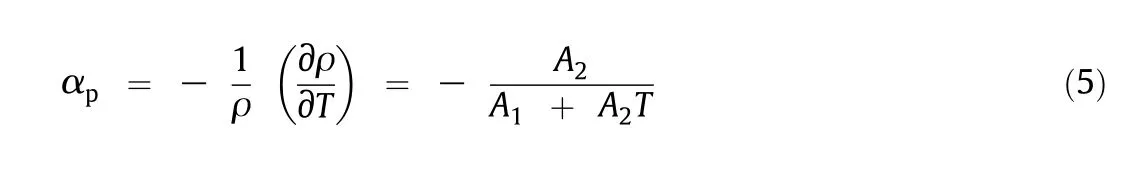
where αpis the thermal expansion coefficient and the values of the thermal expansion coefficients are listed in Table 8.Coefficients of thermal expansion slightly increase with the rise of the temperature and concentration.
The measured values of refractive index of aqueous sodium glycinate solution are presented in Table 9,and its temperature dependence is shown in Fig.4.The refractive index of the sodium glycinate in the aqueous form also increases with increasing concentration of sodium glycinate in the solution and decreases with increasing temperature.For different solvents,the trend of the refractive index with change in temperature and concentration is similar[26,36].The refractive index data are correlated using Eq.(6).

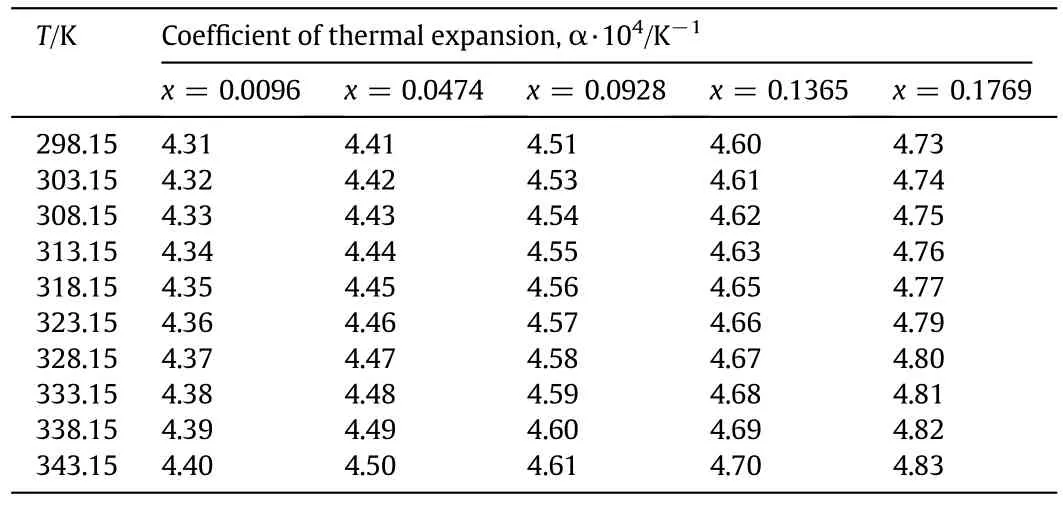
Table 8 Coefficient of thermal expansion of different mass fractions(x)of aqueous sodium glycinate(SG)solutions at different temperatures using Eq.(5)

Table 9 Refractive indices(nD)of different mass fractions of aqueous sodium glycinate(SG)solutions
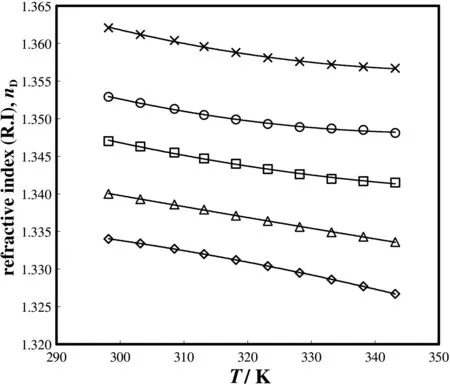
Fig.4.Refractive indices of different mass fractions of aqueous sodium glycinate(SG)solutions as a function of temperature:◊0.0096;Δ 0.0474;□0.0928;○0.1365;×0.1769.
where nDis the refractive index,and C1,C2,and C3,are the correlation parameters listed in Table 10 with the values of AAD.
4.Conclusions
The physicochemical properties of aqueous sodium glycinate solution as a solvent for CO2absorption in non-precipitation regime were studied in the present work at a temperature range of 298.15 to 343.15 K.It wasobserved that the density,viscosity and refractive index of the aqueous sodium glycinate solution increased with increasing concentration of the sodium glycinate in the solution and decreased with increasing temperature.Coefficients of thermal expansion slightly increased with rise in temperature and concentration of sodium glycinate.The trend of temperature and concentration with the measured properties was consistent with the findings of other researchers.The predicted values obtained from correlation equations were in fairly good agreement with the measured values for all properties,and hence can be used in future CO2removal system design.

Table 10 Correlation Eq.(6)parameters and AAD for refractive index(nD)of different mass fractions of aqueous sodium glycinate(SG)solutions
AcknowledgmentsThe authors are thankful to Universiti Teknologi PETRONAS for providing financial support(Grant number YUTP-15-8209-005)and RCCO2C for technical support to complete the present research work.
 Chinese Journal of Chemical Engineering2015年3期
Chinese Journal of Chemical Engineering2015年3期
- Chinese Journal of Chemical Engineering的其它文章
- Micromixing characteristics in a gas-liquid-solid stirred tank with settling particles☆
- An experimental study of drag reduction by nanofluids in slug two-phase flow of air and water through horizontal pipes☆
- Effect of surfactant type on interfacial area and liquid mass transfer for CO2absorption in a bubble column☆
- Effects of bubbly flow on bending moment acting on the shaft of a gas sparged vessel stirred by a Rushton turbine☆
- Enhanced heat transfer in a heat exchanger square-duct with discrete V- finned tape inserts☆
- A novel purification process for dodecanedioic acid by molecular distillation
