Kinetic studies on the dimerization of isobutene with Ni/Al2O3as a catalyst for reactive distillation process☆
Liwei Tong,Lifang Chen,Yinmei Ye,Zhiwen Qi,*
1Max Planck Partner Group at the State Key Laboratory of Chemical Engineering,School of Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
2School of Resources and Environmental Engineering,East China University of Science and Technology,Shanghai 200237,China
Keywords:
A B S T R A C T
1.Introduction
As a commonly used additive of gasoline,methyl tert-butyl ether(MTBE)can not only enhance the octane rating of gasoline but also reduce the amount of exhaust emissions.However,due to its involvement with groundwater contamination[1],MTBE has been banned as a gasoline blend in California and other states of the United States.Many enterprises and research institutions began to find substitutes for MTBE,such as tert-butyl ethyl ether(ETBE),tert-amyl methyl ether(TAME)and isooctane[2].Meanwhile,the shrinking market of MTBE might cause a significant descent in the consumption of IB from fluid catalytic cracking and steam cracking processes.Thus,it is crucial to develop a new route to take full use of the redundant IB.The new route,of which the product can be used as a gasoline additive directly,will be desirable if it can directly take advantageous of the C4 resources and the current MTBE units such as the reactive distillation(RD)column with minimum modification.
Isooctane,one of the branched paraffin with carbon numbers in the range of 8 to 12,is confirmed as a reasonable replacement of MTBE.It is synthesized either by direct alkylation of isobutene with C4 ole fines or by indirect alkylation[3].The process of indirect alkylation of isobutene is divided into 2steps:(1)the production of diisobutenes(DIBs)including 2,4,4-trimethyl-1-pentene(TMP-1)and 2,4,4-trimethyl-2-pentene(TMP-2)by dimerization of IB;and(2)the synthesis of isooctane by hydrogenation of DIBs.
As demonstrated in Fig.1,several byproducts such as triisobutene(TIB)and teraisobutene(TEB)can be produced for the complex seriesparallel reaction network of oligomerization of IB.These byproducts are not only unsuitable as gasoline additives but also possible to promote the catalyst deactivation[4].As a sequence,the selectivity toward DIB is a crucial issue to concern.Dimerization of IB can be carried out in the liquid phase using solid acid catalysts.Hauge et al.[5]and Yaocihuatl et al.[6]studied the reaction on zeolites,and they reached a high selectivity toward DIB.However,the stability of catalyst was not satisfied.Ion exchange resin is also widely used[7-9].Different polar components such as methanol,MTBE,water and tert-butyl alcohol(TBA)[10-12]are used as selectivity enhancers due to its high activity,which causes special costs for the separation of the added components from final products.Besides applying different catalysts,another approach for improving the selectivity to DIB is to employ multifunctional reactors,such as RD.Ouni et al.[13]reported a case study of this reaction by RD with 78 ideal stages,which implied a large investment from an industrial point of view.Kamath et al.[14]studied the feasibility of the dimerization of IB in RD by simulation and found that a hybrid RD was capable of carrying out the reaction and simultaneously separating the products efficiently.Talwalkar et al.[15]made experiments on the dimerization of IB in RD column and reported that the use of polar components as selectivity enhancers was not necessary by RD technology.The above studies on the feasibility of RD were based on the ion exchange resin such as Amberlyst 15.However,catalytic systems consisting mainly of nickel compounds supported on oxides,zeolites and organometallic nickel complexes have been widely used as dimerization catalysts and their feasibility of IB oligomerizaiton in RD is seldom reported.
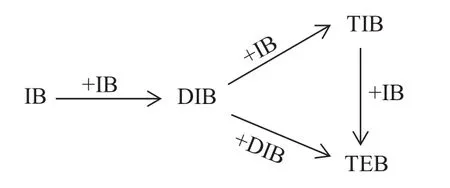
Fig.1.Reaction network of oligomerization of isobutene.
The aim of this contribution was to evaluate the feasibility of IB oligomerization by Ni/Al2O3as a catalyst in RD.The influence of the important parameters(e.g.temperature,the amount of active component(Ni)loading and initial concentration of IB)on the IB conversion and DIB selectivity was investigated based on the catalyst.A kinetic model was developed for such a system under nonpolar conditions based on the laboratory batch reactor data,which was applied to the simulation of DIB productions by RD.
2.Experimental
2.1.Materials
IB(>99.0%,by mass)was obtained from Foshan Shike Air Chemical Ltd.and n-pentane(AR,>98.5%)was supplied by Shanghai Linfeng Chemical Reagents Ltd.,both were used as received.NiSO4·6H2O(AR,>98.5%)and N2(>99.999%)were purchased from Guoyao Chemical Reagents Ltd.The catalyst support Al2O3was supplied by Jiangxi Xinquan Packing Ltd.Its physical- chemical properties are given in Table 1.
2.2.Catalyst preparation and characterization
Ni/Al2O3catalyst was prepared by the incipient impregnation method.The Al2O3support was immersed in the solutions that contained a specific amount of Ni for 4 h at 333 K.The solid was dried for 12 h at 393K and the ncalcined or 8h at 773K.After the above described treatment,the amount of Ni loading was detected by inductively coupled plasma-atomic emission spectroscopy(ICP-AES)(IRIS 1000 Thermo Elemental Co.Ltd.USA).X-ray powder diffraction(XRD)measurements were performed on a Rigaku D/MAX 2550 VB/PC instrument with scan speed of 2(°)·min-1,scan range of 10°-80°at 40 kV and 100 mA.The Thermo Gravimetric Analysis(TGA)pattern was detected by thermos-gravimetric analyzer(SDT Q600 TA Co.USA).The sample was heated from 298 K to 1073 K at a heating rate of 10 K·min-1with air atmosphere in flow of 100 ml·min-1.
2.3.Apparatus and procedure
A500ml stainless steel autoclave(Berghof Ltd.,Germany),equipped with speed and temperature monitoring facilities and an automatic cooling system,was used for all batch reactions.Two calibrated feed containers(150 ml and 75 ml)were used to precisely adjust feed concentration that was pressed into the batch reactor by pressured N2.At the beginning,the specific quantities of the catalyst were charged into the reactor and N2was fed into to exclude air.Then,the solvent n-pentane in the feed container(150 ml)was charged into the reactor.As the mixture was heated to given temperatures,the IB in the feed container(75 ml)was fed into the reactor and the agitation was activated.Meanwhile,the pressure of the reactor was adjusted to a desired value by N2and the corresponding time was regarded as the zero reaction time.Samples were withdrawn at the different time intervals by a self-made micro sampler.All the samples were frozen by ice to avoid the volatilization.
2.4.Sample analysis
All reactants and products were analyzed by a gas chromatograph(Agilent 7890A)using DB-1 column(connected to FID).The column temperature was maintained at 318 K for the first 2 min and then was raised at a rate of 15 K·min-1to 523 K for 5 min.The correction factors of all components were close to 1.0,so the area of peak normalization method was applied to calculate the mass fraction of reactants and products.
2.5.Conversion and selectivity
The conversion of IB(XIB)and selectivity(Si)to DIB,TIB and TEB were calculated on the basis of various products formed:

3.Results and Discussion
3.1.General course of the reaction
The oligomerization of IB is effected by various factors such as active component(Ni)loading,catalyst loading,temperature and initial IB concentration.With the increase of Ni loading,the conversion of IB is dramatically enhanced,while the selectivity to DIB is dropped.The temperature also plays an important role in the oligomerization of IB.As a strong exothermic reaction(ΔHr=-346.9 kJ·mol-1)[16],high temperatures facilitate the desired dimerization of IB as well as the undesirable trimerization and oligomerization.
In this work,the effect of three major reaction conditions(temperature,catalyst loading and initial IB concentration)was examined with a suitable Ni loading decided by experiments.Moreover,properties of catalyst were analyzed through XRD and differential scanning calorimetry-thermogravimetric analysis(DSC-TG).
3.2.Mass transfer effect
Stirrer speeds of200,350and500r·min-1were tested during a preliminary experiment to ensure that external mass-transfer limitations were not present in the experiments.As shown in Fig.2,the masstransfer effect is negligible at the agitation of 500 r·min-1.Thus,in the further experiments,the mixing speed was fixed at 500 r·min-1.

Table 1 Physical-chemical properties of catalyst support
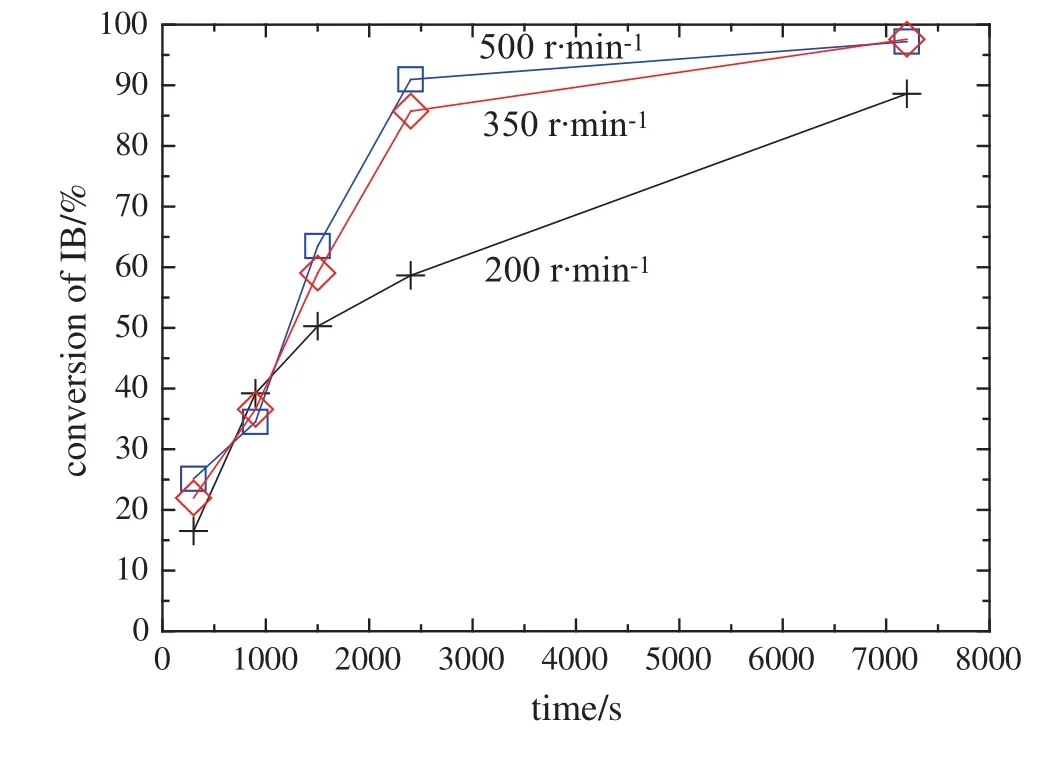
Fig.2.Effect of mixing speed on the IB conversion.(temperature 353 K,pressure 2.0 MPa,catalyst loading 0.12 g·g-1of IB and reaction time 2.0 h).
3.3.Effect of Ni loading
The effect of active components loading(Ni)was studied over the range of 0.5%-3%(by mass).As shown in Fig.3,at high Ni loading,the dimerization and side reactions are simultaneously enhanced and a high conversion of IB can be reached.In addition,as DIB is an intermediate component,it is consumed in the subsequent side reactions to form TIB and TEB.Thus,the selectivity to DIB is significantly declined from 94%to 15.5%with increasing the Ni loading from 0.5%to 3.0%(by mass),while TEB and TIB reveal an opposite trend.In the reactive distillation,DIB could be removed from the column immediately to restrain further side reactions by the effect of distillation.However,at a high Ni loading,namely high activity of catalyst,a large amount of DIB may be produced and remain in the column,which promotes side reactions and further lowers DIB selectivity.Thus,the catalyst should be the moderate activity.

Fig.3.Performance of catalysts with different Ni loading.[temperature 353 K,pressure 2.0 MPa,IB concentration 30%(by mass),catalyst loading 0.12 g·g-1of IB and reaction time 2.0 h].X—conversion of IB;S—selectivity.
Fig.4 further indicates the effect of catalyst activity where the experiments with two different sets of Ni loading(1%and 3%,by mass)were carried out.For the case of 3%(by mass)Ni loading,it can be seen that the selectivity to TIB is the highest one(STIB>60%),which is caused by the high activity of catalyst.However,for the case of 1%(by mass)Ni loading,DIB is the main product(SDIB>75%)and the amount of TIB and TEB show a dramatic decline compared with those of 3%(by mass)Ni loading.Consequently,1%(by mass)Ni loading was selected for the following experiments due to its moderate activity.

Fig.4.General course of the reaction at catalysts with different Ni loading.[temperature 353 K,pressure 2.0 MPa,IB concentration 30%(by mass),catalyst loading 0.12 g·g-1of IB and reaction time 2.0 h].+IB conversion;□DIB selectivity;TIB selectivity;○TEB selectivity;solid line Ni 1%(by mass);dash line Ni 3%(by mass).
3.4.Effect of temperature
A series of experiments were conducted to investigate the effect of temperature over the range of 338 K-383 K.Fig.5 shows the effect of temperature on IB conversion and DIB selectivity.At 353 K,the DIB selectivity reaches a maximum(85%)and then declines at higher temperatures.This is because the activation energy ETIBis less than EDIB(seeTable2),and thus high temperatures facilitate side reaction.Meanwhile,the formation of TIB and TEB depends on the concentration of DIB,and then as the concentration of DIB increases,the reactions to form TIB and TEB will begin,which lowers the concentration and selectivity to DIB.

Fig.5.Effect of temperature on IB conversion and DIB selectivity.[pressure 2.0 MPa,IB concentration 30%(by mass),catalyst loading 0.12 g·g-1of IB and reaction time 2.0 h].X—conversion of IB;S—selectivity.

Table 2 Values of parameters and objective function Φ for the proposed model
3.5.Effect of catalyst loading
The catalyst loading was expressed as the weight ratio of the catalyst to IB charged to the reactor,which was varied from 0.06 to 0.24.As shown in Fig.6,since the oligomerization of IB is a series-parallel reaction,the DIB formed by IB dimerization will be further transferred into TIB by trimerization at high catalyst loading.Therefore,with increasing catalyst loading,the IB conversion is enhanced and the selectivity toward DIB is decreased.The catalyst loading of 0.12 g·g-1of IB is selected for further experiments.

Fig.6.Effect of the catalyst loading on IB conversion and DIB selectivity.[temperature 353 K,pressure 2.0 MPa,IB concentration 30%(by mass)and reaction time 2.0 h].
3.6.Effect of IB concentration
Two different sets of initial IB concentration(30%and 50%,by mass)are conducted to investigate the effect of IB concentration,as shown in Fig.7.The increase in the initial IB concentration will stimulate both the dimerization reaction and side reactions.However,at higher initial IB concentrations,more IB will be adsorbed on the catalyst surface,which leads to strengthen side reactions and more TIB and TEB are produced.Thus,the initial IB concentration of 30%(by mass)is recommended.
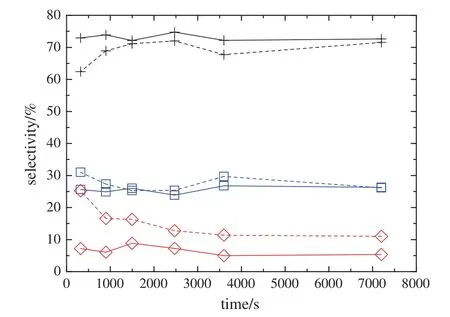
Fig.7.Effect of IB concentration on the product composition.(temperature353K,pressure 2.0 MPa,catalyst loading 0.12 g·g-1of IB and reaction time 2.0 h).Solid line—30%(by mass);dash line—50%(by mass);+DIB;□ TIB;TEB.
3.7.Deactivation of the catalyst
The deactivation of the catalyst was studied by analysis of XRD and TG for the catalyst.The reaction lasted for 12 h at 383 K,2.0 MPa and 0.12 g·g-1of IB catalyst loading.The XRD results shown in Fig.8 indicate the catalyst properties before and after reaction.They both exhibit several2θ peaks around19°,37°,45°,60°,and 66°,in which19°and37°represent Ni,and 45°,60°and 66°represent Al2O3.The two XRD graphs are almost the same,which means the phase state of active component Ni is kept constant after reactions,suggesting that the activation of catalyst remains stable.

Fig.8.XRD patterns of catalysts.
Fig.9 shows the DSC-TGA results for catalyst before and after reaction.In respect of pure NiSO4,DSC curve indicates endothermic peaks at approximate 673 K and 1146 K,resulting from lose of water and decomposition of Ni respectively.As can be seen in Fig.9(a),there is a narrow peak for catalyst before reaction,which is caused by lose of water adsorbed on the catalyst surface.Two peaks appear after reaction,which are also caused by lose of adsorbed water and crystallized water separately.As can been seen in Fig.9(b),the mass of catalyst is lost by 4.8%after reaction.Thus,there exists little coking on the catalyst.
4.Kinetic Models
The Langmuir-Hinshelwood(LH)and the Eley-Rideal(ER)mechanisms are two kinds of popular model for IB oligomerization in which the kinetic model varies with different catalysts and conditions.In this work,the kinetic model of IB oligomerization was conducted in the absence of polar components with Ni/Al2O3as the catalyst.In preliminary experiments,both LH and the ER model were conducted while the results demonstrated that LH model was more suitable for IB oligomerization.The possible products of IB oligomerization were DIB,TIB and TEB.However,in the research of Honkelad and Krause[17],the kinetics were studied in a continuous stirred tank reactor(CSTR)with a commercial ion-exchange resin catalyst.The authors argued that TEB needs not to be considered in the model because of their negligible concentration.The formation of TEB was also ignored in the model since the selectivity to TEB was less than 1.5%in the experiments.Therefore,the reactions involved in this work are as follows:
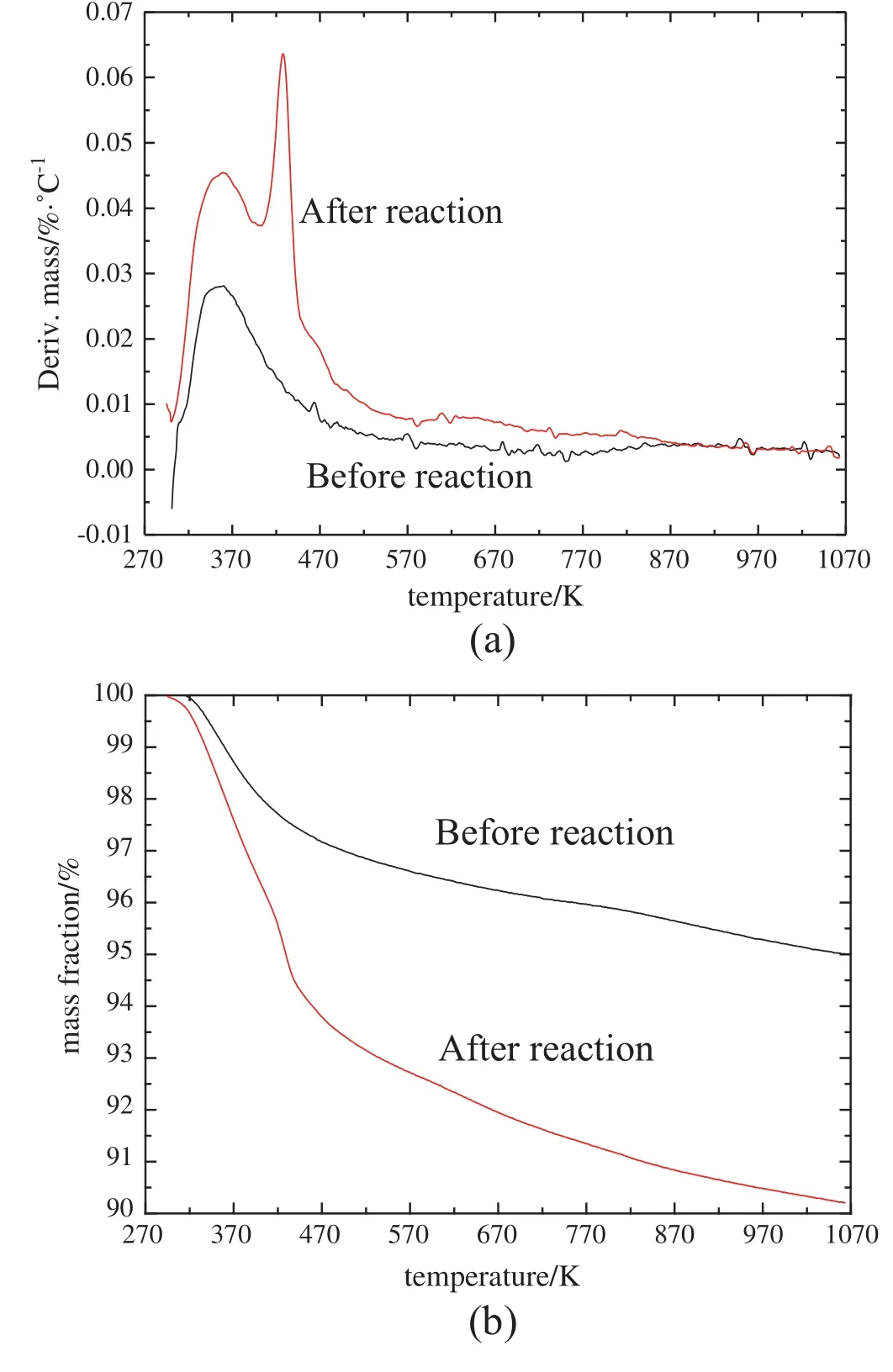
Fig.9.DSC-TG results for catalysts before and after reaction.
Reaction 1:IB+IB→DIB
Reaction 2:DIB+IB→TIB
This means that two active sites are needed for each reaction and the dimerization system consists of the following catalytic steps:
(1)Adsorption on the active site.The IB and n-pentane(NP)are adsorbed on catalyst surface to form the intermediate product

(2)Reactions on the catalyst.
The adjacent adsorbed IBs form DIBs complex which continue to combine with adsorbed IBs to produce TIB complex.The reaction mechanism of C+is assumed as the research of Saus and Schmidl[22].

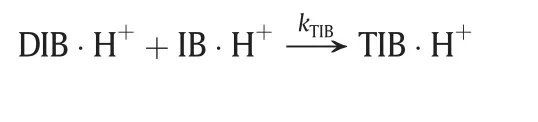
(3)Desorption of the products.
The desorption of all complex to give respective products
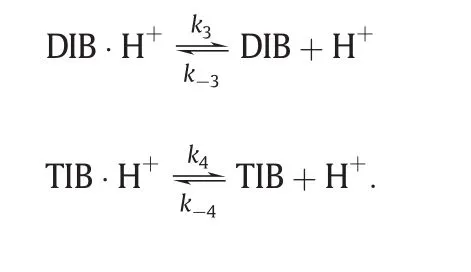
Thus,the reaction rate equations for Reaction 1 and Reaction 2 are:

where kj,0is the pre-exponential factor of reaction j,Ejis the activation energy of the reaction j and Ki′is the relative adsorption equilibrium constant of component i.
The net reaction rates of IB,DIB and TIB can be expressed by r1andr2

4.1.Parameter estimation
Nine sets of experiment are conducted to obtain the data given in Table 2,which are regressed by data- fit in ASPEN PLUS with 95% confidence limits.The overall objective function for optimization applied in data- fit is given by:
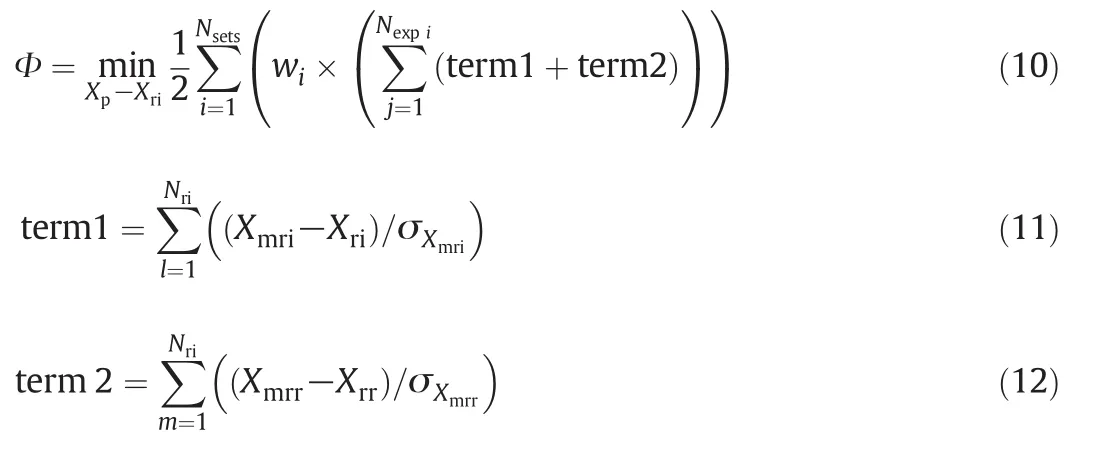
where XplbXpXpub,XrilbXriXrilb.
As can be seen from Eqs.(5)and(6),there are seven parameters to be estimated which are KN′P, KD′IB, KT′IB,kDIB,0,E1,kTIB,0andE2.The result of regression shows that K′NPequaling to 1.0×10-4can be ignored as compared to KD′IBand KT′IB.Therefore,the kinetic model can be simplified as follows:
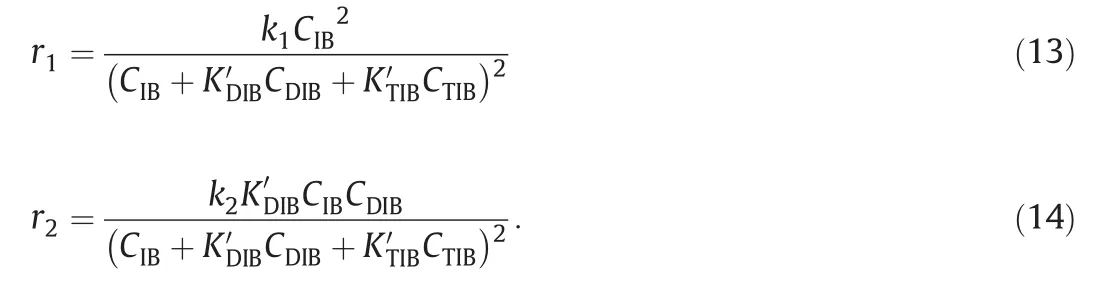

Fig.10.Comparison between modeled and measured kinetics under different temperatures.+IB;TIB;symbol— experimental data;curve— simulation results.
Reh finger and Hoffmann[18]proposed the activation energy of IB dimerization of 40 kJ·mol-1with ion exchange resin Amberlyst-15 as the catalyst.Honkelad and Krause[8]obtained the activation energy of IB dimerization and trimerization,i.e.30 kJ·mol-1and 2 kJ·mol-1,respectively,in the presence of macroporous strongly acidic cation exchange resin.Xu et al.[19]applied strongly acidic cation exchange resin as a catalyst and obtained the activation energy of IB dimerization and trimerization of 54.52 kJ·mol-1and 36.67 kJ·mol-1,respectively.The activation energy of IB dimerization has the same order of magnitude as those above,while the activation energy of IB trimerization is distinct due to different kinds of catalysts.As a result,the laboratory prepared catalyst Ni/Al2O3shows a high activation for IB dimerization.

Fig.11.Concentrations of components calculated by modeled reaction rate versus measured.
The comparison between modeled and measured kinetics under different temperatures is demonstrated in Fig.10.The good agreement suggests the ability of the developed kinetic model for predicting experiments data.The residual analysis for three products in Fig.11 shows very narrow derivations of the measured and modeled concentration at relatively low temperatures(<363 K)and slight derivation at high temperatures(>483 K).Therefore,the developed kinetic model is proper for predicting the performance of RD process with Ni/Al2O3as catalyst.
5.Reactive Distillation Simulations
Typically,a C4 feedstock contains IB and some other C4 components[20].The linear butenes,n-butene and 2-butene could also co-dimerize to produce C8 isomers other than DIB[21]but they are much slower than IB dimerization.Hence,except IB,all other components from C4 cut could be considered as inert substances from a reaction point of view.For the sake of simplicity,all other components are lumped as one component n-butene(NB)due to their similar physical properties.Thus,the system simulated in this work only involved IB,NB,DIB and TIB as components.A steady-state equilibrium stage model was applied to study the process feasibility of the RD process by using the RadFrac model in ASPENPLUS.The nonideality of the liquid phase was described by means of activity coefficients predicted by the modified UNIFAC method.The developed kinetic was edited by Fortran as a subroutine and linked with ASPEN PLUS.
A group of optimal operation parameters is acquired by simulations,as listed in Table 3.In the process,once DIB is formed,it is effectively removed from the reactive zone to the bottom by the effect of distillation.Thus,the concentration of DIB in the reactive zone is maintained at a relatively low level,which suppresses the formation of TIB and higher oligomers[Fig.12(b)].
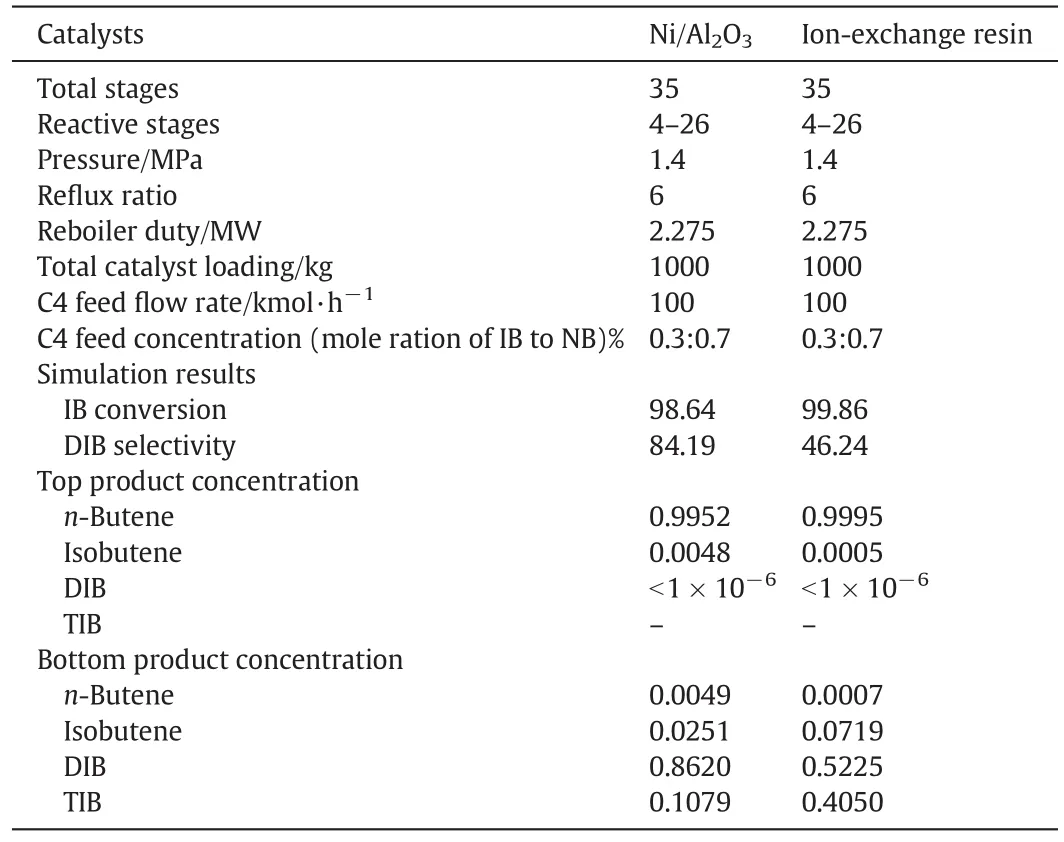
Table 3 Comparison of simulation results of reactive distillation with different catalysts
In Fig.12(a),it is clear that RD is capable of maintaining an almostconstant temperature profile within the reactive zone while it is impossible in a fixed-bed reactor without an external cooling due to the high heat of reaction.The relatively low temperatures in the reactive zone are also helpful for weakening the further oligomerization of isobutene to byproducts.In addition,in the case of RD,the heat of reaction is effectively used to evaporate the liquid phase,which consequently reduces the heat duty of the reboiler.It should be noted that,as the selectivity to DIB is 84.19%,a small amount of heaviest component TIB(0.1079)is collected in the bottom,which causes high bottom temperature(520 K).

Fig.12.Pro files of temperature and liquid mole fraction in the reactive distillation column(a)tray temperature(b)concentration.+IB;
In order to compare the effect of Ni/Al2O3and ion exchange resin as the catalyst in RD process,the kinetic developed from Talwalkar et al.[15]was used,where the effect of polar substance(water)was investigated.However,in this work,the effect of polar substances is not concerned.Thus,the simulations based on Ni catalyst and ion-exchange resin are both in the absence of polar substances.The performance of two catalysts is given in Table 3.As seen,with catalyst Ni/Al2O3,the IB conversion(98.64%)is slightly lower than ion exchange resin;however,the selectivity toward DIB is 84.19%,which is significantly higher than the case with ion exchange resin(46.24%)as the catalyst.This is because ion exchange resin has a higher activity for IB trimerization than Ni/Al2O3(see Table 2 in this work and Table 4 in Talwalkar et al.[15]),and then more DIB would be converted into TIB.Thus,the selectivity to DIB will decrease by catalyst ion exchange resin.
6.Conclusions
Ni/Al2O3catalyst has been prepared in the laboratory and its feasibility for IB dimerization by RD has been investigated.The effect of important factors such as active components(Ni)loading,temperature,
catalyst loading and IB concentration has been studied and it is found that high IB conversion and DIB selectivity could be performed with Ni/Al2O3as the catalyst.A kinetic model has been proposed considering the formation of dimmers and trimers in the absence of polar components.The modified LH model with fitted parameters is able to predict the reaction and is applied in the process of RD to produce DIB.The simulation results show that a high selectivity to DIB and high conversion of IB can be achieved along with the optimal conditions of RD in the absence of polar substances.
Nomenclature
Ciconcentration of component i
Ejactivation energy for reaction j,kJ·mol-1
K′irelative adsorption equilibrium constant of component i
kjrate constant for reaction j,kmol·kg-1·s-1
kj,0pre-exponential factor of component j,kmol·kg-1·s-1
mimass of component i,g
Nexpinumber of experiments in data set i
Nrinumber of reconciled input variables
Nrrnumber of measure results variables
Nsetsnumber of data sets specified on the regression specifications sheet
R gas constant,8.314 J·K-1·mol-1
r1rate of reaction 1,kmol·s-1
r2rate of reaction 2,kmol·s-1
rinet reaction rate of component i,kmol·s-1
Siselectivity to component i
T temperature,K
wimass for data set i specified on the regression specifications sheet
XIBconversion of isobutene
Xmrimeasured values of the reconciled input variables
Xmrrmeasured values of the results variables
Xpvectors and adjusted parameters
Xricalculated values of the reconciled input variables
Xrrcalculated values of the results variables
σ standard deviation specified for the measured variables
Φ objective function for optimization
 Chinese Journal of Chemical Engineering2015年3期
Chinese Journal of Chemical Engineering2015年3期
- Chinese Journal of Chemical Engineering的其它文章
- Micromixing characteristics in a gas-liquid-solid stirred tank with settling particles☆
- An experimental study of drag reduction by nanofluids in slug two-phase flow of air and water through horizontal pipes☆
- Effect of surfactant type on interfacial area and liquid mass transfer for CO2absorption in a bubble column☆
- Effects of bubbly flow on bending moment acting on the shaft of a gas sparged vessel stirred by a Rushton turbine☆
- Enhanced heat transfer in a heat exchanger square-duct with discrete V- finned tape inserts☆
- A novel purification process for dodecanedioic acid by molecular distillation
