Sono-assisted preparation of magnetic ferroferric oxide/graphene oxide nanoparticles and application on dye removal☆
Guodong Jiang,Qing Chang*,Fufu YangXiaoyun HuHeqing Tang*
1College of Chemistry and Chemical Engineering,Hubei University of Technology,Wuhan 430068,China
2College of Chemistry and Materials Science,South Central University for Nationalites,Wuhan 430074,China
Keywords:
A B S T R A C T
1.Introduction
Dyes are widely used with the development of textile,paper,tanning and printing industries.It was reported that over 1.47×106tons of organic dyes were produced in China in 2010[1,2].Dyes usually have complex aromatic molecular structures which make them more stable and difficult to be biodegraded.Moreover,many dyes are toxic and carcinogenic.Therefore,it's an urgent and serious global problem for efficient treatment of these discharged dyes.Many conventional methods,such as coagulation and flocculation[3],chemical oxidation[4],membrane separation[5]and adsorption[6]have been developed to remove dyes from wastewater.Among these methods,adsorption technology is the most extensively used one because of its easy handling,cost-effectiveness and high efficiency[7].
Nanoparticles have extremely small size,high surface-area-tovolume ratio and good mass transfer efficiency and provide a faster rate for the adsorption of substrates from aqueous solutions[8].However,they are difficult to be separated and recycled.Thus,it is necessary to develop a method for the separation of nano-scale particles that does not generate secondary waste[9].
Magnetic separation has attracted increasing interest because the magnetic particle can be effectively and simply separated and recovered by the external magnetic field.Moreover,the power and efficiency of magnetic separation procedure are especially attractive for large-scale operations.And magnetic separation is also the basis of various automatic procedure[10].Due to the ferro-or ferrimagnetic properties,iron oxide nanoparticles such as magnetic materials have been widely used in many fields such as separation of biochemical products[11],targeted drug delivery[12],catalysis[13]and enzyme immobilization[14].The iron oxide nanoparticles can be modified with functional groups or inorganic compounds to obtain magnetic adsorbents,which require the affinity to target pollutants for applications in the environment.
Since the pioneering study of graphene as a new member of carbon nanostructures by Novoselov et al.[15],graphene,two-dimensional graphitic carbon nanomaterial,has attracted increasing interest due to its excellent mobility of charge carrier,a large specific surface area and good electrical and thermal conduction[16].It also has a great promise for potential applications in many fields such as sensors,nanomaterials,electronic devices,solar cells,supercapacitors and hydrogen storage.Graphene oxide sheets,a derivation of graphene,contain a range of reactive oxygen functional groups on the sheet surface,which could render the sheet a good candidate for supporting metal or metal oxide particles[17].For example,Yaoetal.have prepared Fe3O4@graphene composites via a two-step of chemical deposition and reduction method.And they also investigate the adsorption of methylene blue and Congo red on Fe3O4@graphene composites.The maximum adsorption capacity reached 45.27 and 33.66 mg·g-1,respectively[18].Nevertheless,a simple method to prepare a magnetic adsorbent with excellent adsorption capacity for the dye removal is needed to be developed.
In this work,magnetic Fe3O4nanoparticles were successfully deposited on graphene oxide(GO)sheets through co-precipitation with the assistance of ultrasound irradiation for the first time.And then,the as prepared Fe3O4/GO magnetic nanoparticles(MNPs)were used as adsorbent materials.The adsorption performance of the nanocomposite toward a cationic dye Rhodamine B(RhB)in aqueous solution was investigated.The parameters including initial solution pH,adsorbent dosage,temperature and contact time were systematically studied.Furthermore,the adsorption kinetics and isotherms for RhB onto the Fe3O4/GO MNPs were also investigated.This new adsorbent could be easily recovered with a magnetic separation,which could remove dyes from polluted water effectively.The used adsorbent could be regenerated by hydrogen peroxide and reused.
2.Experimental
2.1.Reagents and apparatus
Nature flake graphite(99%)was obtained from Qingdao Lihaofeng Graphite Co.Ltd.(China).All other chemical reagents were purchased from Sinopharm Chemical Reagent Co.Ltd.(Shanghai,China)and were of analytical reagent grade.Double distilled water was used throughout experiments.
2.2.Synthesis of the adsorbent
Oxidized graphite was obtained according to the method of Hummers and Offeman[19].Fe3O4/GO MNPs were synthesized by in situ precipitation method as described below.Graphite oxide was added to distilled water(40 ml)and ultrasonically exfoliated in a bath sonicator for 1h to obtain a light-brown solution,namely GO dispersion.FeSO4·7H2O(3.0 × 10-4mol)and FeCl3·6H2O(4.5 × 10-4mol)were dissolved in 10 ml of distilled water and added to the GO dispersion.The mixed solutions were added drop wise into 10 ml of 12.0 mol·L-1ammonia at 60°C and reacted for 1 h under ultrasound irradiation in an ultrasound cleaning bath operating at 25 kHz with a power of 140 W(KQ-200KDE,Kunshan Ultrasound Instrument Co.Ltd.).Finally,the product was washed with ethanol and distilled water until neutral and then dried to obtain Fe3O4/GO MNPs as adsorbent.
2.3.Characterization of the adsorbent
X-ray diffraction(XRD)patterns of samples were recorded on a Bruker Advance D8 X-ray powder diffractometer with a Cu Kαradiation source generated at 40 kV and 40 mA.The morphology of as synthesized products was evaluated by transmission electron microscope(TEM,FEI Tecnai G2 20).The magnetic properties were analyzed on an ADE 4HF vibrating sample magnetometer at 300 K.The Brunauer-Emmett-Teller(BET)specific surface area was determined using a BELSORP-mini apparatus.The UV-visible absorption spectra of dye were recorded on an EVOLUTION 201 spectrophotometer(thermo scientific).
2.4.Batch adsorption experiments
0.05 g of Fe3O4/GO MNPs and 50 ml of RhB solution of known initial concentration were added to Teflon bottles and then shaken(200 r·min-1)on a thermostatic shaker at 313 K for 270 min in order to reach equilibrium.The initial pH of the solutions was adjusted to the desired value using dilute HCl or NaOH solution.The suspensions were then separated by a magnet to analyze dye concentration.The adsorption capacity of adsorbent toward RhB was calculated using the following formula:

whereqe(mg·g-1)is the amount of the RhB adsorbed on per unit mass of adsorbent at equilibrium;C0,Ce(mg·L-1)are the initial and equilibrium concentrations of RhB in solution respectively;V(L)is the volume of the solution and W(g)is the mass of adsorbent used in the experiments.
2.5.Regeneration experiments
0.5g of the adsorbent after RhB reaching adsorption equilibrium and 200ml of H2O2solution with concentration of 0.08mol·L-1were added to Teflon bottles and then shaken(200 r·min-1)on a thermostatic shaker at 313 K for 360 min.The pH of the solution was adjusted to 4.0 throughout the reaction.The adsorbent was collected by a magnet and reused for adsorption again.
3.Results and Discussion
3.1.Structure of the adsorbent
The XRD pattern of adsorbent shows the characteristic peaks of Fe3O4crystalline particles at 18.2°,30.3°,35.6°,43.4°,53.8°,57.4°and 62.8°,which are ascribed to the(111),(220),(311),(400),(422),(511)and(440)planes of Fe3O4,respectively,as shown in Fig.1,Curve 1.The broad small diffraction peak around 24°corresponds to C(002)reflection of graphite oxide derived from the short-range order in stacked graphene oxide sheets[20].A diffraction peak at 11.0°belongs to(001)crystal of GO(Fig.1,Curve 2).For the Fe3O4/GO MNPs,this diffraction peak disappears indicating that the stacking of GO sheets in the composite was almost disordered[21].
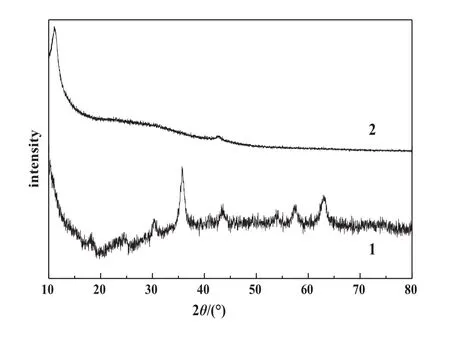
Fig.1.XRD patterns of Fe3O4/GO MNPs(1)and GO(2).
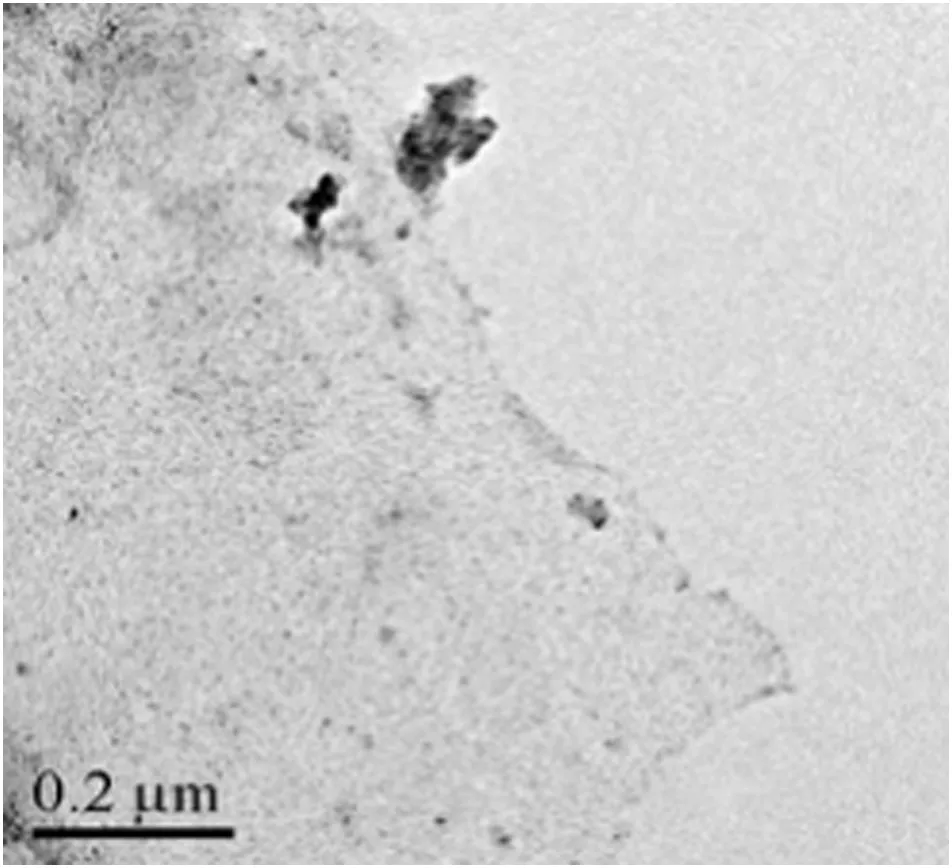
Fig.2.Transmission electron microscopic image of Fe3O4/GO MNPs.
The TEM image is used to observe the morphology of the adsorbent.As shown in Fig.2,it is evident that graphene sheets are uniformly decorated by Fe3O4nanoparticles with the ultrasoundassisted co-precipitation method,little of which aggregate to form larger particles.When the precursor solution is exposed to the ultrasound irradiation,ultrasound may locally produce extremely high temperatures(>5000 K)and pressures(>20 MPa)during acoustic cavitation[22].These factors affect the degree of supersaturation of the solution,which provides enough energy for the formation of crystal nucleus and growth of nucleus to final particles.It is favorable to significantly accelerate the nucleation.The produced high temperature and adsorbed bubbling gases on nucleus surface will decrease the interfacial free energy between nucleus and solution,and consequently inhibit the growth of nuclei at the same time.In addition,the secondary aggregation of particles can be diminished significantly by the intense microscopic turbulence and micro-jet generated from the collapse of bubbles[23].Therefore,the sample has small particle size.
The hysteresis loops of the as-prepared Fe3O4MNPs and Fe3O4/GO MNPs were recorded at room temperature.As shown in Fig.3,they all exhibited negligible coercivity(Hc)and remanence,being typical of superparamagnetic materials.The saturation moment per unit mass,Ms,for the Fe3O4/GO MNPs(Fig.3,Curve 1)is measured to be 26.0 emu·g-1.And the value of Fe3O4MNPs is 68.8 emu·g-1(Fig.3,Curve 2).Fe3O4is estimated as 37.8%(by mass)calculated from the content of Fe3O4/GO MNPs.The superparamagnetism of magnetic nanoparticles is attractive for a broad range of applications because the nanoparticles can be easily separated by external magnetic field in practical use and redispersed rapidly when the magnetic field is withdrawn.
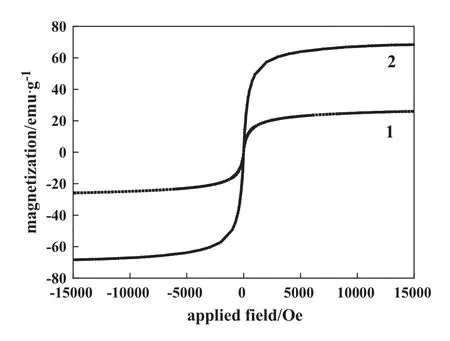
Fig.3.Magnetization curves of Fe3O4/GO MNPs(1)and Fe3O4MNPs(2)measured at 300 K.
The N2adsorption-desorption method was used to investigate the BET surface area and pore characteristic of Fe3O4/GO MNPs.Specific surface area and pore volume were measured to be 191.1 m2·g-1and 0.20 cm3·g-1,respectively.
3.2.The effect of pH
The effect of pH on adsorption of dye was investigated over the range of pH values from 4.0 through 9.0.As shown in Fig.4,the removal efficiency of RhB slightly decreases with the increase of pH value in the range from 4.0 to 7.0.This could be explained as follows.As the pH increases,a cationic and monomeric molecular form of RhB at low pH gradually become a zwitterionic form and aggregate to form a larger molecular form which restrained π-π interactions between the RhB molecules and the aromatic rings of GO[24].And when pH(pH>7)is further increased the removal efficiency slightly increases,which is attributed to the electrostatic attraction between RhB and the negative charged GO at high pH.However,the composites can be used over a broad pH range with adequate efficiency for the removal of RhB.

Fig.4.Effect of pH on RhB adsorption by Fe3O4/GO MNPs.
3.3.Effect of adsorbent dosage

Fig.5.Effect of adsorbent dose on RhB adsorption by Fe3O4/GO MNPs.
The effect of adsorbent dosage on RhB adsorption at a fixed initial RhB concentration of 80 mg·L-1is shown in Fig.5.The removal of RhB increases from 43.9%to 98.9%with the increase of the adsorbent dosage in the range from 0.2 to 1.2 g·L-1.However,RhB adsorption capacity(RhB loaded per unit mass of adsorbent)decreases from 172.1 to 65.9 mg·g-1at the same time,which might be attributed to the overlapping of active sites at higher dosage.Considering the RhB adsorption capacity and the percentage removal of RhB,we optimized the adsorbent dose at 1 g·L-1for the consequent study.
3.4.Effect of temperature
The temperature-dependent RhB adsorption studies on Fe3O4/GO were investigated in the temperature range of 30-70°C,and the results are shown in Fig.6.Obviously,the adsorption capacity of RhB increases significantly when the temperature increase from 30 to 40°C,and the increasement of the adsorption capacity becomes very small when the temperature is above 40°C.Accordingly,the percentage removal of RhB increases from 76.5%to 97.1%when the temperature rises from 30 to 70°C.The result indicates that the RhB adsorption process is endothermic process in nature.

Fig.6.Effect of temperature on RhB adsorption by Fe3O4/GO MNPs.
3.5.Effect of contact time and adsorption kinetics
The influence of contact time on the adsorption of RhB was investigated from 0 to 450 min.As shown in Fig.7,it is observed that the adsorption process is very fast during the first 30m in with a percentage removal of 81.8%and 73.5%for the initial RhB concentration of 40 mg·L-1and 80 mg·L-1respectively.The equilibrium is approached at 270 min and the removal efficiency of RhB reaches 97.5%and 92.7%when the initial RhB concentration is 40 mg·L-1and 80 mg·L-1respectively.The result indicates that the adsorbent could adsorb dye quickly.The extraordinarily fast adsorption rate might be attributed to two factors:(i)the electrostatic attraction between the negatively charged surface of the GO and the cationic RhB;(ii)the π-π interactions between the RhB molecules and the aromatic rings of the GO[25].
Kinetics constants of RhB adsorption on Fe3O4/GO MNPs were determined using pseudo- first-order and pseudo-second-order models.The pseudo- first-order adsorption kinetics model is expressed as:

The rate constant(k1)and theoretical equilibrium adsorption capacity(qe)can be obtained by plotting ln(qe-qt)vs.t.

Fig.7.Effect of contact time on RhB adsorption by Fe3O4/GO MNPs at different initial concentrations.
The pseudo-second-order equation is given as:

The pseudo-second order rate constant(k2)and maximum adsorption capacity(q2)are calculated from the intercept and slope of the pseudo-second-order plots.
Kinetics parameters for two different kinetic models and correlation coefficients with different initial concentrations are summarized in Table 1.The qe,calcalculated from the pseudo- first-order kinetic model is not in a good agreement with the experimental values of qe,expand the correlation coefficient is dissatisfactory with different initial concentrations.However,The kinetics data fit well with pseudo-second-order kinetics model with high correlation coefficients(R2≥0.999)and is in good agreement between qe,expand calculated qe,calvalues.It indicated that the adsorption of RhB onto Fe3O4/GO MNPs complies with a pseudo-second-order rate equation.Similar result was also reported on adsorption of methylene blue(MB)onto Fe3O4/SiO2-GO nanoparticles[26].
In general,the adsorption kinetics of RhB onto solid particles is controlled by different processes,i.e.external mass transfer,adsorption of RhB onto particle surfaces and intra-particle diffusion[27].In order to find the rate-controlling step of the adsorption,we introduce the intra-particle diffusion model as follows:

The intra-particle diffusion rate constant kp(mg·g-1·min0.5)and C of adsorption constant can be obtained from the plot of qeversus t0.5.If the curve is a straight line and through the origin(i.e.,C=0),the intra-particle diffusion is the only rate-controlling step in adsorption;if not,the boundary layer diffusion is also the rate controlling step to some degree.As shown in Fig.8,the kinetic data doesn't fit well with intra-particle diffusion model and the correlation coefficients at initial RhB concentration of 40and80mg·L-1are0.942and0.977,respectively.The plot presents multi-linearity,suggesting that adsorption process is complex and takes place via three steps.The first sharper portion(t0.5
<5.5)represents the boundary layer diffusion of solute molecules.The second portion(5.5<t0.5<16.4)describes the gradual adsorption stage,which attributes to the intra-particle diffusion.The third portion(t0.5
>16.4)is the final equilibrium stage.The multi-linearity curve indicates that intra-particle diffusion is not a fully operative mechanism in the adsorption of RhB on Fe3O4/GO MNPs[24].

Table 1 Kinetics parameters of pseudo- first-order and pseudo-second-order models for adsorption of RhB on Fe3O4/GO MNPs at 313 K with different initial concentrations

Fig.8.Plots of the intra-particle diffusion kinetics equation for the adsorption of RhB onto Fe3O4/GO MNPs with different initial concentrations.
3.6.Adsorption isotherms
Analysis of isotherm data is very important for predicting the mechanism of the adsorption and the adsorption capacity of the adsorbent.Langmuir isotherm and Freundlichisotherm models have been employed to analyze the equilibrium adsorption data.The Langmuir isotherm assumes monolayer coverage of sorbent and all the adsorption sites have equal adsorbate affinity.Langmuir isotherm model linear form can be expressed as:

where qe(mg·g-1)is the amount adsorbed per unit mass of adsorbent at equilibrium,Ce(mg·L-1)is the equilibrium concentration of RhB,qmax(mg·g-1)is the maximum monolayer adsorption capacity of the adsorbent,b(L·mg-1)is the constant term related to the energy of adsorption.
The Freundlich isotherm is based on the multilayer sorption on heterogeneous surface,which can be represented as:

where KFand n are the Freundlich constants related to the adsorption capacity and adsorption intensity.
The calculated isotherm parameters of Langmuir,Freundlich and their correlation coefficients at different temperatures are listed in Table 2.Compared with the Langmuir isotherm,Freundlich isotherm matches better with the experimental data,which are con firmed by correlation coefficient R2(0.960-0.971)of the plot.Therefore,Freundlich isotherm is suitable for modeling the adsorption of RhB,indicating that the adsorption process is likely a multi-layer adsorption process.The Freundlich constant n is found to be 3.26-3.73(n>1),which indicatesthat the adsorption of RhB onto Fe3O4/GO MNPs is a favorable process[28].It can be seen from Table 2 that an increase in temperature from 30 to 50°C promoted an increase of qmax,indicating that adsorption process has an endothermic character.In addition,the maximum adsorption capacities of the GO and Fe3O4were calculated to 330.50 mg·g-1and 10.08 mg·g-1,respectively.The adsorption capacity of Fe3O4/GO MNPs is lower than that of GO,because the Fe3O4account for a certain percentage of the Fe3O4/GO MNPs while they have little adsorption capacity.
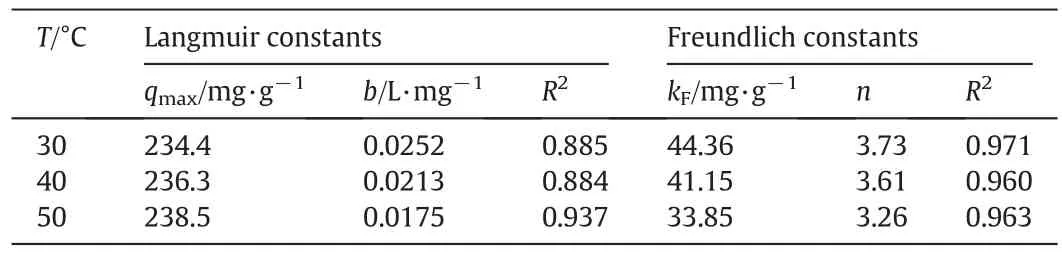
Table 2 Langmuir and Freundlich isotherm parameters for the adsorption of RhB on Fe3O4/GO MNPs at 313 K
3.7.Effects of interfering dyes
The dye-contaminated water may contain several other co-existing dyes that compete with RhB in the adsorption process.In this work,effects of methyl orange(MO)and MB were studied by dissolving appropriate amount of competitive dyes in the RhB solution.In adsorption experiments,the concentrations of the competitive dyes varied from 0 to 40 mg·L-1with initial concentration of RhB 40 mg·L-1.As shown in Fig.9,MO has little interference effect on RhB adsorption.Nevertheless,the RhB removal decreases from 92.7%to 66.7%when the MB concentration increases from 0 to 40 mg·L-1,demonstrating significantly negative influence on RhB adsorption.This may be due to the fact that MB is also a cationic dye and could have been competitively adsorbed on Fe3O4/GO MNPs with RhB.

Fig.9.Effects of MO and MB on RhB adsorption by Fe3O4/GO MNPs.
3.8.Regeneration
Both of Fe3O4nanoparticles and graphene oxide were found to be able to activate H2O2to produceoxidative species,which could degrade the adsorbed RhB on Fe3O4/GO MNPs[23,29].Thus,H2O2was used to regenerate the adsorbent for reuse.The maximum adsorption capacity was obtained to be 167 mg·g-1after regeneration of the adsorbent,showing about 70%of the dye removal efficiency.It can be retained and the adsorption is a reversible process from the results.It is concluded that Fe3O4/GO MNPs can be recovered and reused for the RhB adsorption.The studies on more effective desorption methods for further use are in progress.These results show that the adsorbent can be potentially used as a magnetic adsorbent to remove dye contaminants from water.
4.Conclusions
A nano-adsorbent material,Fe3O4/GO MNPs has been synthesized by a simple ultrasound-assisted co-precipitation method.The obtained composites were characterized by X-ray diffraction,transmission electron microscopy,vibrating sample magnetometer and Brunauer-Emmett-Teller.The RhB quickly adsorbed onto Fe3O4/GO MNPs and adsorption process followed the pseudo-second-order kinetic model.The equilibrium data were better represented by the Freundlich isotherm to explain the adsorption behavior of RhB on Fe3O4/GO MNPs.In addition,the adsorbents can be separated and recovered by the external magnetic field easily.Their reusability could be achieved by treating the used Fe3O4/GO MNPs with H2O2.The Fe3O4/GO MNPs can find their efficient application in environmental science and technology.
 Chinese Journal of Chemical Engineering2015年3期
Chinese Journal of Chemical Engineering2015年3期
- Chinese Journal of Chemical Engineering的其它文章
- Micromixing characteristics in a gas-liquid-solid stirred tank with settling particles☆
- An experimental study of drag reduction by nanofluids in slug two-phase flow of air and water through horizontal pipes☆
- Effect of surfactant type on interfacial area and liquid mass transfer for CO2absorption in a bubble column☆
- Effects of bubbly flow on bending moment acting on the shaft of a gas sparged vessel stirred by a Rushton turbine☆
- Enhanced heat transfer in a heat exchanger square-duct with discrete V- finned tape inserts☆
- A novel purification process for dodecanedioic acid by molecular distillation
