Kinetics of xylose dehyd ration in tofurfural in acetic acid
Zhou Chen,W eijiang Zhang,Jiao Xu*,Pingli Li
Institute of Chemical Engineering,Tianjin University,Tianjin 300072,China
Keyw ords:Xylose Furfural Acetic acid
ABSTRACT In thispaper kineticsofxylose dehydration in tofurfuralusing acetic acid as catalystwasstudied com prehensively and system atically.The reaction order of both furfural and xylose dehydration was determined and the reaction activation energy was obtained by nonlinear regression.The effect of acetic acid concentration was also investigated.Reaction rate constants were gained.Reaction rate constan t of xy lose dehyd ration is k1=4⋅189× 1010reaction rate constan t of fu rfu ral degradation isand reaction rate constan t of condensation reaction isased on this,the kinetics equation of xylose dehyd ration intofurfu ral in acetic acid was set up according to theory of Dun lop and Furfural generating rate equation is
1.Introduction
Furfural is a very basic heterocyclic organic com pound.Furfu ral has very im portant industrial application and large numbers of derivatives are developed by hyd rogenation reaction,oxidation reaction,ch lorination reaction,nitrification reaction,and condensation reaction.M ore than 1600 types of chemical products are direct or indirect derivatives of furfural,which are widely usedin medicine,pesticide,resin,spinning and oil etc.[1-5]Up to now,plan t fiber is the only raw material to produce furfural,such as corn cob and bagasse[6].
Due to the existence of side reaction,fu rfural yieldis much low er than 100%.According to Root[7],it is considered that low fu rfu ral yieldis on account of the condensation reaction between furfural andits precu rsors.The furfural yield cou ld be increased by timely rem oval of the generated furfu ral.The condensation reaction only occu rs in the liquid phase,therefore,high fu rfu ralyieldis usually obtained by con tinuous pumping gas in to the reactor and then the furfural is removed out of the reactor.
Xylose is the main pen tose generated by hemicellu lose hyd ro lysis.Therefore,pure xylose is usedin this work instead of pentose.In most of the citations,kinetics of xylose dehyd ration in tofu rfu ral is studied[8-13].How ever,there is lim ited work about the kineticsof transform ation process of xylose intofurfural in acetic acid.Acetic acidis one kind of organic acids,and also is the byp roduct of hemicellu lose hyd rolysis into pentose,the corrosion of which is much low er than su lfuric acid or hyd roch loric acid.There is advanced technology to recycle the acetic acidin industry[14],which makes the using of acetic acid environm entally friend ly.In this work,the kinetics of transform ation of xylose tofu rfu ral with acetic acid as catalyst is w ell studied.The main factors affecting fu rfu ral yield are investigated and the kinetics equation of transform ation of xylose in tofurfural is estab lished.The curren t study provides theoretical guidelines for furfural producing technology with pentose as raw materials.
2.Experim ental
The schem e of experimental setup for reaction kinetics study is show n in Fig.1,which main ly consists of sam pling system,high pressure reactor,m agnetic stirring system and temperature con trol system.The main device is the magnetic stirring high pressure reactor with 150 ml volum e heated by electric jacket,and made of stain less steel of grade 316 L for to lerance at conditions of 300°C and 10 MPa.The temperature con trol system is regu lated by proportion in tegral derivative(PID)and displayed by Pt resistance in the reactor with an accu racy of±0.1 K.The rotating speed of magnetic stirring is in the range of 0-1200 r·m in-1.The pipeline of sam pling system is made of 316 L stain less steel with a specification of 3.0 mm OD×1.0 mm ID.The length of coo ling pipeline after Valve 4 is app roxim ately 100 cm.
Experim ental procedure is as follow s:
(1)Add 110 ml raw material in to the reactor,then fasten the reactorflange.
(2)Close the Valve 2,and then open Valves1 and 3 and valveofN2to conduct air replacement,and finally shut dow n Valves 1 and 3.
(3)Set the rotating speed of magnetic stirring to 400 r·m in-1and the required reaction temperature,then start to heat.
(4)Take the sam ples w hen the preset temperature is achieved as required.Make sure the residual sam ples in the sam pling line are replaced and the sam pling volum e is 10 ml.(Due to the fast incrementof temperature,the com position change du ring the process of heating is not taken in to account.)
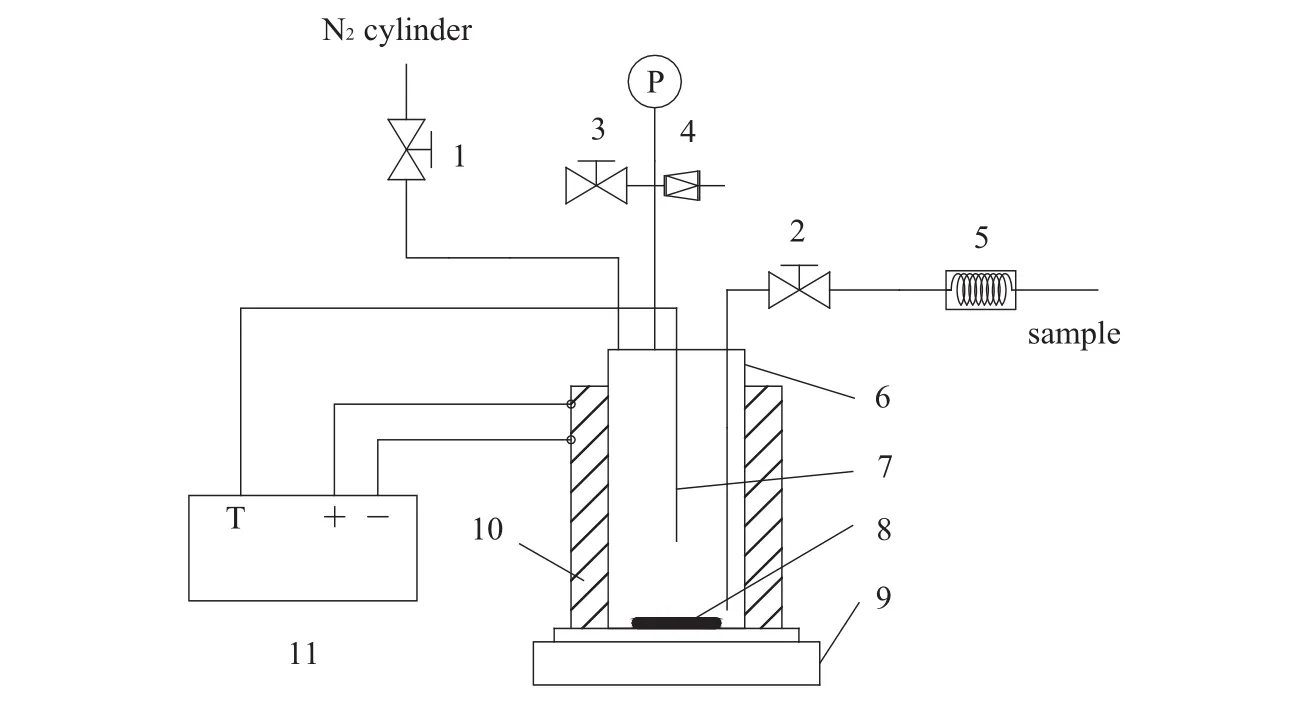
Fig.1.Laboratory setup for dehyd ration reaction.
(5)Stop heating after the experiment ends and let the system cool dow n naturally.
(6)Analyze the sam ples,p rocessdata,and clean the setup to prepare for the next experiment.
3.Results and Discussions
The mechanism of xylose dehyd ration in tofu rfu ral has not been confirm ed even though a lot of related and analogous work has been done[15-17].The most typical reaction route is Dun lop's.According to Dun lop[18],the xylose generates in to an in term ediate substance and the certain interm ediate substance further generates intofurfu ral.Under its generating conditions,the condensation reaction will occu r between furfuraland precursor in addition tofurfuraldegradation reaction.The following reaction route(Fig.2)is considered reasonab le in the process of xylose transform ation in tofurfural.

Fig.2.Reaction route.X—xy lose;F—furfural;D—p roduction of esterification reaction;I—in term ediate substance;P—p roduction of condensation reaction.
In this paragraph,it is aim ed to get the reaction rate constant ofeach reaction based on Dun lop route and ob tain the op tim al condition of furfural product.
3.1.Dynam ics study on xylose dehyd ration
Xylose dehyd ration reaction will take place under high temperature and acid environm en t.The purpose of the research in this section is to study the dynam icsofxylose dehyd ration and establish the corresponding equation w hen acetic acidis used as a catalyst.
3.1.1.Determination on the order of xylose dehydration reaction
In a constan t condition of acetic acid concentration of[A]=0.5 mol·L-1and reaction temperature of 463.15 K,the variation of xylose dehyd ration rate on reaction time is studied w hen initial xylose concentrationsare20 g·L-1,50 g·L-1,80 g·L-1and 120 g·L-1,respectively.The experimental results are show n in Fig.3.It is found that the xylose dehyd ration rate only depends on the reaction time and has no connection with the initial concentration.This characteristic con form s to the first order reaction.

Fig.3.Effect of reaction time on xylose conversion for different initial xylose concentrations(T=463.15 K,[A]=0.5 mo l·L-1).
3.1.2.Dynamics equation fitting of xylose dehydration
According to Dun lop,the in term ediate substance cannotbe detected by experiment.Therefore,the in term ediate substance is considered to exist instan taneously.By the principle of steady-state app roxim ation the reaction process cou ld be described as:

Due to the characteristic of first order reaction,the equation of xylose dehyd ration reaction is:

The reaction constant k1cou ld be expressed as:

where k10,term ed as pre-exponen tial facto r,is a constan t with no relation with temperature,R is the gas constan t,J·m o l-1·K-1,E1is the activation energy,kJ·m ol-1,[A]represents the acetic acid concentration,m ol·L-1,and α is the index factor of acetic acid concentration.
3.1.2.1.Fitting of xylose dehydration activation energyE1.In a constan t condition of acetic acid concentration[A]=0.5 mo l·L-1andin itial xylose concentration of[X]0=80 g·L-1,the variation of xylose dehydration rate on reaction time is studied at different reaction temperatures.The experimental results are show n in Fig.4.

Fig.4.Effectof reaction timeon the conversion ofxy loseatdifferent reaction temperatures([A]=0.5 mo l·L-1,[X]0=80 g·L-1).
Xy lose dehyd ration process is a first order reaction.The expression-ln(1-x)which is show n in Fig.5 has a linear relation with time through the origin poin t,where x rep resen ts xy lose conversion.The corresponding slopes are xy lose dehyd ration rate constants at different temperatures.

Fig.5.Fitting of xy lose dehyd ration rate constants at different reaction temperatures([A]=0.5 mol·L-1,[X]0=80 g·L-1).
The xylose dehyd ration constants are presentedin Table 1.
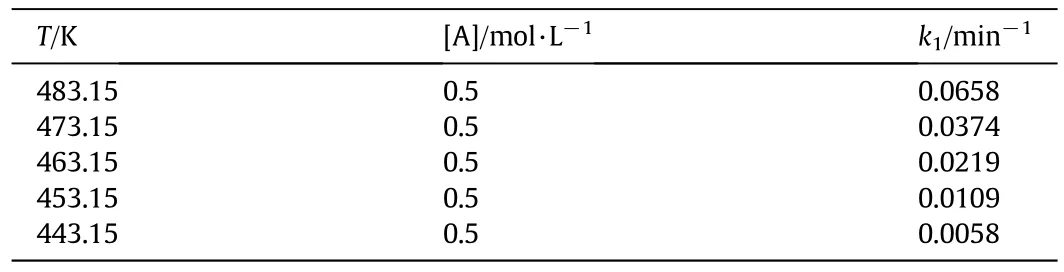
Tab le 1 Results of xylose dehyd ration rate constants at different reaction temperatures
Taking logarithm for both sides of Eq.(3),Eq.(4)is obtained
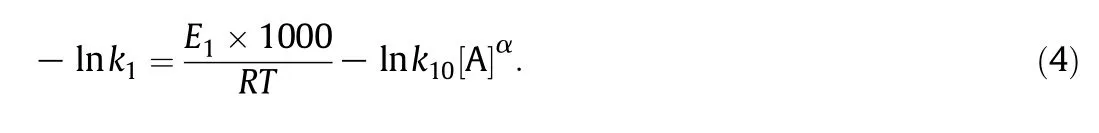
Plotting-ln k1against 1000/RT,Fig.6 can be reduced.The corresponding slope is just E1.

Fig.6.Fitting of activation energy of xylose dehyd ration reaction.
According to the fitting result of Fig.6,the activation energy of xylose dehydration is E1=108.6 kJ·m ol-1.
3.1.2.2.Fitting of aceticacidconcentration indexα.In a constant condition of reaction temperature of 463.15 K andinitial xylose concentration of[X]0=80 g·L-1,the variation of xylose dehyd ration rate on reaction time is studied w hen acetic acid concentrations are 0,0.17 mol·L-1,0.5 mo l·L-1,1 mol·L-1and 1.5 mol·L-1,respectively.The experim ental results are show n in Fig.7.
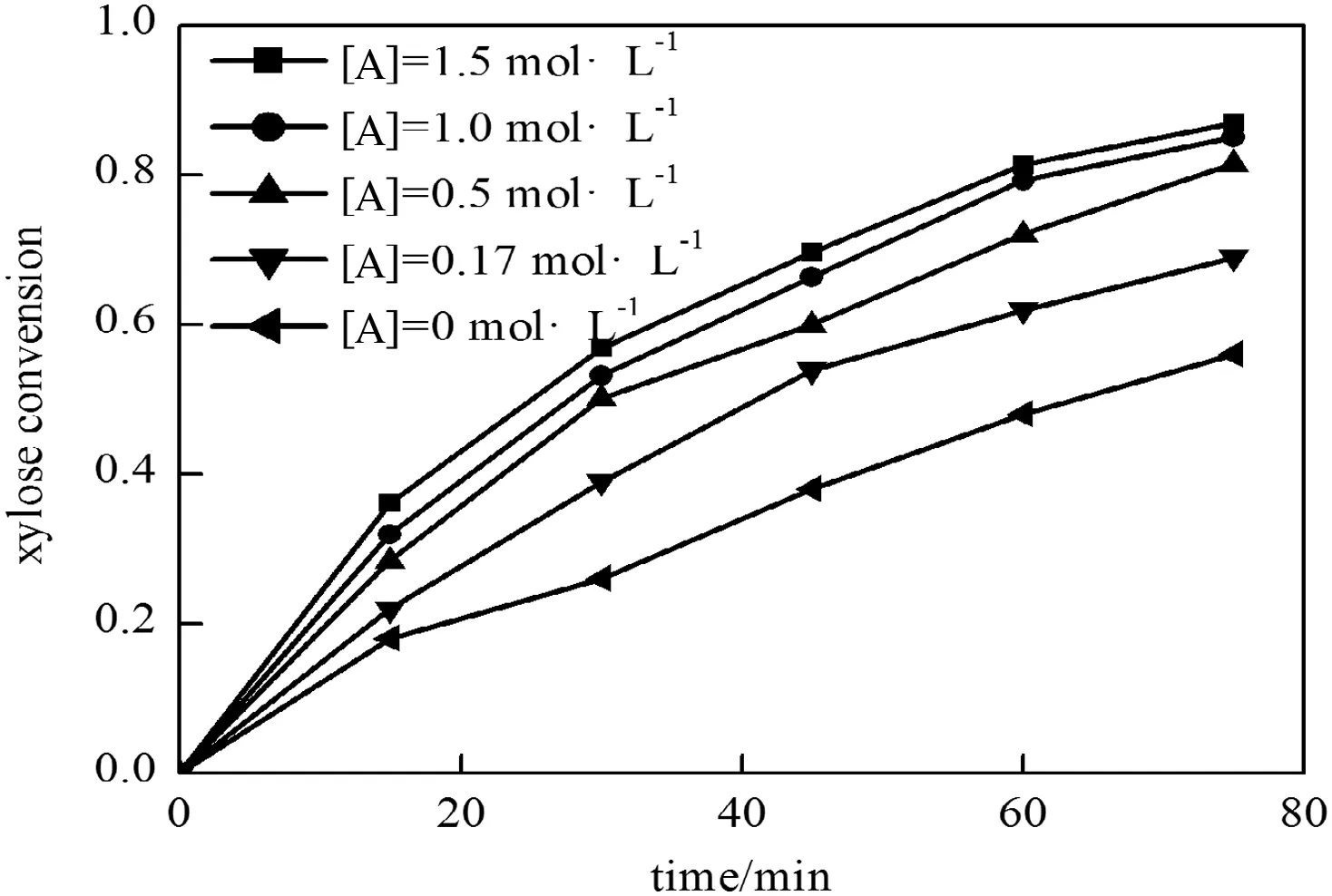
Fig.7.Effectof reaction time on the conversion ofxylose with differentacetic acid concentrations(T=463.15 K,[X]0=80 g·L-1).
The experimental results are conducted by the sam e fitting method of Section 3.1.2.1.The fitting results are presentedin Table 2.
Rearranging Eq.(4),w e can get


Table 2 Results of xylose dehyd ration rate constants with different acetic acid concentrations
Plotting ln k1against ln[A]on different acetic acid concentrations,the obtained corresponding slope is α.The results are show n in Fig.8.

Fig.8.Fitting of acetic acid concentration index α.
According to the fitting result of Fig.7,the acetic acid concentration index α of xylose dehyd ration is α =0.1676.
3.1.2.3.Xylose dehydration rate equation.Based on the aforementioned work,the activation energy of xylose dehyd ration E1and the index factor of acetic acid concentration α are obtained.By substitu ting E1and α into Eq.(3),the pre-exponential factor k10could be figured out.The results are show n in Tab le 3.
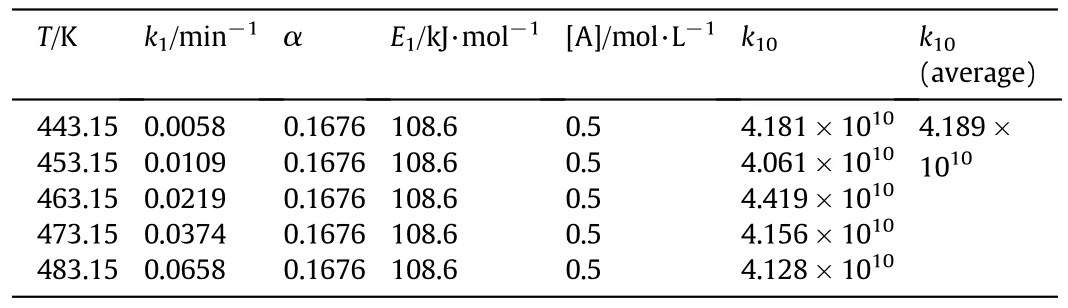
Tab le 3 Results of exponen tial factor k10
The summ ary of xylose dehyd ration rate constants is presentedin Tab le 4.

Tab le 4 Summ ary of xylose dehydration rate constants
Therefore,the dehyd ration rate constan t k1with acetic acid as catalyst cou ld be expressed as:
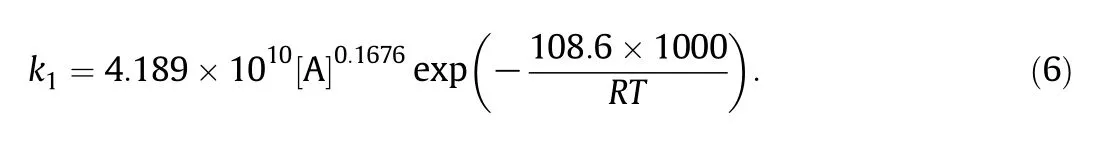
The in tegral equation of Eq.(2)cou ld be expressed as:

By substitu ting Eq.(6)in to Eq.(7),w e can get:
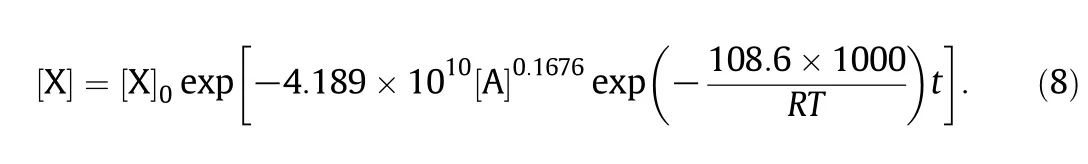
Under the experim ental conditions,it is able to accurately describe the xylose dehyd ration process with acetic acid as catalyst.
3.2.Dynam ics study on furfural degradation
Du ring the generating process of fu rfu ral,furfural degradation reaction occurs.It is necessary to investigate the dynam icsof furfuraldegradation using acetic acid as catalyst.In this section,it is aim ed to study the dynam ics of furfu ral degradation and estab lish corresponding equations.
3.2.1.Determination of the furfural degradation reaction order
In a constan t condition of reaction temperature of 453.15 K and acetic acid concentration of 0.17 mo l·L-1,the variation of furfu ral degradation rate on reaction time is studied.The experim ental results are show n in Fig.9.It is found that the fu rfu ral degradation rate only depends on the reaction time and has no relevancy with the fu rfu ral concentration.This characteristic con form s to the first order reaction.

Fig.9.Effect of furfural conversion rate vs.time with variousinitial furfuralconcentrations(T=453.15 K,[A]=0.17 mol·L-1).
3.2.2.Dynamics equation fitting of furfural degradation
The degradation reaction of furfural is a com plex process and black resin will be generatedin addition toform ic acid.
Assum ing the productof furfural degradation as a certain substance,the reaction process cou ld be described as:

Due to the characteristicoffirstorder reaction,the equation of fu rfural degradation reaction is:

The reaction constant k2cou ld be expressed as:

where k20,a constan t with no relation with temperature,is term ed as pre-exponen tial factor.R is the gas constant,J·m ol-1·K-1,E2is the activation energy,kJ·m ol-1,[A]represents the acetic acid concentration(m ol·L-1)while λ is the exponen tial factor of concentration.
3.2.2.1.Fitting of furfural degradation activation energyE2.In a constan t condition of initial furfu ral concentration,where[F]0=2%(by mass)and acetic acid concentration is 0.17 mol·L-1,the variation of furfu ral degradation rate on reaction time is studied at different temperatures(443.15,453.15,463.15,473.15 and 483.15K).The experim entalresults are show n in Fig.10.
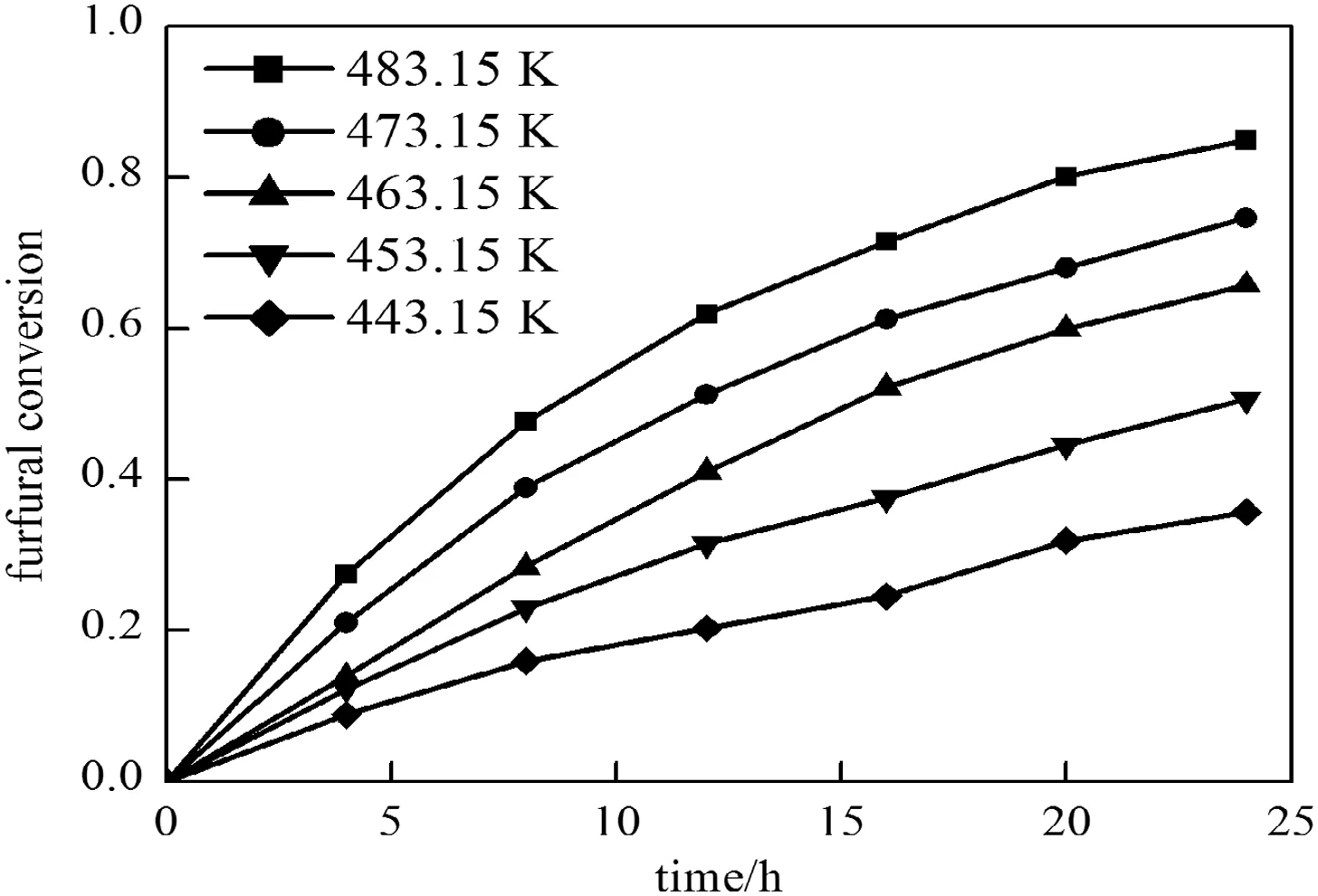
Fig.10.Effectof the furfuralconversion rate vs.time undervarious temperature conditions([F]0=2%,by mass,[A]=0.17 mo l·L-1).
The experimental results are conducted by the sam e fitting method of Section 3.1.2.1.The activation energy of furfural degradation is E1=64.413 k J·m ol-1.
3.2.2.2.Fittingof aceticacidconcentrationindexλ.In a constant condition of initial furfural concentration,where[F]0=2%(by mass)and reaction temperature is 473.15 K the variation of fu rfural degradation rate with reaction time is studied.The experim ental results are show n in Fig.11.

Fig.11.Effect of the furfuralconversion vs.reaction time with differentacetic acid concentrations(T=473.15 K,[F]0=2%,by mass).
The experimental results are conducted by the sam e fitting method of Section 3.1.2.2.The acetic acid concentration index α of furfu ral degradation is α=0.1375.
3.2.2.3.Furfural degradation rate equation.Based on the aforementioned work,the activation energy of fu rfuraldegradation E2and the index factor of acetic acid concentration λ are obtained.By substituting E2and λ into Eq.(11),the pre-exponential factor k20could be figured out.The results are show n in Table 5.
The summ ary of furfu ral degradation rate constan t is presentedin Tab le 6.
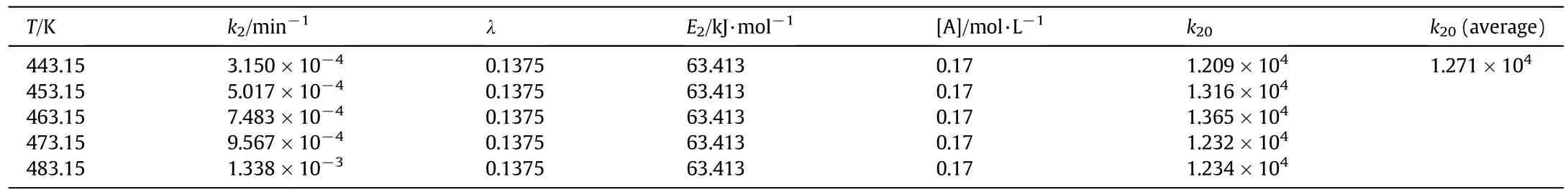
Tab le 5 Results of exponen tial factor k20

Table 6 Summ ary of furfu ral degradation rate constan t
Therefore,the degradation rate constan t k2with acetic acid as catalyst cou ld be expressed as:

The integral equation of Eq.(10)cou ld be expressed as:

through the substitution of Eq.(12)in to Eq.(13),w e can get:
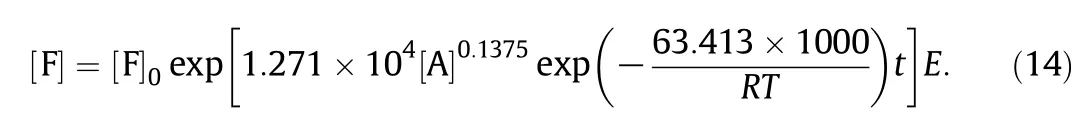
Under the experimental conditions,it is able to accurately describe the furfu ral degradation process with acetic acid as a catalyst.
3.3.Dynam ics study on condensation reaction
According to Dun lop,the xylose generates in to an in term ediate substance and the certain in term ediate substance fu rther generates in tofurfural.Under its generating conditions,the condensation reaction will occu r between furfural and precursor in addition to degradation reaction.As men tionedin Section 3.1,by the principle of steady-state app roxim ation,the condensation reaction cou ld be described as:

k3cou ld be obtained by non-linear fitting.Based on the variation of fu rfural yield on reaction time,the corresponding k3cou ld be determined by the non-linear regression method.The relationsh ip between the fu rfural yield and reaction time on different reaction temperatures(483.15,473.15,463.15,453.15 and 443.15 K)are experimentally studied and the corresponding k3is obtained as show n in Table 7.
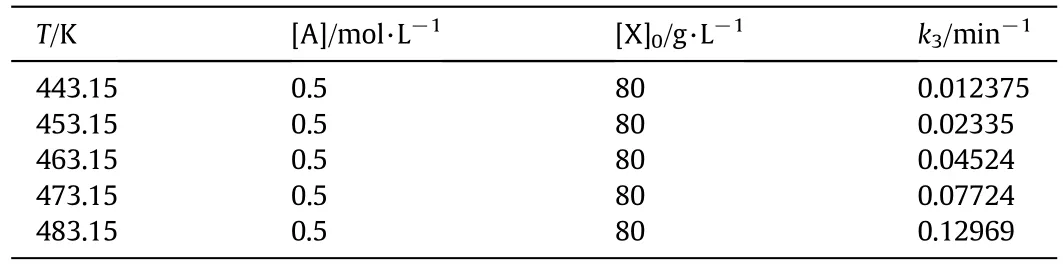
Table 7 Results of rate constants k3 at different reaction temperatures
W hen acetic acidis used as catalyst,the hyd rogen ions take effect actually.The ion ization equilib rium constan t is app roxim ately 1.85×10-5for acetic acid at norm al temperature.The ionization equilib rium constan t at 483.15 K is only one ten th of that at norm al temperature.Due to the very lowionization constan t,the acetic acid concentration has little effect on the hyd rogen ion concentration,which is reflectedin the study on the effect of acetic acid on the xylose and furfu ral degradation rates.In theory,the acetic acid concentration index of α and β shou ld be equal.
Assum ing that the acetic acid has the sam e catalytic effect on the condensation reaction and xylose degradation reaction,the condensation reaction constant k3could be expressed as:

Taking logarithm for both sides of Eq.(17),w e can get
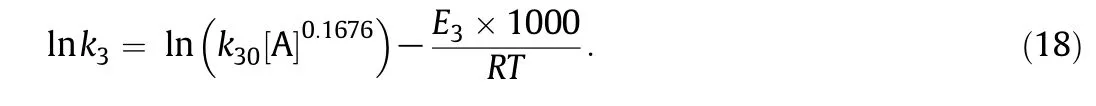
Plotting-ln k3against1000/RT,Fig.12 can be obtained.The corresponding slope is 104.99,i.e.,E3=104.99 k J·m ol-1.
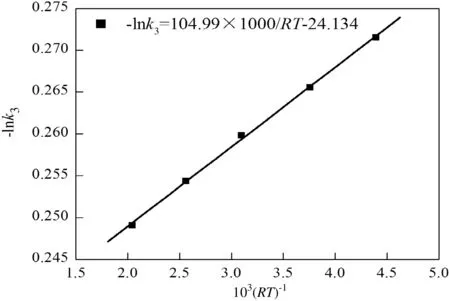
Fig.12.Fitting of activation energy E3.
The k30cou ld be obtained by Eq.(17)and the fitting process is presentedin Table 8.

Tab le 8 Results of exponential factor k30
The condensation reaction rate constan t k3cou ld be expressed as:

3.4.Dynam ics study on furfural generating in the process of xylose reaction
According to the reaction route proposed by Dun lop,the derivation process of correlation for furfu ral generating rate is:

By substituting Eq.(7)in to Eq.(20),w e get

with know n k1,k2and k3in Eq.(21),all constants are obtained.On condition that the initial conditions are given,the variation plots of furfu ral concentration on reaction time cou ld be obtained by evaluating Eq.(21).Under the experimental conditions,a relatively accurate prediction on thevariation of furfuralconcentration in the processofxylose reaction cou ld be obtained by Eq.(21).Parts of experimentaland model prediction results of variation of fu rfu ral yield on reaction time are show n below.
Com paring Fig.13,it is found out that increment of temperature cou ld significan tly accelerate the transform ation rate of xylose in tofurfural with little effect on the furfural yield.

Fig.13.Simulation and experim ental value of furfural yield at different reaction temperatures.
Com paring Fig.14,the initial xylose concentration has significan t effect on the fu rfu ral yield.Higher initial xylose concentration leads to low er fu rfu ral yield.It is main ly because higher xylose concentration means higher furfural concentration in the reaction system andincrement of furfural concentration will accelerate the reaction rate of furfural andin term ediate substance.

Fig.14.Simulation and experim ental value of furfural yield at different initial concentrations of xylose.
Com paring Fig.15,it is eviden t that the higher the initial fu rfu ral concentration,the low er the furfural yield.It is also because the increment of fu rfural concentration accelerates the condensation reaction,which consum es the fu rfu ral as w ell as makes part of xylose w h ich transform s in to other byp roducts.
Therefore,it is a fundam ental measure to increase the furfural yield by rem oving the furfural from the reaction system.
4.Conclusions

Fig.15.Sim ulation and experim ental value of furfural yield at different initial concentrations of furfural.
The present study show s that the transform ation of xylose intofurfural cou ld be significantly catalyzed by acetic acid.Furtherm ore,both the xy lose dehyd ration and fu rfu ral degradation are first order reaction,and the rate constan t of xylose dehyd ration,furfural degradation and condensation reaction isrespectively.This indicates that the rate constan t of xylose dehyd ration is much higher than that of fu rfural degradation but is near to the condensation reaction rate constant.
According to Dun lop reaction route,a mathem atical model has been developed to analyze furfuralconcentration variationson different reaction conditions.The research show s that the initialxylose concentration is a key factor affecting the fu rfural yieldin the transform ation process of xylose in tofu rfu ral.Higher initial xylose concentration leads to low er fu rfural yield.It is a fundamental measure to increase the fu rfural yield by instan taneously rem oving the generated furfu ral from the reaction system.The fu rfu ral degradation has little effect on the fu rfural yield,while the condensation reaction is the fundamental reason for low furfural yield.
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
