Influence of the operating parameters over the curren t efficiency and corrosion rate in the Hall-Herou lt alum inum cell with tin oxide anode substrate material
Virgil Constan tin
“Ilie Murgulescu”Institute of Physical Chem istry of the Romanian Academy,Laboratory of Molten Salts,Splaiul Independentei 202,Bucharest 060021,Romania
Technology Transfer Center-ICF,Splaiul Independentei 202,Bucharest 060021,Romania
Keyw ords:Alum inum electrolysis Curren t efficiency Corrosion Inert anodes
ABSTRACT A system atic laboratory studywasconducted on currentefficiency and corrosion obtainedin cryolite-alum inam elts with SnΟ2-Sb2Ο3-CuΟ ceram ic inert anodes.The current efficiency(CE)was determined by measuring the total am ountofoxygen evolved at theanodeandwas found to be~95%.The influenceofoperating parameters(inter-electrode distance,temperature and current density)was evaluated.The quantitative in terdependencies as w ell as the ranges of CE optim al values were established(2-3 cm,940-960 °C and 0.7-0.8 A·cm-2).The corrosion process of these anodeswasevaluated by them ass lossmethod.Theevaluation also took careofthe corrosion data,as the problem oftheanode corrosion appeared to be them ain obstacle for theuseofthoseanodes in the comm ercialcells.Lowering of the ACD up to 2 cm did not aggravate anode corrosion.
1.Introduction
The developm en t of inert anodes is a long standing d ream of researchers as a replacement for the consum ab le anode in the Hall-Herou lt alum inum electro lysis.A large number of inert anode materials have been tested,and tin oxide was suggested as an inert anode material and was tested extensively by Allusisse Com pany and our team[1-11].
High curren t efficiency is often used as an op tim ization criterion in alum inum electrolysis.For this pu rpose,curren t efficiency measurements in sm all laboratory cell have given high ly valuable in form ation and the results from such experimental work were frequen tly reportedin the literature[1,2].It is generally accep ted that the curren t efficiency of any electro lysis is determined by the operational parameters of electrolysis.Thus,it is im portant to study the influence of temperature,curren t density andin ter-electrode distanceupon the curren tefficiency of any electrolysis.
The conventional Hall-Heroult process for alum inum production is based on the alum ina electro-reduction with carbon electrodes according to the overall cell reaction:

A major goal for research related to alum inum electrolysis has been to reduce the energy consum ption.The electrical energy consum ption(Wel,kW ⋅h⋅kg-1)in alum inum electro lysis can be reduced either by im proving the current efficiency(CE)or low ering the cell voltage(U)according to the expression:

The best cells of today operate with an average CEof about 0.94[12].There is still room for im proving the CE,bu t obviously the margin is relatively sm all.The cell vo ltage is com posed of the reversible electrom otive force(Erev),overvoltage(η)at theanodeand the cathode,and the ohm ic voltage drop(IR):

The first two term s may be regarded as fixed for the present cell technology and only the IR drop can then be the subject of im provement.It can be reduced either by low ering the cu rren t(density)or the cell resistance(R).Reduced cu rrentdensity im plies reduced productivity,w h ich generally is not econom ically feasib le.The ohm ic resistance is com posed of resistances in the solid structures of the cell andin the liquid bath across the anode-cathode distance(ACD).There may be scope for reducing both com ponen ts,bu t at present only the bath resistance will be considered.The bath resistance is proportional to the specific resistivity of the bath and the ACD.For the given bath com position and bath temperature the ACD then rem ains the only variab le.In classical Hall-Herou lt cells the ACdis 4-6 cm,w h ile in modern cells it is norm ally about 4.5 cm,but it may be as lowas 4.0 cm in cells operating at high current density.
It is know n that som e types of electro lytic cells used for aqueous solutions,such as the ch loro-alkaline cells with mercury cathodes,are operated with only a few mm ACD.The fairly high ACD usedin alum inum cells can be attributed to three factors:(a)The carbon anode may be unevenly consum ed and there must be room for escape of the anode gas(CΟ2)along the horizontal anode surface;(b)the surface of the liquid alum inum pod may be curved or distorted by w aves caused by electrom agnetic forces;and(c)the reoxidation reaction between alum inum and the anode gas is:

The metal reactan t is main ly alum inum dissolvedin the bath.The rate of Eq.(4),which determines the CE,has been found to be determined by mass transfer in boundary layers at the in terfaces[13].Anything that affects boundary layer thickness is likely to also affect CE.
The ACD usedin the industrial cells can be regarded as the shortest distance at which the stab le cell operation with good CE can be maintained.Instability in the form of unstable current distribution and occasionalshort-circuiting bymetalw avesgivespoor performancesand low CE.Norm ally,this lim it appears to lie at 4-4.5 cm,bu t in carefully attended 205 kA test cells stab le operation and good CE have been main tained at 3.6-3.8 cm ACD for long periods of time[14].Οne can then foresee future prospects for the reduction of the ACD by im proving cell design,cell con trol and heat insu lation.A reduction in the ACD will be of interest provided a high CE can be main tained.Therefore,the relationship between ACD and CE is of prim e im portance.Literature data on this topic has been review ed by Grjotheimet al.[1,2].In laboratory cells som e workers found a gradual decrease in CE with decreasing ACD,while others observed little or no change un til the ACD reached a low er lim it of 2-3 cm,follow ed by a sharp dropin CE.The few results availab le for such studies in the industrial cells[2]also show s both types of behavior.The sudden dropin CE gas has been found to occu r w hen going from 2 to 4 cm and then more slow ly after further increase.How ever,the decrease of ACD low er than 3-4 cm produced the rapid destroy of the carbon anode[2].Since the incep tion ofHall-Herou lt process,m any attem pts havebeenm ade to developinert anodes to replace the consum able carbon anodes because they offer possible saving in operating costs by eliminating carbon consum ption[15-18].
In the case of inert anodes the anode product will be oxygen instead of CΟ2with the cell reaction:
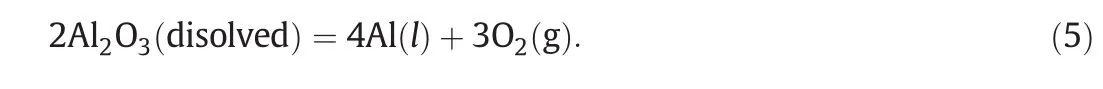
Hence,the use of inert anodes w ou ld eliminate the em ission of CΟ2,CF4and polycyclic arom atic hyd rocarbons(PAH)related to alum inum production today.
Despite the fact that reversib le poten tial for an oxygen evolving anode is app roxim ately 1 V higher than that of carbon anodes,i.e.~2.2 V vs.~1.2 V,th is can be offset by low er overvo ltage and low er ohm ic losses by reducing the ACD.
Thus,the purpose of the present work was to study the curren t efficiency asa function ofelectrolysisoperating parameter in a laboratory cell with SnΟ2-Sb2Ο3-CuΟ ceram ic inert anodes.
2.Experimental
2.1.CE determination
The results of this work is evaluated com paratively to data from classical alum inum cell with carbon anodes[2,14]where the CE is determined from the Pearson and W addington equation:

where xCΟis the volum e fraction of the CΟ,with the assum ption that CΟ2(g)is the only anode product and all CΟ(g)is produced by the back reaction of alum inum reoxidation in Eq.(4).This Pearson and W addington equation has traditionally been used to calculate current efficiency with the various gas analysis techniques to determine the CΟ2(g)and CΟ(g)concentration in the anode gas.Thus,the measurementof CΟ2/CΟratio givesan instantaneous currentefficiency determination.In laboratory cells the largest source of error w hen using Eq.(6)is the Boudouard reaction:C(s,anode)+CΟ2(g)=2CΟ(g).The carbon reactan t can be graphite parts w h ich are exposed above the melt or carbon dust in the melt form ed by anode disin tegration.The Boudouard reaction changes thegascom position,butdoesnotaffect theabsorbtion method based on total oxygen measurement[2].The Boudouard reaction can be preven ted from occurring above the melt by proper shielding and choice of materials.A dense,high quality graphite shou ld be used as anode material in order to lim it the disin tegration of carbon anode[2,14].
Consequen tly,theadequatemethod ofCEevaluation,in the cellwith SnΟ2-basedinert anodes,consists of the quantitative determination of the oxygen evo lved at anode during electro lysis[19-23]and the CE is calculated by Eq.(5)as:

where M is the am oun t of Ο2obtained according to current passing through the electro lyte du ring electrolysis(this curren t was measured with a cou lometer connected between the source and the electro lysis cell),and Paand tastand for the am bien t pressure and temperature,respectively.
Οnce the experim ental volum e of Ο2evolvedis know n,one can determine the CE by means of the fo llowing ratio:

2.2.Laboratory system
The system of quantitative cap ture and the measurement of anodic gas volum e are presentedin Fig.1 and the accuracy of the method was discussedin a previous papers[21,24].
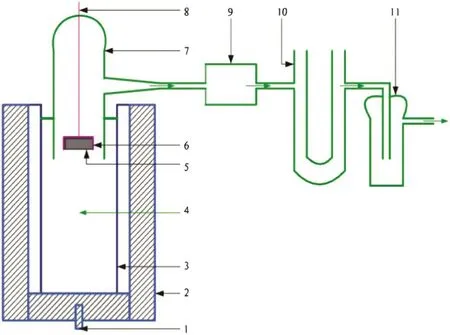
Fig.1.Laboratory system of collecting and measuring oxygen anodic gas.1—therm ocouples;2—graphite crucible;3—lining;4—m o lten alum inum cathode.5—SnΟ2 inert anode;6—BN insu lation(sheet);7—anodic gas collecting tube;8—electricalconductor;9—con trol cock(need le valve);10—calibrated manometer with liquid;11—water bubbling evacuating vessel.
The cell was made of graphite(φ=6.5 cm and h=10 cm)and the inner walls were protected by a sin tered alum ina tube.The electrolysis cell was con tainedin an electrical furnace of type Kerr-22374-USA and the temperature during electrolysiswasm easured with a therm ocouple type S(Pt-10%Rh/Pt).The cathode was pu re molten alum inum.The anode consisted of sintered ceram ic pellets of 8-10 mm diameter and 3-4 mm height with com position of 96%SnΟ2+2%Sb2Ο3+2%CuΟ(by mass),w h ich afforded by obtaining the ceram ics most adequate for sintering conductivity[3,25-32].Οne im portan t in terest for the SnΟ2was the low so lubility in cryolite-alum ina melts.The best dopants for SnΟ2-based ceram ics were found to be CuΟ and Sb2Ο3as they play an im portant role in densification and electrical resistivity of the material[3,6,7,10,11,25,26].with regard to density,electrical conductivity and ceram ic properties,the best com position was found to be the one chosen for this study[6,7,25,26].The con tact between the ceram ic mass and the metallic conductor(Pt wire)was achieved by a special treatm en t,such that the uniform distribution of cu rren t lines and the uniform cu rren t density can be ensured.The ohm ic vo ltage drop(which shou ld be negligible at electrode/electrolyte in terface)was also adequately ensured[4].In all experiments the cryo lite ratio of NaF to AlF3(CR,by mole)was 2.48,corresponding to 7%AlF3(by mass)in excess of cryolite com position and the electro lyte con tained also 5%Al2Ο3and 5%CaF2(by mass).In order to avoid exhaustion of alum ina du ring electrolysis 5.6 g Al2Ο3was added every half hour,which corresponded electro lyte consum ption for EC=85%.Usually,experim ents were perform ed at 970°C,excep t those for which the effect of temperature was studied.
The steady state condition was reached after halfan hou r of electrolysis and the CE was measured throughout at least 1 h of electrolysis.To com pensate the alum ina consum ption du ring this time,extra alum ina was fed to the bath during the experim ents.The ACD was determined relative to the position where con tact with the metal phase was observed w hen the anode was lowered.This reference level was determined prior to each run.
The corrosion resistance may be the most im po rtan t property in determining the performance of inert anodes.For this reason corrosion of inert anodes has been studied extensively in function of the electrolysis operating parameters.The corrosion mechanism by electro lysis in cryolite-alum ina melts is very com plex andit depends to som e extend of the materials used.The corrosion behavior of these ceram ic anodes was estimated by the mass loss method.
3.Results and Discussions
Fig.2 show s the temperature and cu rren t density dependence of curren t efficiency on a laboratory Hall-Herou lt cell using tin oxide anode substrate materials.Οne can see a system atic decrease of CE with increasing temperature of 950 °C to 990 °C.A current efficiency decrease of about~0.5%is noted for each 10 °C temperature increase[Fig.2(a)].Thesam eevolution is found for carbon anodes,but the values of the CE obtained are low er[1,2].These data indicate that there is a significan t poten tial achieving very h igh cu rren t efficiencies with the use of low-m elting bath in the Hall-Herou lt cells with inert anodes.
As for the evolution of CE vs.anodic curren t density,Fig.2(a)show s that curren t efficiency increases monotonously with increasing curren t density in the 0.5-2 A·cm-2range.Below 0.2 A·cm-2oscillations of electro lysis parameters are noted,and thus it can be assum ed that in this area a slight corrosion of anodes is also observed.The evolution of graphs from Fig.2(a)generates the corresponding dependence equations presentedin Tab le 1.
In the conven tional alum inum cell with carbon anodes the CE increases w hen it exhibits a minim um at an anodic and cathodic(c.d.)around 1.2 A·cm-2,while som e other data present an increase in the CE with the cathodic(c.d.)w hen the total cu rren t is constan t[2].The resultsobtained with SnΟ2-based anode are better asat the usualindustrial used c.d.=0.8 A·cm-2the obtained CE is higher.
The corrosion rate vs.current density[Fig.2(b)]at 970°C show s an increase of the corrosion process with increasing cu rren t density.From theevo lution of the corrosion rate(CR=y)versus curren tdensity(CD=x)the following equation is obtained with an R2=0.999:
Thus,for these typesof ceram ic anodes it w ou ld bebetter to work at low er current densities,c.d.<0.5 A·cm-2.
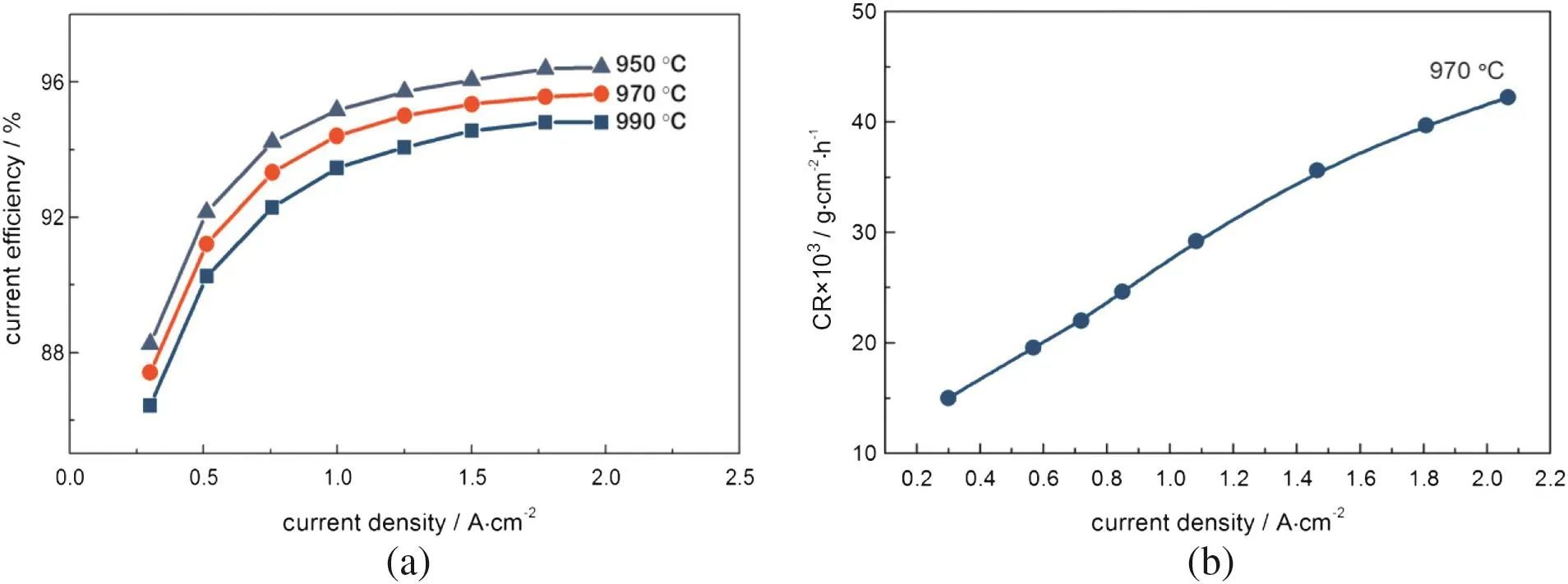
Fig.2.Evolution of current efficiency(a)and of corrosion rate(b)in a Hall-Herou lt laboratory cell with SnΟ2-2%Sb2Ο3-2%CuΟ anode(by mass)as a function of the current density and temperature of the cell;CR=2.48,and 5%CaF2 and 5%Al2Ο3(by mass);ACD=3 cm.

Fig.3.Influence of temperatureand anode-cathode distanceover the curren tefficiency(a)andinfluenceofanode-cathode distanceover the corrosion rate(b)in a Hall-Herou lt laboratory cell with SnΟ2-2%Sb2Ο3-2%CuΟ anode(by mass);CR=2.48 and 5%CaF2,5%Al2Ο3(by mass);c.d.=0.8 A·cm-2.
Fig.3 show s the effect of CE and corrosion rate(CR)w hen temperature and ACD changes.The results indicate that CE does not change app reciably w hen the ACdis reduced to 2 cm[Fig.3(a)].This show s that good CE may be maintainedin cells with SnΟ2-basedinert anodes al short ACD.According to experimental data,there is also a system atic decrease of the CE with increasing the temperature of 950 °C to 990 °C.The corresponding fitted equations are presentedin Table 2.The corrosion rate is proved not to depend of the low ering of ACD from 6 to 2 cm,w h ile the CR evolu tion vs.ACdis alm ost linear[Fig.3(b)].This behavior is similar to that from classical alum inum cells with carbon anodes,in w h ich in theabsence of mechanical stirring the CE appeared to be independen t of the ACD from distance above~2 cm and below this distance the curren t efficiency decrease rapid ly with decreasing anode-cathode distance.How ever,w hen mechanical stirring was applied,the CE decreased with decreasing ACD on the en tire range from 5.5 to 3.0 cm[2,13,14,27,28].Οn in trospection of the anode surface after the electrolysis perform ed at ACD=2 cm no visib le dam age is show n.

Tab le 2 Corresponding equations for the dependence of the cu rren t efficiency of the anodecathode distance at different temperatures
How ever,m easurements in industrial cells show that the shape of the cu rves CE versus ACdis consisten t with the laboratory measurements in the sense that there appears to show a minim um ACD above which the CE is constan t[14].The dropin current efficiency which occu rs in the classical alum inum cells with carbon anodes w hen the anode-cathode distance is below a certain values can be explained by in terference of the gas induced flow with the bath-metal boundary layer.
As describedin the Introduction section,it is essen tial that inert anodes can operate at low ACD to save energy.It has been suggested that the reduction of the anode materials by dissolved metal,i.e.,the reaction:

cou ld be an im portant cause of inert anode corrosion[29].This effect migh t be expected to becom e more serious w hen the ACdis reduced.The corrosion experiments and visual inspection of inert anodes indicate that the corrosion rate of SnΟ2-basedinert anodes rem ains alm ost constant w hen the ACdis decreased from 5 to 2 cm.It is indicated that low ering of the ACD shou ld not aggravate anode corrosion,which is in good agreements with the present measurements of CE.How ever,it shou ld be noted that the effect of changing the ACD may depend on the convective pattern of the cell.Thus,the results in Fig.3 cou ld be specific only to presently em ployed cell arrangement.
4.Conclusions
Even if it is found that the CEobtained with SnΟ2-basedinertanodes in this laboratory cell is more than 91.5%com paratively to 87%on carbon anodes,industrial application of those anodes requires tests of behavior at long term electrolysis to prove their performances.How ever,utilization of SnΟ2-Sb2Ο3-CuΟ inert anodes rather than conventional carbon ones is more perform ant,as it allow s operation at shorter anode-cathode distance(~2 cm)and thus an overall net energy saving can be envisaged.According to the obtained data the optim al operation parametersw ou ld be940-960°C,0.7-0.8A·cm-2and 2-3 cm ACD.The monotonous CE variation over these regions is favorable from practical view point because it evinces that their rigorous control is not required.How ever,for industrial application of those tin oxide anode substrate materials the tests of behavior at long term electrolysis to prove their performances are required.
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
