Therm odynam ic analysis of manufacturing polysilicon from SiHCl3,SiCl4 and H2☆
Peilong Li,Tiefeng W ang
Beijing Key Laboratory of Green Reaction Engineering and Technology,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
Keyw ords:Therm odynam ics Polysilicon Fluidized bed Trich lorosilane Silicon tetrach loride
ABSTRACT Production of polysilicon by chemical vapor deposition of SiHCl3 with a fluidized bed reactor is a com petitive technology.As equilibrium conversion can be approachedin a fluidized bed reactor,a reliable therm odynam ic analysis is very im portan t.How ever,inconsistent therm odynam ic analysis results have been reportedin the literature.The present work studied the effects of therm odynam ic data and species selection,and recomm ended that JANAF was the best Cp data source and the minim um set of species included the following eigh t species:H2,HCl,SiCl4,SiCl2,SiHCl3,SiH2Cl2,SiH3Cl and Si.Then,the influence of operating conditions on the equilibrium was studied.For the SiHCl3-H2 system,both the yield of silicon and selectivity to silicon reached their maxim um at(up to 1100°C),and low pressure and high H2 feed ratiowere ofbenefit for silicon production.For the SiHCl3-SiCl4-H2 system,silicon cou ld be produced only at 900-1400°C,and reducing pressure andincreasing H2 feed ratio enhanced the yield of silicon.M eanw h ile,the operation map for zero net by-p roduction of SiCl4 by directly recycling the produced SiCl4 was determined.
1.Introduction
In recent years,the photovoltaic(PV)industry hasexperienced an explosion developm ent with an average annual grow th rate of up to 30%.Many efforts have been devoted to the production of solar grade silicon to meet the rising dem and for silicon-based solar cells.Now adays about 70%-80%w orldwide polysilicon is produced by the modified Siemens process using SiHCl3.How ever,the Siemens process is a batch operation,and hasa low productivity and high energy consum ption[1].In addition,the present polysilicon industry faces severe overcapacity,and the key issue for the po lysilicon industry is changing from production expansion to techno logy improvement that enhances the efficiency and decreases the production cost.The fluidized bed process is considered a promising alternative to the Siemens process for its much low er cost and high efficiency[2].It has been demonstrated that the direct power consum ption of the fluidized bed process using SiHCl3as feedstock is less than half of that of the modified Siemens process[3].
The SiHCl3hyd rogenation reduction system isa Si-H-Cl ternary system.In the fluidized bed process,the Si-H-Cl system is usually close to therm odynam ic equilib rium because the seed particles provide very large area of reaction surface[4].Therefore,a reliable therm odynam ic analysis is very im portan t for the design of a fluidized bed process.The therm odynam ic analysis of the Si-H-Cl system has been reportedin many works.Sirtl et al.[5]revealed the equilibrium com position diagram of the Si-H-Cl system.The effects of temperature,p ressure andinitial feed com position on equilibrium were studied[5-7].Diana et al.[8]studied the equilibrium considering som e radicals and species that are only stable at high temperature.
Although the therm odynam ic analysis has been studied by many researchers,significan t inconsistencies exist in the results.The first difference is the source of therm odynam ic data.The heat capacity Cp,standard enthalpy of form ation ΔfHϴand standard absolute entropy Sϴ(or standard entropy of form ation ΔfSϴ)are the basic data for therm odynam ic calculations.Allen et al.[9]found that the value of ΔfHϴhad a significan t effect on the equilibrium com positions,while the value Sϴhad a less significan t effect.How ever,the effects of Cpvalues have not been carefully studied.Tw o main heat capacity sources are used,nam ely therm odynam ic databooks[10-17]and chemical softw are in which som e of Cpdata are even tually from databooks,as show n in Tab le 1.The other inconsistency is resulted from the selection of species for the therm odynam ic analysis.In the Si-H-Cl system at conditions relevan t for silicon production,silicon exists as solid state and the gas phase may con tain H2,HCl,SiCl4,SiCl3,SiCl2,SiCl,SiH4,SiHCl3,SiH2Cl2,SiH3Cl and som e radical species.Differen t sets of species have been usedin therm odynam ic analysis of the Si-H-Cl system in the literature[4-8,18-23],as listedin Tab le 2.
In this work,the effects of Cpdata and species selection on therm odynam ic analysis of the Si-H-Cl system were system atically studied.JANAF was recomm ended as the best database for Cp,and the minim um and necessary set ofeightspecieswasobtained.The calculationmethod was validated by experimental data,and was then used to study the effects of reaction temperature,p ressure and feed com position on theequilibrium conversion and selectivity for both the SiHCl3-H2and SiHCl3-SiCl4-H2system s.The feasibility of zero net by-p roduction of SiCl4by directly recycling the produced SiCl4was discussed.

Tab le 1 Som e widely used therm odynam ic data resources

Tab le 2 Different species selection in the therm odynam ic studies of Si-H-Cl system
2.calculation Method
Therm odynam ic calculations are based on the therm odynam ic equilibrium theory.The Gibbs free energy of a reaction system under conditions of T,P and specific com position is[7]:

where ni,yiand Φiare the moles,m olar fraction and fugacity of species i,respectively,is the standard molar Gibbs free energy,and Pϴis the standard pressure.According to the Gibbs energy min im ization principle,the partial molar Gibbs energy of i is zero w hen the system reaches the therm odynam ic equilibrium:

At temperature T,the standard molar Gibbs free energy ΔfGiϴis:

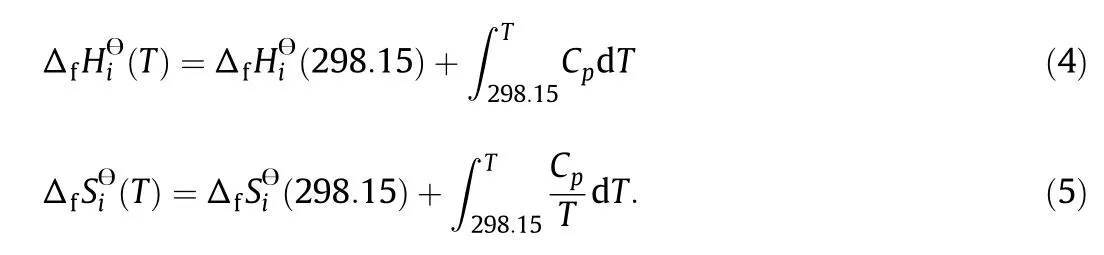
Tofacilitate the following discussion,som e variables are defined.W hen the reactants are SiHCl3and H2,the equilibrium conversion of SiHCl3(X),selectivity to Si(S)and yie ld of Si(Y)are defined as follow s:

where n rep resen ts the equilibrium mo le com position of a specific species.
W hen the reactants are SiHCl3,SiCl4and H2,the change rate of SiHCl3(R)isdefined as the ratio of consum ed SiHCl3to the total am ount of SiHCl3and SiCl4in the feed,and the yield of Si(Y)is defined as the ratio of the produced silicon to the total am ount of SiHCl3and SiCl4in the feed:

Because SiCl2and som eother speciesare stab le only athigh temperature and convert to other species at low temperature,the equilibrium com positions are difficu lt to measure at high temperature,but can be easily obtained by therm odynam ic calculations.
3.Results and Discussions
3.1.Calculation method and validation
3.1.1.Effects of heat capacity
Fig.1 com pares the heat capacities of H2,Si,HCl and SiCl4from different databases.The results show that the Cpdata from different sourceshave som e inconsistencywith each other.Theequilibrium compositionsare calculated using different Cpdata tofurther study the influence of Cp.The data from JANAFare used asbasis,and the Cpdatahaving the largest deviation are com pared.For exam ple,the Cpdata of H2in Ye[15]have the largestdeviation from JANAF,the relative changesofequilibrium com positions using these two sources are calculated to study the influences of Cpdeviation from different sources.Tab le 3 show s the relative changes in the equilibrium com positions of SiCl4,SiHCl3and Si.At 900°C,the relative change in SiCl4equilibrium com position is 0.48%,which is defined as the ratio of the difference of SiCl4equilibrium com positions calculated using Cp(H2)data from Ye[15]and JANAF to that using Cp(H2)data from JANAF.In the temperature range of 900-1200°C,the differences in the Cpdata of H2,Si,HCl and SiCl4do not cause significan t changes in the equilibrium com positions,and most of the relative changes do not exceed 3%.At T>1200°C,the influences of Cpbecom e more eviden t,especially on the con ten t of SiCl4,with the relative change being 10.4%at 1400°C.The effects of the Cpdata of other species are not considered because these species have either very low equilibrium concentrations or very similar Cpvalues in different databases.The operating temperature of a fluidized bed reactor for silicon production does not exceed 1200°C,and therefore the effects of different Cpdata on the equilibrium can be neglected.
JANAFis them ostwidely used therm odynam ic database andit is the major Cpdata source of comm ercial softw are such as Aspen and HSC.In addition,com pared with other therm odynam ic databases,JANAF contains more species of the Si-H-Cl system,including SiH3Cl that has a significant effect on equilibrium and som e trace substances such as SiCland SiCl3.Som eof these speciesarenot includedin other databases.Thus,JANAF is recomm ended as a reliab le database for Cpdata of the Si-H-Cl system.
Fig.1.comparison of C p data from different sources.HSC;Aspen;Chem kin;Kubaschew ski[17];DIPPR[13].JANAF[10];Ye[15];○Gm elin[14].
3.1.2.Effects of species selection
In the therm odynam ic study of the Si-H-Cl system,the species H2,HCl,SiCl4,SiHCl3,SiH2Cl2and Si are considered by most researches.How ever,the selection ofother species isvery different in the literature.The effects of SiCl2,SiH3Cl and other trace species on therm odynam ic equilibrium are discussedin this section.Fig.2(a)show s the effects of SiCl2on the equilibrium com positions in a system of H2,HCl,SiCl4,SiHCl3,SiH2Cl2and Si.The results show that the inclusion of SiCl2in the system has an insign ifican t effect below 900°C,bu t significan tly changes the equilibrium com positions at higher temperatures.The equilibrium am ount of Si monotonically increases with increasing temperature w hen SiCl2is not included,but it show s a maxim um at(up to 1150 °C)w hen SiCl2is considered.At1200 °C,theneglectofSiCl2causes a positiveerror of53%for theequilibrium am ountofSi,30%for SiCl4and 22%for SiHCl3,indicating that SiCl2must be included.

Tab le 3 Effect of Cp data source on equilib rium com positions
with the species H2,HCl,SiCl4,SiHCl3,SiH2Cl2,Si and SiCl2,the effects of further including SiH3Cl are studied.The results are show n in Fig.2(b).The equilibrium am oun t of Si is decreased by 21%at 1100°C and 15%at 1200°C w hen SiH3Cl is includedin the calculation.This show s that SiH3Cl must also be includedin the therm odynam ic analysis of the Si-Cl-H system.
The effects of further including other species[SiCl,SiCl3,SiH4,SiH3,SiH2,SiH,Si(g),Si2(g),H and Cl]on the equilibrium are studied on the basis of H2,HCl,SiCl4,SiCl2,SiHCl3,SiH2Cl2,SiH3Cl and Si(s).The calculation resultsshow that the relative changes in theequilibrium am oun ts of major species are within 0.5%w hen including SiCl and SiCl3,and the inclusion of other trace species leads to much sm aller changes in the equilibrium com positions.
Based on the above results,a reliable therm odynam ic analysis of the Si-H-Cl system shou ldinclude at least eigh t species,nam ely H2,HCl,SiCl4,SiCl2,SiHCl3,SiH2Cl2,SiH3Cl and Si(s),and others are trace species that can be neglected.Som e previous works did not include all the above eigh t species,as listedin Tab le 2.This can cause significan t errors of the results.
3.1.3.Experimental validation
The therm odynam ic calculations with eight species(H2,HCl,SiCl4,SiHCl3,SiH2Cl2,Si,SiCl2and SiH3Cl)and their therm odynam ic data from JANAF are validated with the experimental data.Fig.3(a)show s the comparison between the experimental[24]and calculated results.The calculated equilibrium yields(Y)agree w ell with the experimental data.Them easured selectivity to silicon(S)ishigher than the calculated equilibrium values at T<1000°C,and both Y and S agree w ell with the experimental data at T>1000°C.with increasing temperature,the equilibrium conversion(X)increases,but the measured conversionfirst increases and then decreases.

Fig.2.Effects of including(a)SiCl2 and(b)SiH3Cl on equilibrium com positions.(Initial feed com position:H2=4 mo l,SiHCl3=1 mol;P=1×105 Pa.)with SiCl2 or SiH3Cl:SiCl4;SiHCl3;Si.without SiCl2 or SiH3Cl:SiCl4;SiHCl3;Si.
The calculated equilibrium conversion of SiHCl3(X)is different from the measured curve.At temperature below 1000°C,the Si-H-Cl experim ental system is controlled by reaction kinetics and deviates from therm odynam ic equilib rium.As a result,the measured conversion of SiHCl3(X)is significantly low er than the equilibrium value.The Si-H-Cl experimental system reaches therm odynam ic equilib rium at T>1000°C[4].The inconsistencies between the experimental and equilibrium conversions are caused by the measuring method.In the experiments,the conversion is determined based on concentration of stab le species(SiHCl3,SiCl4,SiH2Cl2and HCl)in the gas product measured by gas ch rom atograph.The con ten t of SiCl2increases with increasing temperature,reaching 33%of the total silicon con tain ing gas at 1200°C,bu t it is only stable at high temperatures.W hen the hot equilibrium gas product flow s to the GC colum n at low er temperature,SiCl2converts to stab le species such as SiHCl3,SiCl4and Si.The reactions involving SiCl2are as follow s:

Because silicon deposition is not observed during cooling of the hot equilibrium gas,the possible reactions are Eqs.(11)and(12).Fig.3(a)show s the calculated conversions with different ratios of reaction[Eqs.(11)and(12)],where fEq.11is the ratio of SiCl2converted to SiHCl3via Eq.(11).For exam ple,“fEq.11=0.75”m eans that 75%of SiCl2converts to SiHCl3via Eq.(11)and the rem ain ing 25%of SiCl2converts to SiCl4via Eq.(12).According to the definition of X,the calculated X isunchanged w hen allSiCl2converts to SiCl4(fEq.11=0).At T<1000°C,the curves with different fEq.11are very close to each other because the equilibrium am ountofSiCl2isvery sm all.The comparison of the calculated and experimental results show s that 25%-50%of SiCl2converts to SiHCl3and the rem aining converts to SiCl4.
In the chemical vapor deposition(CVD)of SiHCl3,SiCl4is a major byproduct.Οur previous study[25]show ed that it is feasible to directly recycle SiCl4to the reactants in a fluidized bed reactor.Therefore,the equilibrium analysis of the Si-Cl-H system that in itially con tains SiHCl3,SiCl4and H2is of in terest.Fig.3(b)show s the comparison of the calculated and experimental yield of Si[24]with feed com position SiHCl3/(SiHCl3+SiCl4)of 75%and 50%.Οverall,the trends of calculated equilibrium yield of Si agree w ell with the experimental results.The maxim um yield occurs at 1100-1200°C,which is similar to that with the initial feed of H2and SiHCl3.The addition of SiCl4significantly decreases the Si yield with the maxim um yield decreases from 21%for pu re SiHCl3to 14%for 25%SiCl4+75%SiHCl3in feed and to 7%for 50%SiCl4+50%SiHCl3.
The calculated equilibrium com position is also consistent with the experimental results of W ood ru ff and Sanchez-M artinez[26],as show n in Fig.3(c).In that work,the measured equilibrium is expressed by Si/Cl vs.Cl/H.The calculated Si/Cl values are w ell consistent with the experimental results with the initial feed of H2and SiHCl3.
3.2.Thermodynam ic analysis for silicon production
3.2.1.Effects of reaction conditions
Them ost im portantparameters in the silicon production processare the reaction temperature T,operating pressure P andinitial feed com position.Because the therm odynam icequilibrium isapproachedin afluidized bed reactor under most conditions,the influence of T,P and feed com position can be discussed based on therm odynam ic calculations.The effects of T on the SiHCl3conversion(X),silicon selectivity(S)and yield(Y)are show n in Fig.3(a)(fEq.11=0).The equilibrium am oun t of SiHCl3decreases with increasing temperature,leading to an increase in X.The system has thehighestsilicon selectivity(28%)and yield(22%)at(up to 1100°C).The calculation result isconsisten twith theoperating temperature usedin the industrial process.
In the modified Siemens process where the reactions do not reach equilibrium,elevated operating pressure up to 0.5-0.6 MPa is often used to increase the reactor efficiency and production.In con trast,the elevated pressure is un favorable for a fluidized bed reactor,which is determined by therm odynam ic equilib rium.Fig.4(a)show s that increasing pressure results in an increase of SiHCl3conversion but a decrease in the selectivity to silicon,which is consisten twith the results of Chernyavsky et al.[7].In a fluidized bed process,increasing pressure is un favorable for the yield of silicon,buta higher gasvelocity can effectively increase the reactor production.To assure good fluidization quality at a higher gas velocity,larger silicon particles shou ld be used.
The H2/SiHCl3ratio of the feed(H2feed ratio)is also an im portan t operating parameter.Fig.4(b)show s the effects of the H2feed ratio on the equilibrium.with increasing H2feed ratio,the conversion of SiHCl3first decreases and then increases with the minim um present at H2/SiHCl3=4.Meanwhile,both the silicon selectivity and yield monotonically increasewith increasing H2feed ratio.Based on the therm odynam ic calculations,a higher H2feed ratio is favorab le for producing more silicon in a fluidized bed reactor.How ever,with increasing H2feed ratio in the modified Siemens process that the deposition rate is determined by reaction kinetics,decreasing concentration of SiHCl3results in a decrease in silicon deposition rate and an increase in energy consum ption.
The fluidized bed process and modified Siemens process are comparedin term s of SiHCl3conversion and silicon selectivity.In the later process,only~20%of reacted SiHCl3converts to Si while most of the rest converts to SiCl4.Generally,the SiCl4byp roduct is 15-20 kg·kg-1silicon,which causes both increasing production cost and severe environmental issues[27].Reducing the am oun t of SiCl4byp roduct is very im portan t for the silicon industry.Ding[28]studied the effect of adding SiCl4to the feedin the Siemensprocess,and found that the silicon productionwasdecreased and the power consum ption per kg silicon significantly was increased.In con trast,the fluidized bed process can reach therm odynam ic equilibrium,and thus it can efficien tly use SiCl4.

Fig.3.comparison of the experiment and calculated results.
Fig.5(a)show s that with the feed mo lar ratio of H2:SiHCl3:SiCl4=4:0.5:0.5,solid silicon is form edin a certain temperature range with the maxim um yield of silicon occurring at(up to 1150 °C).At T < 900 °C,no solid silicon is form ed.The effects of pressure at T=1200°C are show n in Fig.5(b).Both the change rate of SiHCl3and silicon yield monotonically decrease with increasing pressure,which is different from the trend of the conversion of SiHCl3with initial feed of H2:SiHCl3=4:1.Fig.5(c)show s the results at T=1200°C and P=1×105Pa.The change rate of SiHCl3(X)has its minim um at H2/(SiHCl3+SiCl4)=~4,while the silicon yield(Y)increases with increasing both H2/(SiHCl3+SiCl4)and SiHCl3/(SiHCl3+SiCl4)ratios.W hen SiHCl3/SiCl4=3 and H2/(SiHCl3+SiCl4)=20,the silicon yield reaches 33%.
In the Si-H-Cl system,the silicon etching reactions occu r under som e conditions.As show n in Fig.5(c),no silicon form s w hen H2/(SiCl4+SiHCl3)<4 and SiHCl3/SiCl4=1/3.The silicon etching reactions occur as follow s:


Fig.4.Effects of P and H2 feed ratio on equilibrium conversion of SiHCl3(X),silicon selectivity(S)and silicon yield(Y)(feed:H2-SiHCl3).
To better discuss the conditions for silicon etching and deposition,an am ount of solid silicon is includedin the initial feed.As show n in Fig.5(d),the temperature and feed com position have significan t effects on deposition or etching of silicon.W hen SiHCl3/(SiCl4+SiHCl3)>25%,the silicon yield has its maxim um at 1100-1200°C,and silicon etching reactions may occur at both high and low temperatures.At SiHCl3/(SiCl4+SiHCl3)<25%,the silicon etching reaction is therm odynam ically favoredin all temperature range.These results show that the SiHCl3/(SiCl4+SiHCl3)ratio of the feed cannot be too low,otherwise there w ou ld be no silicon deposition and the silicon seed particles w ou ld be consum ed by etching reactions.

Fig.5.Effects of reaction conditions on equilibrium(feed:H2-SiHCl3-SiCl4).
3.2.2.Feasibility of zero net by-production of SiCl4
In the Siemensp rocess,two app roaches are usedin the treatm ent of SiCl4:one is to convert SiCl4to other chemical products such as w hite carbon black,and the other is to convert it to SiHCl3by hyd rogenation,which is then used as feed of the bell jar reactor[29].The therm odynam ic calculations show that it is feasib le to directly mix the byp roduct SiCl4in the feed to realize net zero SiCl4by-production.This has been validated by ou r previous experimental work in a fluidized bed process[25].
In the modified Siemens process,the mixture of SiCl4and SiHCl3from the outlet of the bell jar reactor is separated by distillation,and SiCl4is then converted to SiHCl3through eithera cold orhothyd rogenation process,as show n in Fig.6(a).Because zero net by-p roduction of SiCl4is feasible in a fluidized bed reactor,the produced SiCl4and unconverted SiHCl3can be directly recycled to the feed stream after rem oving H2and HCl from the gas product without separation of SiCl4and SiHCl3and hyd rogenation of SiCl4to SiHCl3,as show n in Fig.6(b).In this w ay,the silicon production process is significan tly sim plified and the overall energy consum ption can be reduced.

Fig.6.comparison of two processes with different treatm ents of SiCl4.
The regim e map of SiCl4by-p roduction is show n in Fig.7.The three curves show the operating conditions for zero net by-production of SiCl4w hen feed molar ratio SiHCl3:SiCl4is 3:1,1:1 and 1:3,respectively.Οn the righ t side of each curve,the net by-p roduction of SiCl4is negative because SiCl4is consum ed,while on the left side of the cu rve SiCl4is produced from SiHCl3.with increasing SiHCl3/SiCl4ratio of the feed,a higher temperature is needed to realize zero net by-p roduction of SiCl4.
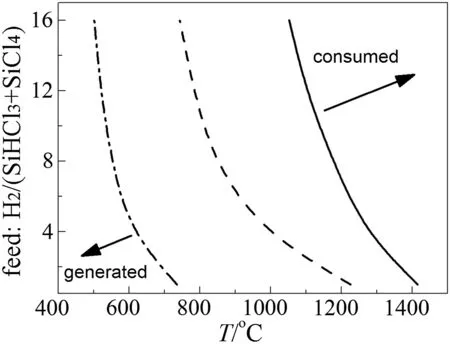
Fig.7.Regim e map of SiCl4 by-production(P=1×105).SiHCl3:SiCl4:
4.Conclusions
A detailed therm odynam ic analysis has been carried for the Si-H-Cl system with focus on the production of polysilicon from SiHCl3,SiCl4and H2.The effects of the heat capacity data and the species selections are discussed to determine the proper setting of therm odynam ic calculations.The effects ofoperating conditionsof temperature,pressure and feed com position on the equilibrium are studied,and the feasibility of zero net by-production of SiCl4in a fluidized bed reactor is discussed.The main conclusions are as fo llow s:
(1)The JANAF database is recomm ended as the best Cpdata source for the Si-H-Cl system.A reliab le therm odynam ic analysis of the Si-H-Cl system must include at least eigh t species,nam ely H2,HCl,SiCl4,SiCl2,SiHCl3,SiH2Cl2,SiH3Cl and Si(s),while other trace species can be neglected.
(2)with a feed of H2:SiHCl3=4:1,the conversion of SiHCl3monotonically increases with increasing T,bu t the silicon selectivity and yield have maxim um values at 1100-1200°C.Higher pressure and low er H2feed ratio result in a low er silicon yield.
(3)The addition of SiCl4to the feed decreases the change rate of SiHCl3,bu t sign ifican tly decreases the am oun t of the SiCl4byp roduct.Decreasing pressure andincreasing H2/(SiHCl3+SiCl4)and SiHCl3/(SiHCl3+SiCl4)ratios are favorab le to increase the silicon yield.At SiHCl3/(SiCl4+SiHCl3)>25%in feed,the silicon yield has its maxim um at 1100-1200°C,while at SiHCl3/(SiCl4+SiHCl3)<25%the silicon etching reaction is therm odynam ically favoredin all temperature range.
(4)A regim em ap for the am oun tofSiCl4byp roducthasbeen obtained,which can be used to guide the choosing of operating conditions to realize zero net by-p roduction of SiCl4in a fluidized bed reactor.
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
