Numerical study of pore structure effect on SO2-CaO reactions☆
Liang Ma ,Liyong Cao ,Rong He ,*
1 Department of Thermal Engineering,Tsinghua University,Beijing 10084,China
2 Dongfang Electric Corporation,Chengdu 611731,China
Keyw ords:Desu lfurization model Gas diffusion Fractal Pore structure
ABSTRACT The effect of varying pore structures on the kinetics of SO2-CaO reactions is not fully understoodin the previous studies.Com bining fractal pore model,gas molecular movement model and two-stage reaction model,a new desu lfurization model is estab lishedin this paper.Fractal pore model is used to simulate CaO particle and gas molecular movement model is used to sim ulate gas diffusion in pores.Fractal dim ension is used to characterize com plexity of pore structure instead of tortuosity factor.It is found that the reaction is significan tly affected by pore structures.A modu lus φ is in troduced to characterize the relationship between varying pore structures and apparen t reaction parameters.And this relationshipis verified by therm o-gravimetric analysis(TGA)data.Com paring to the previous models,the effectof varying pore structure on the kinetics of the reaction isdescribed more accurately by the desulfurization model.
1.Introduction
SO2is a pollu tan t in flue gas and desu lfuration has been experim entally and numerically investigated by som e researchers[1-3].In SO2em ission con trol,lim estone is w ell-know n and comm only used to reactwithfluegas.The lim estone(CaCO3)iscalcined(heated to decompose in to CO2and porousCaO)and then su lfated[4].The su lfation reaction ofporousCaOwith SO2isa typicalnon-catalyticand heterogeneous reaction,and a com plex physicaland chemicalp rocess.In general,there are two stages du ring the su lfation reaction[5,6].In the early stage,the reactan t gases diffuse into pores and react with CaO on the CaO surface.The first-stage reaction can be represented by:

And the reaction rate-lim iting step rep resen ts the com plex coupling of chemical reaction and gas diffusion in pores[7].How ever,w hen a con tinuous CaSO4product layer form ed on the CaO surface,the Ca2+and O2-diffuse to the gas-so lidin terface through the product layer and react with SO2as:

In the second-stage reaction,the rate-lim iting step changes to ion diffusion through product layer[8].The resistance of diffusion through product layer is far greater than that of gas diffusion in pores[9,10].The first stage reaction plays a dom inan t role in the fluegasdesu lfurization.Therefore,the study of the first stage su lfation reaction of CaO is more im portant for engineering application.
Gas diffusion in pores is strongly affected by pore structure[11]and pore diffusion has an im portant effect on gas-solid reaction kinetics[12].The mo lar vo lum e of the product CaSO4is about 3 times of the mo lar vo lum e of the reactan t CaO.The form ation of CaSO4product layer on CaO surface has two effects.First,the volumetric expansion results in pore structure variation,even pore closure.Pore structure variation during the reaction has an im portant effect on gas diffusion and the kinetics of such reaction[13].Second,CaSO4covers the fresh CaO surface and b locks the first-stage reaction.Therefore,the key of studying the reaction mechanism is the effect of varying pore structures on the kinetics of the reaction.
To include the effect of varying pore structures on the kinetics of the su lfation reaction,porous CaO particle model shou ld be able to reflect pore characteristics of natural CaO and the gasdiffusion in pores shou ld be simulated accurately at the molecu lar level.The previous desu lfu rization models can be grouped as un reacted shrinking core models[14,15],grain models[16-19],and pore models[9,20-24].The un reacted shrinking core models assum e that a CaO particle is a nonporous solid.It is mathem atically sim ple and can be applied to nonporous solid reactants,bu t it cannot be applied to studying the effect of varying pore structures on the kinetics of the reaction.The grain models consider a porous CaO particle as assem b lage of sm all nonporous spherical CaO grains surrounded by in tergranu lar voids.The pore models,such as distributed-pore model[20,21]and random-pore model[9,22-24],assum e that a CaO particle contains num erous pores of a size distribu tion or random ly in tersecting pores.Grain models and pore models have made greater progress than un reacted sh rinking corem odels.How ever,pore structures of CaO particle models of both grain models and pore models are too idealized to reflect the topography and morpho logy of pore structures of natural CaO[25].Pore structures of natural CaO have fractal characteristics[26,27].Gas diffusion in fractal pores,which is anom alous,has been show n to con travene Fick's law and Knudsen's law[28-30].But the diffusion in pores is based on Fick's law and Knudsen's law and modified em pirically by tortuosity factor in grainm odelsand porem odels.The twom odelshave difficulties in accurately investigating the effect of varying pore structures on the kinetics of the reaction.Although num erous studies have investigated the sulfation reaction of CaO,the effect of varying pore structures on the kinetics of the reaction rem ains vaguely understood because porous CaO particles and gas diffusion in pores cannot be simulated accu rately.Therefore,to investigate the effect of varying pore structures on the kinetics of the reaction,a new andim proved desu lfurization model needs to be built to simulate the su lfation of CaO more accurately.
Liang et al.presented a fractal po re model using random w alk method[31].The fractal pore model can present fractal properties,m orpho logy and topography of natural CaO.Cao and He presented a gas diffusion model for fractal pores[32]based on classic mo lecu lar kinetic theory[33].In this paper,the fractal pore models are used to simulate porous CaO particles and the gas diffusion model for fractal pores is im proved to describe the gas molecu lar movement in pores.A new desu lfu rization model,com bin ing fractal pore models,gas mo lecu lar movement models and two-stage reaction models,is deve loped to simulate the SO2-CaO reactions.through numerical simulations of su lfation reaction of 30 po rous CaO models,it is found that the su lfation reaction of CaO is strong ly influenced by pore structure.Evo lu tion of pore parameters andinternal CaO active surface can be accu rately predicted by the desu lfu rization model.The relationsh ip between varying pore parameters and the apparen t reaction parameter is obtained.
2.Numerical M odels
2.1.Fractal pore model
An accurate pore model shou ld be able to simulate all the pore properties of CaO particles.Natural po rous geometries are too com plex to simulate perfectly,bu t fractal po re models w h ich are generated by using a random w alk algo rithm can get very good results[31].Th is paper created three-dim ensional fractal po re mode ls with 100×100×100 cubic grids.A typical fractal po re model is show n in Fig.1 where the b lack cubes are so lid CaO grids and the w h ite cubes are po re grids.Visually,the con tou r shapes of the fractal po res are similar to real po res.The three parameters used to describe the po re structures are po rosity,specific surface area and fractal dim ension.Porosity,θ,is defined as the ratio of the pore vo lum e to the total particle vo lum e.Specific surface area,s,is defined as the pore surface area per unit solid volum e.Fractal dim ension is defined as[34]:

where r is the pore radius,S(r)is the accumulated surface area for this pore radius,d S(r)/d r is the surface probability distribu tion function and Dfis the fractal dim ension.Fractal dim ension of real CaO particle pores can be experimentally measured by mercu ry porosimetry.Th is definition reflects the pore geometry characteristics.A larger fractal dim ension of pores indicates a more com plex pore with a greater gas diffusion resistance[34].

Fig.1.Fractal pore model(NO.1).The b lack cubes are the so lid elements and the w hite cubes are pore elements.
2.2.Gas molecular movement model
The su lfation reaction ofCaO isstrongly influenced by pore diffusion.So the gas diffusion in fractal pores shou ld be accurately modeled.This paper im proved the gas diffusion model proposed by Cao and He[32].Fig.2 show s a pore element grid.The classic mo lecu lar kinetic theory of gases is used to describe the mo lecu lar movement in fractal pores.The distribution of molecu lar free paths follow s Eq.(4)[35].Thus,the number of mo lecu les in the pore element moving out through x-axis righ t border without a collision per un it time can be calculated by Eq.(5).

where N0is thenumber ofm olecu les in one pore element,N is thenumber of molecu les with the free paths greater than x in the pore element,λ is the mean free path(m),L is the edge length of one cubic element(m)and v is the mean molecu lar velocity(m·s-1).As can be seen in Fig.3,there are four kinds of boundary conditions,and the governing equation of mo lecu lar movements for each is established based on Eq.(5).

Fig.2.One pore element grid.
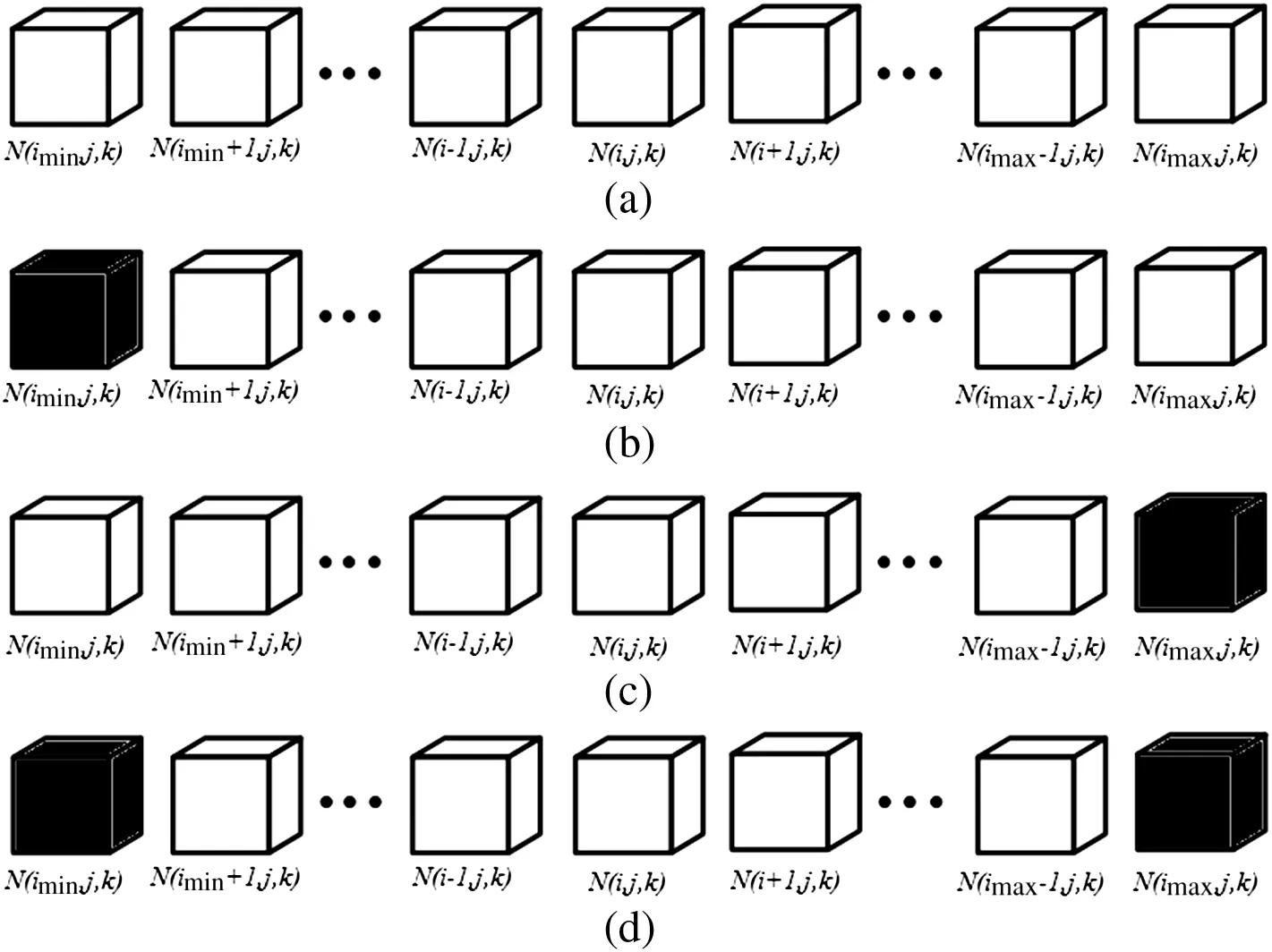
Fig.3.Illustration of cubic grids,where b lack cubes represent solid cubes.(a)Case 1:perforated pores;(b)Case 2:connect to outside in right direction and blocked by a solid cube in left direction;(c)Case 3:connect to outside in left direction and b locked by a solid cube in righ t direction;and(d)Case 4:b locked by two solid cubes at both directions.
2.2.1.Case 1
If along the x-axis of the grid(i,j,k)all grids are pore grids,as Fig.3(a)show s,the number of mo lecu les moving to the grid(i,j,k)from all other grids along the x-axis per unit time is:

2.2.2.Case 2
The number of mo lecu les moving to the grid(i,j,k)from all other pore grids and rebound mo lecu les of hitting the so lid grid(i min,j,k)per unit time is:
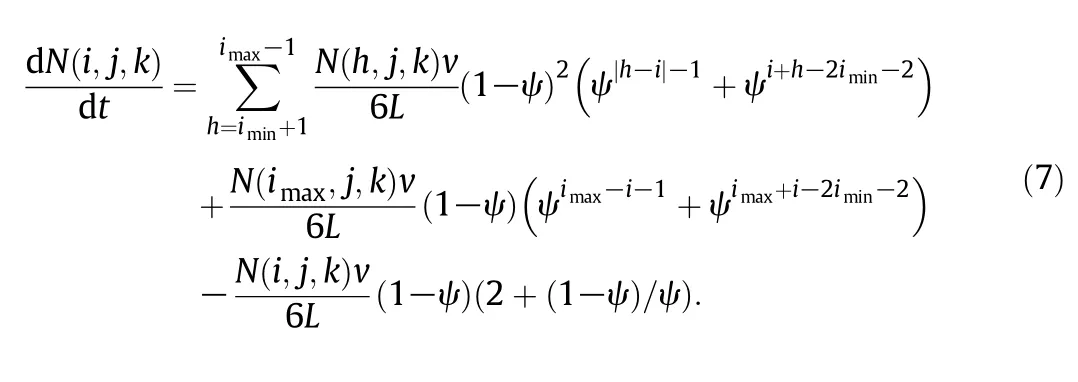
2.2.3.Case 3
Analogously,the number of mo lecu les moving to the grid(i,j,k)per unit time is:

2.2.4.Case 4
If along the x-axis of the grid(i,j,k)there are two solid grids,as Fig.3(d)show s,the total number of mo lecu les moving to the grid(i,j,k)per unit time is:

where ψ =e-L/λ,(i,j,k)is the coordinate of grid,i min andi max rep resent the sm allest and the largest coordinates along x-axis,respectively.The above governing equations describe mo lecu lar movements along x-axis.Analogously,m olecular movements along y-axis and z-axis can be derived,respectively.The advantage of the gas molecu lar movement models can accu rately describe how the pore structure of CaO particle affects SO2and O2molecu lar diffusion,and calculate the number of SO2and O2molecu les colliding solid surface.
2.3.Two-stage reaction model
Thereare two stages in the su lfation reaction ofCaO.Sim ple collision theory(SCT)[36-38]is used to describe the first-stage reaction.SCT based on molecu lar-kinetic theory assum es that the reactions cannot occur un less the molecu lar collision in tensity exceeds the threshold energy[34].Because the solid molecu les vibrate in a very sm all range near thefixed location,the translational energy of gas molecu les can be used as the collision in tensity between the gas molecules and the solid molecules.According to SCT,the sulfation reaction of CaO occurs w hen the translational energy of SO2mo lecu les co lliding with CaO mo lecu les is greater than the th reshold energy.Theanalysis isbased on the following assum ptions:
(1)There is enough O2for the reaction so that the O2concentration does not affect the reaction.
(2)There is no adsorption during the process.
(3)The velocities of the SO2molecu les follow the Maxw ell velocity distribution.
(4)The th reshold energy is 56000 J·m o l-1[9].
In the second stage,the ion diffusion through the product layer follow s Fick's law and the ion diffusion coefficient is affected by many factors[39].There are many studies of ion diffusion coefficient.Duo etal.[40]studied the reaction kineticsafter the form ation of the product layer,and claim ed that the ion diffusion coefficient can be represented by an equation of the Arrhenius form as:

In this paper,Eq.(10)is used to describe ion diffusion through product layer.
The su lfation reaction of CaO can be simulated at the molecular level by com bining fractalporem odels,gasm olecu larm ovementm odelsand two-stage reaction models.For a given fractal pore model,the gas molecu larm ovementm odels can accurately describe SO2and O2molecular diffusion,and calculate the number of molecu les colliding solid surface.Then,the number of the molecules reacting with CaO can be calculated by the two-stage reaction models.
3.Results and Discussion
3.1.Relationship between the apparent reaction parameter and pore parameters
Fractal pore models with different pore structures can be obtained by changing the parameters of the random w alk.Tab le 1 presents the pore parameters of 30 fractal pore models.In this paper,the su lfation reactionsof30 fractalporem odelsare simulated at the range of temperatures 973 to 1173 K and SO2concentrations 1500 to 3500 μl·L-1.The CaO particle temperature is assum ed to be isothermal and the sam e as the temperature of the am bien t flow.
Fig.4 show s the conversion-time curves of the su lfation reaction of model NO.30 at 1123 K and 3000 μl·L-1SO2.The analysis of the reaction rates for the two stages indicates that the reaction begins to sh ift to the second stage w hen the reaction rate decreases and app roaches a constan t.The first-stage reaction rate is far greater than that of the second-stage.The first-stage reaction kinetics is the em phasis of this paper.
Marsh and Ulrichson gavea conclusion that thefirst-stage reaction is the first order in SO2concentration through TGA experiments[41].The global reaction rate in the first-stage reaction can be described by:

where C is the bu lk SO2concentration(m o l·m-3),k is the apparen t reaction rate constan t,S0is the initial value of CaO specific surface area(m2·m-3),VCaOis the CaO molar volum e(m3·m ol-1),X is the CaO conversion and α is the ratio of active surface of un reacted CaO and the in itial CaO surface.The apparen t reaction parameter can be calculated by Eq.(11)w hen the reaction is at initial time,α=1.
Three pore parameters(porosity,specific surface area and fractal dim ension)are used to describe pore structuresofCaO particles.Thus,the relationship between the apparen t reaction parameters and three pore parameters cannot be determinedintuitively.To solve this problem,a modu lus φ is in troduced to present the relation.The gas diffusion inpores affects the apparen t reaction parameters.Therefore,the modu lus shou ld be a function of the three pore parameters and can reflect gas diffusion characteristics in pores.The results of Cao and He[32]show that the diffusion coefficient is proportional to θ2/3e-Df.And the Knudsen diffusion law show s that the diffusion coefficient is proportional to the mean pore radius rmor 2ε/s[11].Thus,the modu lus φ can be defined as[42]:

The simulation results in Fig.5 show the relation between theapparent reaction parameter k and the modu lus φ.The relationship presents:

Analyzing the relationship,w hen ε→ 100%,φ → ∞,which means that there is no diffusion resistance in pores,thus k→ks,that is c=ks.Bu t w hen ε→ 0%,φ → 0,which means that there is no diffusion and reaction in pores,thus k→0,a=-ks.So the relationship can be expressed as:

Fig.4.Conversion-time behavior of model NO.30.

Fig.5.Relation between apparent reaction parameter and the modu lus φ.

where ksis the in trinsic reaction rate constan t(m·s-1)and b is a parameter relating to the particle diameter.The path of reactant gas molecu le diffusion from bu lk flow to the reaction surface in pores is greater than the sm aller diameter CaO particle.The apparen t reaction rate constantdeceaseswith increasing CaO particle diameter.Thefitting results show ks=0.0045 m·s-1and b=2.43 × 108for 1 μm diameter CaO particle at 1123 K.The in trinsic activation energy is Es=56,000 J·m o l-1du ring the simulations.Thus,the in trinsic preexponen tial factor is As=1.81.Both the in trinsic pre-exponen tial factor and the in trinsic activation energy are close to the results of Sim onsand Garm an[7].
The modu lus in Eq.(12)is a function of the three pore parameters and characterizes the gas diffusion properties in fractal pores.Eq.(14)show s that the relationship between the apparen t reaction parameters and the modu lus and the su lfation reaction is strongly affected by pore structures.
3.2.Evolution of pore structures
CaO pore structures change con tinuously as the reaction proceeds.The varying pore structure affects the kinetics of the su lfation reaction.The ability of a model to accurately predict the evo lution of pore structures is critical to the analysis of the effect of varying pore structure on the kinetics of the su lfation reaction.

Fig.6.Porosity evolution with the CaO conversion for NO.11,NO.17,and NO.30 fractalpore models.
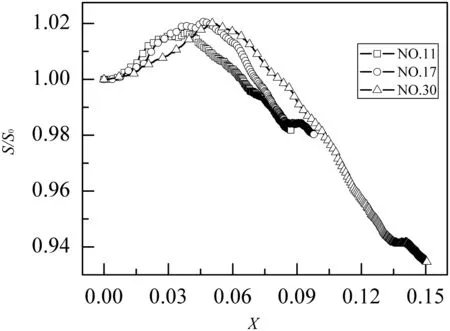
Fig.7.Specific surfacearea evolution with theCaO conversion forNO.11,NO.17,and NO.30 fractal pore models.
Fig.6 show s that the linear relationship between porosity and CaO conversion.This observation is also consisten t with the analysis of Geogakis et al.[43]and the experimental data of Hartm an and Cough lin[16].Fig.7 show s that specific surface area of CaO particle firstly increases and then decreases with the CaO conversion,and the evolution of the specific surface area is different for various porous CaO.The results are in qualitatively agreement with Bhatia and Perlm utter[22,23].Fig.8 show s that the fractal dim ension is rough ly constan t w hen the CaO conversion increases.The results suggest that the com plexity of the pore geometry is also rough ly constan t du ring the reaction.The similar trends of three pore parameter evo lu tion can be foundin other 27 models.Pore parameter evolu tions are investigated to study the effect of varying pore structures on the kinetics of the reaction.In the previous section,the modu lus φ was in troduced to present the relation between the pore structures and apparent reaction parameters.Therefore,the modu lus evo lu tion is critical to study the effect of varying pore structures on the kinetics of the reaction.The modu lus evolution is calculated supposing that fractal dim ension does not change during the reaction.The numerical results are fitted as:
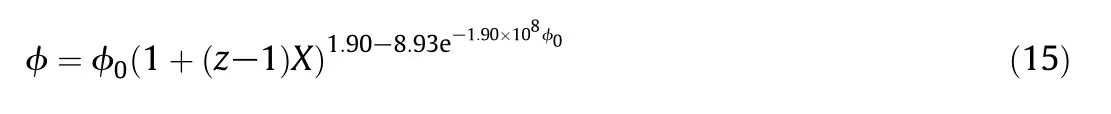
whereφ0is the initialm odu lusφand z is ratio ofm olar volum eofCaSO4and CaO.Fig.9 show s theevolution of them odu luswith CaO conversion and comparison of φ/φ0predicted by Eq.(15)with the numerical results.Eq.(15)can accu rately predict the modu lus evo lution or pore diffusion evolution because of pore structures evo lu tion with the reaction proceeding.
3.3.Evolution of the CaO active surface
Eq.(11)p resen ts the global reaction rate in the first-stage reaction.The active surface area of un reacted CaO is proportional to the global reaction rate.The active surface of un reacted CaO is covered by the product layer inch by inch du ring the reaction.The product layer b locks the first-stage reaction and then the global reaction rate decreases.Evolution of the CaO active surface is crucial to the prediction of the conversion-time behavior.These 30 fractal pore models are simulated at various temperatures and SO2concentrations.The ratio of active surface of un reacted CaO and the initial CaO surface α is calculated and the numerical results are fitted as:
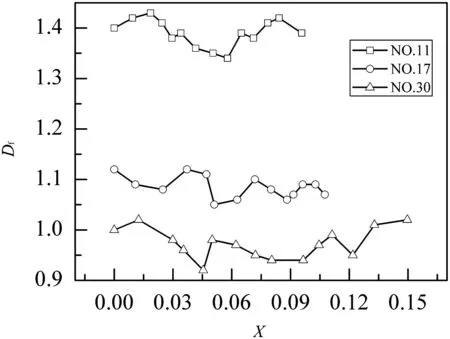
Fig.8.Fractal dim ension evolution with the CaO conversion for NO.11,NO.17,and NO.30 fractal pore models.

Fig.9.comparison of φ/φ0 predicted by Eq.(15)with numerical results of NO.11,NO.17,and NO.30 fractal pore models.The curves are the results of by Eq.(15)while the discrete points rep resent the numerical results.

Fig.10 show s that the ratioαdecreaseswith CaO conversion and the comparison between the ratioαp redicted by Eq.(16)and numericalresults.Eq.(16)can accurately predict active surface of unreacted CaO evo lu tion because of the product layer coverage.
3.4.Comparison with TGA experiments
TGA measurements were used to verify the results of the effect of varying pore structures on the kinetics of the reaction.three kinds of CaO sam ples were usedin this study.Three pore parameters,porosity,specific surface area and fractal dim ension,were measured by the mercury porosimetry and listedin Table 2.During the TGAexperiments,any residual water was evaporated fully before the TGA measurements by heating the sam ple with N2as the sw eeping gas to 573 K at 1×105Pa.After about 10 min,the N2flow was cut off and the reactant gas including SO2and O2was in troducedin to the TGA.The reaction runs at 973 K and 3000 ppm SO2.The sam ple w eigh t was recorded every 1 s and then the CaO conversion-time curves cou ld be obtained.
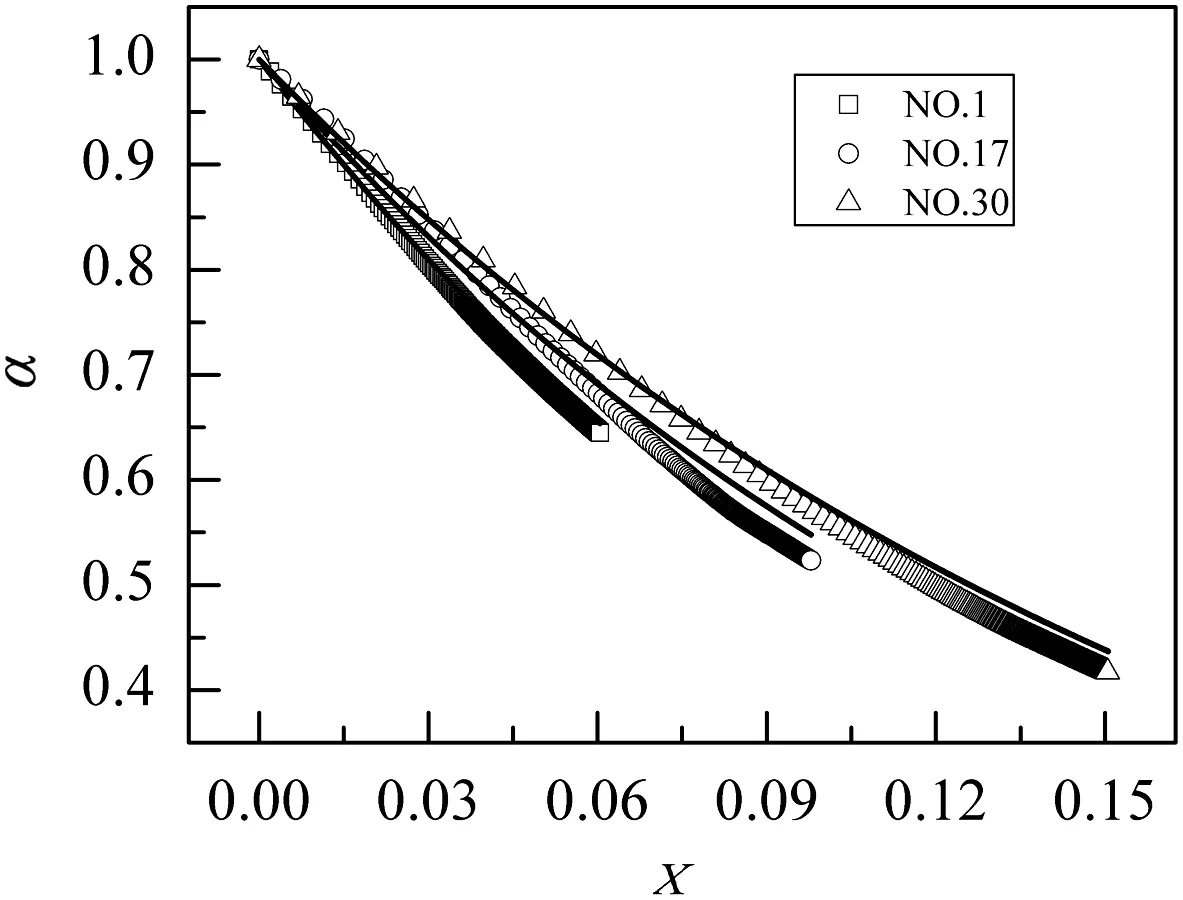
Fig.10.comparison of α predicted by Eq.(16)with numerical results of NO.1,NO.17,and NO.30 fractal pore models.The cu rves are the results of by Eq.(16)while the discrete points rep resent the numerical results.
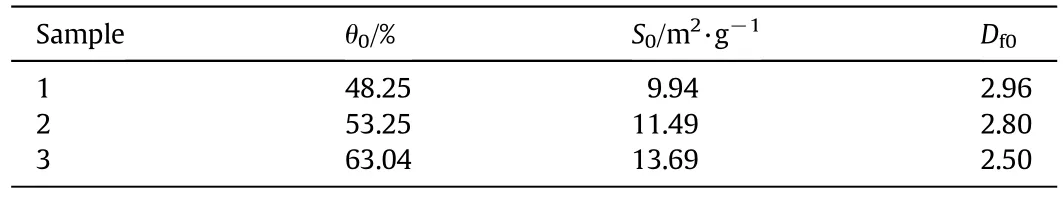
Table 2 Pore parameters of TGA sam ples
The conversion-time behavior for a given CaO particle can be predicted by Eqs.(11)-(16).Through comparison of the conversion-time behavior,TGA experimentsareused to verify the resultsof the desu lfurization model that are the effectof varying pore structures on the kinetics of the reaction.The parameter,b,in Eq.(14)p resen ts the effect of particle diameter on the apparen t reaction parameter.The average particle diameter of numerical CaO particles is 1 μm and the average particle diameter of experimental CaO sam ples is about 300 μm.The particle sizes of experim ental CaO sam ples are close to engineering application.Therefore,the parameter b shou ld be modified with TGA experimentsfirstly.The parameter b is fitted by comparison of the conversion-time behavior predicted by com bining Eqs.(11)-(16)with TGA data.Thefitting results show b=1.22 × 106for 300 μm diameter CaO particles.TGA data and the predicted conversion-time behavior are show n in Fig.11.The predicted results of the first-stage reaction are in a very good agreementwith the TGA data.Thesealso show that thenew desu lfurization model can accurately present the effectof varying pore structure on the kinetics of the first-stage reaction.

Fig.11.comparison of the conversion-time behavior predicted by Eqs.(11)-(16)with TGA data of three sam ples.The curves are the predicted results of by Eqs.(11)-(16)while the discrete points rep resen t TGA data.
4.Fu rther Discussion
Although num erous studies have investigated the sulfation reaction of CaO,the reaction mechanism rem ains vaguely understood.Firstly,the previous models,such as the random pore model,pore vo lum e,pore surface area and pore length are adopted to characterize the pore structure.Three pore parameters can be calculated for an ideal porous media model.Bu t pore length is difficu lt to be calculated or measured for a real CaO particle.Second ly,the classic diffusion law(Fick's law and Knudsen law)is used to describe gas diffusion in fractal pores and modified by the tortuosity factor.But the classic diffusion law has much difficu lty for describing fractal pore diffusion.And the tortuosity factor is an em pirical parameter and difficu lt to be independen tly measured by experiments.Therefore,the traditional models have difficu lties in accurately describing the effect of varying pore structures on the kinetics of the sulfation reaction.
The present model is distinct from the previous models in its treatm ent of porous CaO particle models and gas diffusion model for fractal pores.Three pore parameters,porosity,specific surface area and fractal dim ension,describe the pore structures.Fractal dim ension characterizes the pore com plexity instead of the tortuosity factor,andit can be w ell-defined andindependen tly measured experimentally.The gas molecu lar movement models can accurately describe the effect of the pore structures on the gas diffusion and no modification by em pirical parameters.An im portan t advan tage of the gas molecu lar movement models is that the governing equations are estab lished according to pore structures.Gas diffusion results are obtained from so lid physics background.Theeffectofallthe three pore parameterson pore diffusion is com prehensively considered.The modu lus φ is a function of all the three pore parameters and characterizes the gas diffusion properties in fractal pores.The modu lus acts as a bridge between the apparent reaction parameter and pore structures.Eq.(15)quan titatively show s theeffectofvarying pore structureson pore diffusion.Eq.(14)quantitatively indicates the influence of pore diffusion on apparen t reaction parameter.Com pared to the previous models,one of the im portan t features of the present model is that it can accurately predict the effect of varying pore structures on pore diffusion and then on the apparen t reaction parameter.
5.Conclusions
Fractal pore models can better reflect pore properties of natural CaO and gasm olecular movementm odels can accurately sim ulate gas diffusion in fractal pores based on physical principles.The new desu lfurization model,com bin ing fractal pore model,gas mo lecu lar movement model and two-stage reaction model,simulates the su lfation reaction of CaO.Fractal dim ension is used to characterize the com plexity of the pore structures instead of the tortuosity factor.A modu lus φ is introduced to characterize the effect of pore structures on the diffusion in pores.The numerical results show the relationship between the apparen t reaction parameter and the modu lus,the evo lu tion of the pore structures,and the evolution of CaO active surface.The desu lfurization model is distinct from previous models in its treatm en t of porous CaO particle models and gas diffusion model for fractal pores.This model can accurately predict theeffectofvarying pore structureson the kinetic behavior without any em pirical parameters.Com paring to the previous models,the new desu lfurization model more accu rately presents the reaction mechanism.
Nomenclature

 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
