Use of chemically activated cotton nut shell carbon for the rem oval offluoride contam inated d rinking water:Kinetics evaluation☆
Rajan Mariappan ,Raj Vairam uthu ,Alagum uthu Ganapathy
1 Department of Natural Products Chem istry,School of Chem istry,Madurai Kamaraj University,Madurai 625 021,Tam il Nadu,India
2 Advanced M aterials Research Laboratory,Departm ent of Chem istry,Periyar University,Salem 636 011,Tam il Nadu,India
3 PG and Research Centre of Chem istry,Sri Paramakalyani College,Alw arkurichi 627412,Tam il Nadu,India
Keyw ords:Activated carbon Cotton nut shell Fluoride Isotherm Kinetics
ABSTRACT Chemically activated cotton nu t shell carbons(CTNSCs)were prepared by different chemicals and they were used for the rem oval of fluoride from aqueous solu tion.Effects of adsorption time,adsorben t dose,pH of the solution,initial concentration of fluoride,and temperature of the solution were studied with equilibrium,therm odynam ics and kinetics of the adsorption process by various CTNSC adsorben ts.It show ed that the chemically activated CTNSCs can effectively remove fluoride from the solution.The adsorption equilibrium data correlate w ell with the Freund lich isotherm model.The adsorption of fluoride by the chemically activated CTNSC is spontaneous and endothermic in nature.The pseudofirst order,pseudo second order andin tra particle diffusion kinetic models were applied to test the experimental data.The pseudo second order kinetic model provided a better correlation of the experiment aldata in comparison with the pseudo-first-order and intraparticle diffusion models.Am echanism offluoride adsorption associating chem isorption and physisorption processes is presented allowing the discussion of the variations in adsorption behavior between these materials in term s of specific surface area and porosity.These data suggest that chemically activated CTNSCs are promising materials for fluoride sorption.
1.Introduction
It is w ell know n thatfluorine is an indispensable element for hum an healthy.Fluoride related health hazards are a major environmental problem in many regions of the world.According to the American Association for the Advancement of Science,fluoride is considered as the third most serious pollutant in the country after SO2and ozone.As per the WHO standards,the permissible limit of fluoride is 1.5 mg·L-1.W hen the fluoride concentration is more than the perm issible lim it,it causes dental,skeletal fluoros is and lesions of the thyroid,liver and other organs[1].Therefore,it is essential to control the concentration of fluoride in water.Several methods and technologies have been tried to remove fluorides from water,nam ely adsorption,precipitation,electrodialysis,ion exchange,electrocoagu lation and reverse osmosis[2-6].Am ong these methods,adsorption methodis still one of the most extensively used for rem oval of fluoride in water.
Arangeof adsorbentshasbeen examined for the rem ovaloffluoride,such asnanom aterials[7]alum sludge[8],fly ash[9],granular activated alum ina[10],calcite[11],m on tm orillon ite[12],charcoal[13]and activated carbon from agricu ltural waste materials[2,14].The activated carbon exhibit is one of the good adsorption capacity for the rem oval offluoride and other pollutants from the water.The activated carbon is low-cost,often naturally occu rring products which have good adsorbent properties.It has been widely used to treat industrial and household water[15]because of its excellen t adsorption properties,characterized by a high specific surface area[16].It is also used to removemetalions from solution[17].The increasing variety and am oun ts of potentially hazardous im pu rities in water have led to the increased use of activated carbon.The prob lem associated with its use as a water purifier is largely econom ic;activated carbon is expensive.Activated carbons are non-hazardous carbonaceous products,having a porous structure and a large internal surface area.These materials can adsorb a wide variety of undesired species from the gaseous or liquid phase in order to ensure effective purification[18,19].The adsorption capacity of the activated carbon is main ly based on the porosity of the carbon;surface area and chemical functionality present on these surfaces[20].Its chemical structure influences its in teraction with adsorbate,andits active sites determine w h ich chemical reactions occu r with other molecules.
All can be custom designed by selecting the righ t precursor and choosing the pyrolysis and activation conditions.High volum e lignocellu losic byp roductsm ay offer inexpensive and renew able sources ofspecial granu lar activated carbons.This op tion is particu larly attractive for crops grow n w o rldwide yield considerab le quan tities of agricu ltural byp roduct wastes.Their conversion in to activated carbons w ou ld add value to agricu ltural comm odities,reduce waste disposal costs and provide inexpensive replacement of comm ercial adsorbents derived from non renew ab le sources capacity[20].Activated carbons are usually made from coal,peat,coconut shells,Tam arind fruit shell or w ood[21,22].Nut shells are renew able low-value agricu ltural wastes that are activated carbon sources.Nu tshells are preferred precu rsors and they are hard and do not require a binder.Nut shell derived carbons rep resen tagreener technology than using coal.In the presentstudy,thefluoride is one of the very serious risky prob lem s to hum an health.Thefluoride rem oval is a necessity in fluoride con tam inated water by the waste indigenous materials.Hence the study focused on fluoride rem oval from con tam inated ground water using waste cotton nut shell in the form of physical activation carbon and chemically activated carbons.Since,w e have been engagedin developing low cost activated carbons using low-value agricu ltural byp roducts and wastes for water rem ediation.
2.Materials and Methods
2.1.Adsorbent preparation
The cotton nut shells(CTNS)(Gossipium hirsutum)were collected from local agricu ltural area andit was washed with deionized water to remove dust,d ried at 80°C to get rid of the moisture and other volatile im purities.The dried sam ples were groundin laboratory blender and sieved to <210 μm.Raw materials were directly im pregnated with chemical activation agents.Ground and sieved 100 g of CTNS was treated separately with 40 g of ZnCl2,K2CO3and H3PO4at room temperature.Con tinuous mixing of the precursor with chemicals for 24 h was maintained by using a magnetic stirrer.After mixing,the solutions were allow ed to d ry at room temperature for 24 h and then d ried at 85°C for 72 h in a temperature con tro lled oven to prepare im pregnated sam ples.After this period,the raw CTNS and chemical im pregnated CTNSs were ready for the carbonization and activation which were carried outsim u ltaneously.Im pregnated sam pleswere then carbonizedin a M u ffle fu rnace at 600 °C and at a heating rate of 10 °C·m in-1and held at this temperature for 1 h.After being cooled,all the carbonized sam ples were washed several times with hot doub le distilled water un til pH becam e neutral and finally washed with cold DD water to remove residual chemicals.W ashed sam ples were d ried at 105°C for 24 h to obtain thefinal activated carbons.The raw CTNSC and chemically activated CTNSCs were classified as R-CTNSC,Z-CTNSC,K-CTNSC,and P-CTNSC rep resen t ZnCl2,K2CO3and H3PO4im pregnated CTNSCs,respectively.
2.2.Sorption experiments
The sorption isotherm and kineticsstudieswere perform ed by batch adsorption experiments and were carried out by mixing 1.75 mg of sorben t with 100 ml of sodium fluoride con taining 3 mg·L-1as initialfluoride concentration.As most of the fluoride endemic areas,the fluoride level was found to have a maxim um of 3 mg·L-1in d rinkingwater and hence it is decided tofix the in itial fluoride concentration as 3 mg·L-1for further studies.The mixture was agitatedin a therm ostatic shaker ata speed of200 r·m in-1at room temperature.Filter them ixture andfluoride concentration of the solutionwasanalysed usingfluoride ion selective electrode(Model:Eutech-cyberscan 2100).The defluoridation studies were conducted for the op tim ization of various experimental conditions like contact time,pH,initial fluoride concentration andinfluence of co-ions with fixed dosage.Kinetic studies of sorben t were carried out in a temperature con trolled mechanical shaker.The effect of different initial fluoride concentrations viz.,2,4,6,8 and 10 mg·L-1at four different temperatures viz.,303,313,323 and 333 K on sorption rate were studied by keeping the mass of sorbent as 1.5 mg and volum e of solution as 100 ml at neutral pH.The pH at a zero poin t charge(pHzpc)of sorben t was measured using the pH d rift method[23].The pH of the solution was ad justed by using 0.01 mol·L-1sodium hydroxide or hydrochloric acid.Nitrogen was bubbled through the solution at 30°C to remove the dissolved carbon dioxide.50 mg of the adsorbent was added to 50 ml of the solution.After stabilization,the final pH was recorded.The graph of final pH versus initial pH was used to determine the zero poin t charge of the activated carbon.The mechanism has been confirm ed by using isotherm models like,Langm uir[24];Freundlich[25];Tem kin[26]and Red lich Peterson[27]and the reaction rate was determined using kinetic equation like Pseudo first order[28];Pseudo second order[29]and interaparticle diffusion model[30].
2.3.Characterization of sorbents
Fou rier Transform In ferred(FTIR),X-ray diffraction(XRD),Scanning Electron Microscope(SEM)and Energy Dispersive X-ray Analyzer(EDAX)were used for the characterization of adsorbents.The XRD pattern of the activated andim pregnated carbon was obtained using a Bruker AXS D8 Advance,Inst ID:OCPL/ARD/26-002 X-ray diffractometer.Examination of surface morphology of the adsorben ts which was studied by HITACHI-S-3400N model fitted with an EDAX allow s a qualitative detection and localization of elements in the adsorben ts.Fourier transform in frared spectra were recorded using the spectrophotometer-Nicolet 6700,Therm o Electronic Corporation,the USA.Com putations were made using Microcal Origin(Version 6.0)softw are.The accuracy of fit was perform ed using regression correlation coefficient(r)and Chi-square analysis(SSE).
3.Results and Discussion
3.1.Characterization of the adsorbents
3.1.1.SEM studies
SEM technique was used to observe the surface morphology of the adsorbents.SEM im age of R-CTNSC and chemically treated Z-CTNSC,K-CTNSC and P-CTNSC sam ples are given in Fig.1.It is seen that a thick wall structure of R-CTNSC exists along with no porosity on the raw material[Fig.1(a)].It is obvious that thick wall gets opened and a wider porosity is created,thus the external surface areas of the chemically activated carbons are full of cavities.Pores of different size and different shapes were obtained from different chemical activation agen ts.Honeycom b like morpho logy ob tained by H3PO4treated CTNSC sam ple[Fig.1(d)].K2CO3activated adsorben t form s of spongelike morpho logy[Fig.1(c)]and ZnCl2leads only to a surface deform ation of raw materialand alm ostno pore form ation obtained after activation,thus the surfaceareawasvery low for ZnCl2im pregnated activated carbons.SEM im ages of the chemically treated sam ples show s that the surface of CTNSC has very high porosity on the surface after activation.
3.1.2.XRD studies
The XRD patterns of the R-CTNSC and chemically treated Z-CTNSC,K-CTNSC and P-CTNSC exhibit significant variation as show n in Fig.2.The in tensity of the peaks corresponds to the 2θ values of 14°,16°,26°,46°and 50°of XRD patterns of various adsorbents showing considerable differences.The XRD patternsofR-CTNSC,Z-CTNSC,K-CTNSCand P-CTNSC adsorben ts 2θ values registered a markedincrease and this occurs to create pores on the surface of the R-CTNSC by the chemical activation method.The miller indices of 010 plane and the monoclinic crystal system of the raw sam ples appeared as 001 plane and triclin ic system after the activation.It is presum ed to be due to the form ation of a crystal like structure of the raw material surface.similar results were reported by Venkata Mohan et al.[31]in their adsorption studies on activated carbon and coal based adsorben ts.The physical characterization of the prepared four adsorbent results is given in Table 1.Based on the physical characterization andinstrumental analysis of the four adsorben ts,the defluoridation study carried out only two adsorbents nam ely,K-CTNSC and P-CTNSC,because other two adsorbents R-CTNSC and Z-CTNSC have no significan t effect on the primary study on the fluoride rem oval.
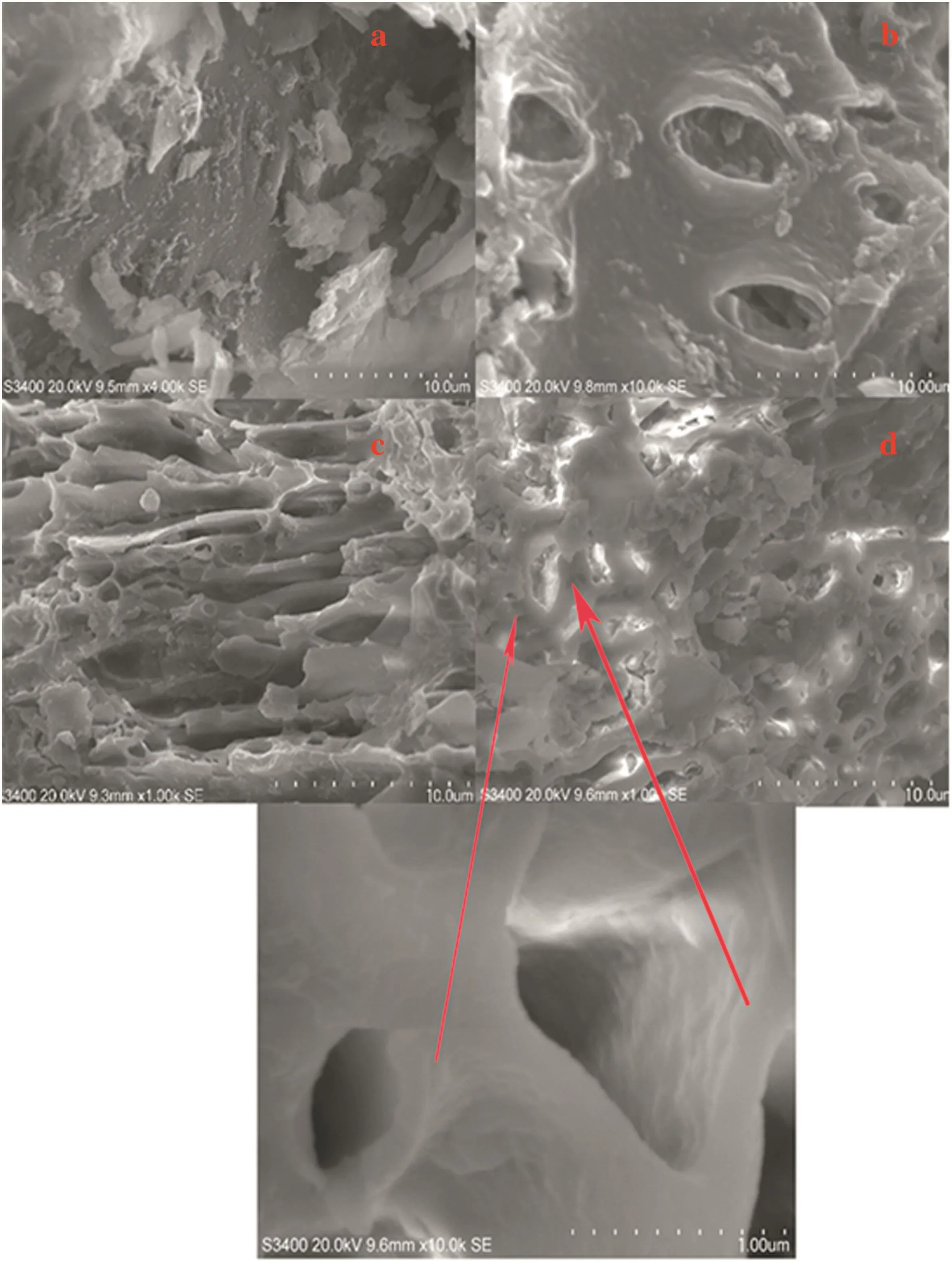
Fig.1.SEM im ages of(a)R-CTNSC,(b)Z-CTNSC,(c)K-CTNSC and(d)P-CTNSC.
3.2.Defluoridation capacity of K-CTNSC and P-CTNSC
3.2.1.Effect of contact time
The effect of con tact time on fluoride rem oval by K-CTNSC and P-CTNSC studied with 3 mg·L-1as an in itial fluoride concentration under neu tral pH at room temperature is show n in Fig.3.From the figure,the fluoride rem oval by sorbents increases with increased time and finally reached the saturation.Both of the sorben ts reached saturation at 180 min to attain equilibrium.For subsequen t experiments,180m in was fixed at the optimum con tact time for both of the sorbents.In the present study,the percentage rem oval of fluoride for K-CTNSC and P-CTNSC are 74.33%and 83.67%respectively at 180 min.The initial peak portion revealed the high sorption up take of the fluoride ions on adsorbents.The second stage designated the slow up take of fluoride ions that show ed the consum ption of all active sites over the adsorbent surface and attainm en t of saturation or equilibrium stage.The third stage indicated the equilibrium stage in which,the sorption up take was relatively sm all.similar findings were reported by Ramanaiah et al.[32]for fluoride rem oval on biom ass of Spirogyrasp.The initial fast sorption was perhaps due to the participation of specific functional groups,and active surface sites on adsorbent surfaces.The presence of such surfaces and function group on biom ass based adsorbents andinitial fast sorption was reported by other researchers as w ell[33].Decreased rem oval rate,particu larly tow ard the end of the experim ent,indicates the possible mono layer of fluoride ions on the outer surface,pores of both the adsorbents,and pore diffusion onto inner surface of adsorbent particles[31].This happened through the film,due to con tinuous shaking main tained during the experiment.
3.2.2.Effect of dosage

Fig.2.XRD patterns of(a)raw material of CTNSC,(b)Z-CTNSC,(c)K-CTNSC and(d)P-CTNSC.
The effect of the dosage on the percentage of fluoride rem oval was studied with a fixed time of 180 min and the results are given in Fig.3.The results revealed that there isan increase in percentageoffluoride rem ovalwith the increasing dosageof the adsorbents.It isobserved that the percentage rem ovaloffluoride increased from 31.33%to 74.00%for K-CTNSC and from 42.67%to 84.33%of P-CTNSC with the increase in adsorben t dose of 0.50-2.5 g of K-CTNSC and P-CTNSC.How ever,in more than 1.75 mg of adsorbents,there was no significan t change in percentage rem oval.It may be due to the overlapping of active sites at higher dosage as w ell as the decrease in the effective surface area resulting in the conglom eration of exchange particles[34].In the present study,the fluo ride level was reduced from the in itial concentration of 3.0 to 1.0 mg·L-1by the 1.75 mg of adsorben ts.Since the perm issib le lim it for fluoride in d rinking water is fixed as 1.0 mg·L-1,it is concluded that only a maxim um of 1.75 mg of K-CTNSC and P-CTNSC is needed for the efficien t rem oval of fluoride from groundwater.
3.2.3.Effect of pH
Several researchers reported[7,15,22]that biosorption process is relian t on the aqueous phase pH,and the functional groups on the bioso rben t,and their ion ic states(at particu lar pH).These biom acrom olecu les on the biosorben t surfaces have several functional groups(such as,am ino,carboxy l,thio l,su lfyd ry l,alcoho l,pheno l,and phosphate groups)and biosorption phenom ena depend on the protonation or dep rotonation of these functional groups,on the surface of the biosorbent.The ionic form of fluoride in solution and the electrical charge of the surface of biosorben t(i.e.,functional groups carrying po lysaccharides and proteins)depend on the solution pH.Fig.3 illustrates the effect of pH on fluoride adsorption by K-CTNSC and P-CTNSC at room temperature(299± 2)°C.As the pH of the fluoride solu tion increased from 3.0 to 12,the percentage of fluoride rem oval waschanged.In the presentstudy,only a sligh tdifference in percentage rem oval of fluoride by K-CTNSC and P-CTNSC was observed between pH 3 and pH 7.Bu t beyond pH 7,the adsorption capacity of K-CTNSC and P-CTNSC d rastically decreased and the adsorben texhibited negligib le adsorption rate of 36.00%for K-CTNSC and 21.33%of P-CTNSC at pH 12.0.This is consistent with the results obtained for the other adsorbent system s nam ely,iron(III)-tin(IV)bimetal mixed oxide[35].The quick reduction of the am oun t of fluoride adsorbedin the alkaline pH range shou ld be attribu ted to com petition of hyd roxyl ions with fluoride for adsorption sites[36].The fluoride is removed much faster at low er pH.This can be attributed to the fact that at low er pH there is astrong d riving force for the rem ovaloffluoride ions[37].The rem ovaloffluoride ions from aqueous fluoride solu tion was high ly dependen t on the solu tion pH in many cases as it altered the surface charge on the adsorben ts.It is im portan t to men tion here that the pH 7.0 is fixed as the op tim um pH which is high ly suitab le for application in natural system s.Further experim ent was main tained at neutral pH.

Tab le 1 Characterization of the adsorbents

Fig.3.Effect of contact time(a),dosage(b),pH(c)andinitial concentration(d)of K-CTNSC and P-CTNSC adsorbents for percentage of fluoride rem oval.
3.2.4.Effect of initial fluoride concentration
Fig.3 explains the effect of initial fluoride concentration on the processofadsorption.It can be seen that the percentage rem ovaloffluoride increased with the increasing initial fluoride concentration.Based on the results,a maxim um of 7.38 mg·L-1and 7.89 mg·L-1of fluoride was absorbed by 1.75 mg of K-CTNSC and P-CTNSC respectively with the fluoride initial concentration of 10.0 mg·L-1.It is possible to treat the water as per the d rinking water standard quality(1.5 mg F·L-1)by P-CTNSC.The fluoride adsorption capacity of K-CTNSC and P-CTNSC was com pared with those of other comm only used fluoride adsorben t for 100 ml of 10 mg·L-1fluoride standard test solution with a K-CTNSC and P-CTNSC adsorben t dose of 1.75 mg and 180 min agitation time given in Table 2.

Tab le 2 Com parative study of fluoride adsorption onto various adsorbent
3.2.5.Effect of co-ions
A variety of anions in addition to fluoride are generally present in water.The effects of HCO3-,SO42-,NO3-,and Cl-on defluoridation were examined.In this experim ent,an initial fluoride concentration of 10 mg·L-1was used with different concentrations of other anions which vary from 10 to 50 mg·L-1.Fig.4 indicates that the fluoride adsorption ratio decreased to≥25%w hen the HCO3-concentration increased from 10 to 50 mg·L-1.W hen SO42-and NO3-ions were also present in the system,the adsorption capacity is slightly decreased.In the case of Cl-,it had less im pact on fluoride adsorption as com pared to other anions studied.This is due to the Cl-ions which form ed outer-sphere com plex,while SO42-form ed both outer-sphere andinner sphere com plexes with surface active sites[12].Therefore,the im pact of major anions on fluoride adsorption follow ed the order of HCO3->SO42->NO3->Cl-reflecting the relative affinity of theseanions for K-CTNSC and P-CTNSC.These results agreed with the defluoridation property of activated alum ina[15].
3.2.6.Sorption isotherm
Equilibrium data of fluoride adsorption onto K-CTNSC and P-CTNSC was obtained at neu tral pH and at various temperatures(303,313,323 and 333 K).The isotherm deals with the relationship between the equilibrium am oun t of so lute on the adsorben t and the solute concentration in solutions.

Fig.4.Effect of other co-ions of(a)K-CTNSC and(b)P-CTNSC adsorben ts.

Tab le 3 Langm uir,Freund lich,Tem kin and Red lich Peterson isotherm valuesat different temperatures on K-CTNSC

Tab le 4 Langm uir,Freund lich,Tem kin and Red lich Peterson isotherm valuesat different temperatures on P-CTNSC
3.2.6.1.Langmuir isotherm.The linear form of Langm uir isotherm data is representedin Tab les 3 and 4 and show n in Fig.5 for K-CTNSC and P-CTNSC.From this study,the qm(m g·g-1)and b(dm3·m g-1)are the Langm uir isotherm constants referring the capacity and the energy respectively.The qmvalues are found to increase from 1.379 to 1.503 mg·g-1and from 2.47 to 3.359 mg·g-1for K-CTNSC and P-CTNSC respectively with rise in temperature from 303 K to 333 K.The results show that the sorption capacity increased with the increasing temperature.This is because of the rise in temperature that results in decreasing theescaping tendency of them o lecu les from interfaceand thereby increasing the extent of adsorption[13].The Langm uir equilibrium sorption constan t(b)varied from 1.89 to 3.5 dm3·m g-1for K-CTNSC and 3.19 to 5.56 dm3·g-1for P-CTNSC at 303 K to 333 K.The equilibrium constan t b increases with increasing temperature for both of the adsorben ts.The equ ilib rium parameter RLvalues are given in Table 5,which indicates that the adsorption of fluoride on K-CTNSC and P-CTNSC is favorab le(0<RL<1)for the concentration range studied.The data of fluoride rem oval by K-CTNSC and P-CTNSC gave a straigh t linewith a relatively good correlation coefficient(r2)indicating the accep tability of the model(Fig.5).
3.2.6.2.Freundlich isotherm.The Freund lich parameters,KFand 1/n refer em pirical constants and the results are tabu latedin Tab les 3 and 4 and the trends are show n in Fig.5.The adsorption capacity(KF)of K-CTNSC was found to be 1.87 mg·g-1and 0.549 mg·g-1for P-CTNSC.The magnitude of KFshow ed a good fluoride adsorption capacity by K-CTNSCand P-CTNSC from aqueous so lution atall the temperaturesstudied.The condition for the validity of a Freund lich type adsorption model is that adsorption shou ld be on heterogeneous surfaces[13,38].Freund lich adsorption intensity(1/n)for fluoride/K-CTNSC system was obtained as 0.755-0.720 and for fluoride/P-CTNSC system it was 0.806-0.772 at different studied temperatures.For both the system s,adsorption in tensity(1/n)values lie in the range between zero and one,which indicates a favorab le adsorption.It reveals that the CNSC and ZICNSC had good adsorption intensity.Therefore it indicates that the K-CTNSC and P-CTNSC system s are suitab le for fluoride adsorption[39].From the plots it is found that the related correlation co-efficien t(r2)for the Freund lich mode is 0.99 and 0.98 for K-CTNSC and P-CTNSC respectively at 303 K.
3.2.6.3.Temkin isotherm.The calculated Tem kin isotherm constants(KT)and(BT)for K-CTNSC and P-CTNSC system s are presentedin Tab les 3 and 4.The straigh t line plots of Tem kin are show n in Fig.6.The values of equilibrium binding energy of the system s increase with the increasing of temperature.similarly the equilibrium constant(KT)value of K-CTNSC and P-CTNSC also increased with increasing temperature.similar resultshave been reported by Y.S.Ho[40].Low er valuesof KTindicate a w eak in teraction between adsorbate and adsorben t supporting an ion-exchange mechanism for the present study.

Fig.5.Plot of the Langm uir and Freund lich isotherm study of fluoride adsorption on K-CTNSC(a,c)and P-CTNSC(b,d)respectively.
3.2.6.4.Redlich-Peterson isotherm.The Red lich-Peterson isotherm plots for sorption of fluoride on K-CTNSC and P-CTNSC are presentedin Fig.6.The Red lich-Peterson isotherm constants,KR,α,β and r2are presentedin Tab les 3 and 4.All the values increase with temperature.Based on the β value,Red lich-Peterson isotherm was fo llow ed by both the system s of Langm uir and Freund lich isotherm s and r2and SSE values are also eviden t.
3.2.6.5.Chi-square analysis.To measure the degree of fitness of each isotherm model with the experimental data,r2and SSE values were com puted for Langm uir,Freund lich,Tem kin and Red lich-Peterson isotherm models.From the chi-square values,the best fit for the sorption of fluoride on K-CTNSC is in the fo llowing order Freund lich>Langm uir>Tem kin>Red lich-Peterson(Tab les 2 and 3).The best fit for the sorption of fluoride on P-CTNSC on the basis of chi-square values is in the following order Freund lich>Langm uir>Red lich-Peterson>Tem kin.Both of the adsorben ts fit w ell with the Freund lich isotherm model which indicates the Heterogeneous adsorption.
3.2.7.Sorption dynamics
3.2.7.1.Pseudofirst order kinetics.The mechanism of fluoride sorption on K-CTNSC and P-CTNSC is explained by pseudofirst and second order equations.The results of pseudofirst order kinetics are listedin Tab les 6 and 7.The graphical rep resen tations are show n in Fig.7.The rate constants(K1)of fluoride sorption on P-CTNSC system are found to be 0.016,0.014,0.020,0.019 and 0.019 min-1and for K-CTNSC system,0.020,0.014,0.019,0.016 and 0.016 min-1at an in itial fluoride concentration of 2,4,6,8 and 10 mg·L-1respectively.The pseudofirst order equation gives a higher regression value showing the adherence to the pseudofirst order rate law.It is found that there is a direct linear relationsh ip between the rate constan t(K1)and the initial fluoride concentration.The equilibrium sorption capacity(qe)values of P-CTNSC are found to be sligh tly higher than the value of K-CTNSC.The correlation coefficient(r2)values are nearer to the un ity.
3.2.7.2.Pseudo second order kinetics.The straigh t line plots of t/qtagainst t have been tested to ob tain rate parameters(Fig.7).The pseudo second o rder kinetics parameters,K2,h and r of fluoride rem oval by K-CTNSC and P-CTNSC were calculated and given in Tab les 6 and 7.The equ ilibrium rate constan t(K2)values for fluoride rem oval by K-CTNSC and P-CTNSC are found to be 0.014,0.063,0.002,0.001,and 0.0008 g·m g-1·m in-1and 27.22,3.15,0.804,0.258,and 0.091 g·m g-1·m in-1respectively in the concentration range of fluoride used for the adsorption study.It is observed that(K2)values of P-CTNSC are larger than those of the K-CTNSC adsorben t.The initialsorption rate(h)increased from 1.21 to 4.97 g·m g-1·m in-1for K-CTNSC and decreased from 2.6 to 34.7 g·m g-1·m in-1for P-CTNSC.K2and h values increased with the increase of initial fluoride concentration.Ou r results agreed with the study of Sundaramet al.[41].This cou ld be probab ly due to different availab le surface sites with initial concentration resulting in variation of rate constants.Thecorrelation coefficients(r2)for both adsorbents are found as near to unity for the different initialfluoride concentrations.The correlation coefficient(r2)values of the pseudo second order are higher than those of the pseudofirst order for these two adsorben ts.

Tab le 5 R L values at different temperature on K-CTNSC and P-CTNSC
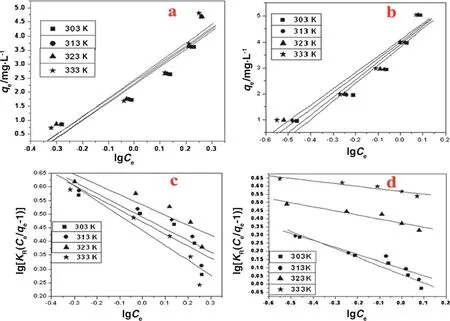
Fig.6.Plot of the Tim ken and Red lich-Peterson isotherm for fluoride adsorption on K-CTNSC(a,c)and P-CTNSC(b,d)respectively.
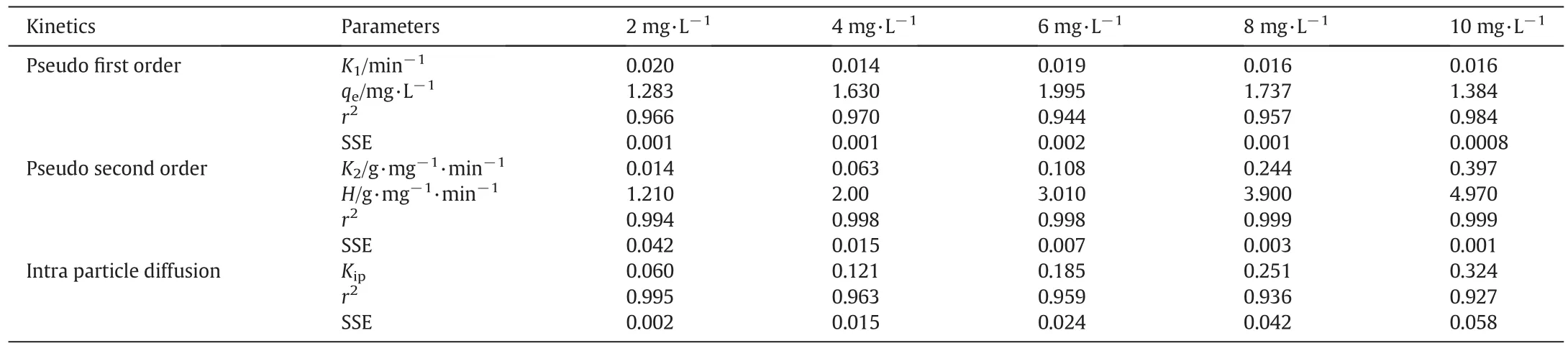
Tab le 6 Kinetic parameters of fluoride adsorption on K-CTNSC

Tab le 7 Kinetic parameters of fluoride adsorption on P-CTNSC
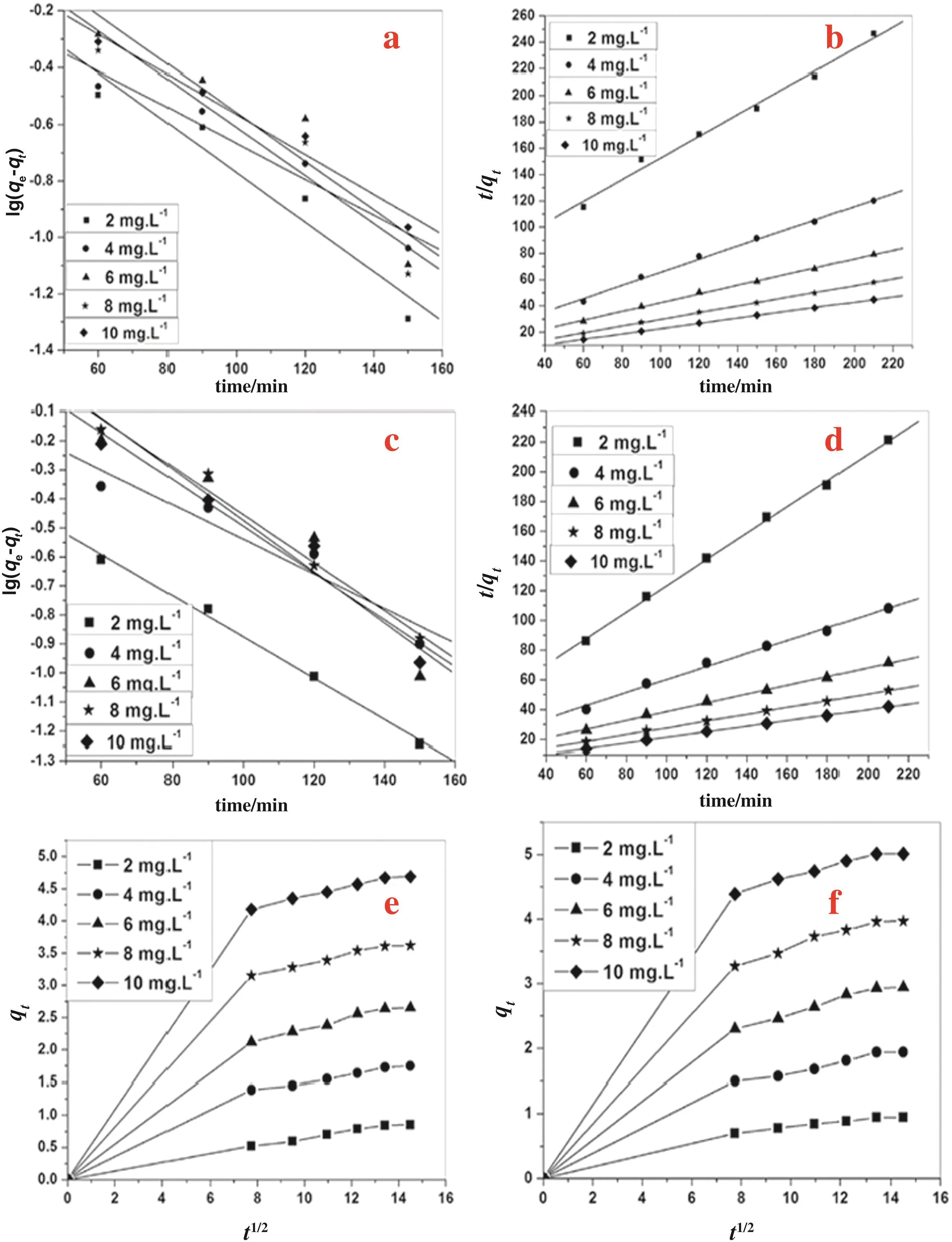
Fig.7.Plot for kinetic of fluoride adsorption on K-CTNSC(a,c,e)and P-CTNSC(b,d,f).

Tab le 8 Therm odynam ic parameters of fluoride sorption on K-CTNSC and P-CTNSC
3.2.7.3.Intra particle diffusion.Fig.7 explains the straigh t line rep resen ting the con tro l of sorption by in tra-particle diffusion.The data are given in Tab les 6 and 7.The first linear po rtion of the plot re fers the macro po re diffusion and the second linear portion of the plot depicts the micro pore diffusion[42,43].The ex trapo lation of the first linear portion of the plots back to the axis gives a positive in tercep t(qt)suggesting the boundary layer thickness.It is indicated that the ex trapolation of the first linear portion of the plots shou ld not pass through the origin.Hence the adsorption rate of fluoride on both K-CTNSC and P-CTNSC is not so lely con tro lled by pore diffusion.Thus the adsorption data indicated that the rem oval of fluoride from the aqueous phase onto K-CTNSC and P-CTNSC is a rather com plex process involving both boundary layer diffusion andintra particle diffusion.The increase of Kidwith increasing concentration of fluoride indicated the higher pore sorption possibility of fluoride on to the adsorben t at room temperature.
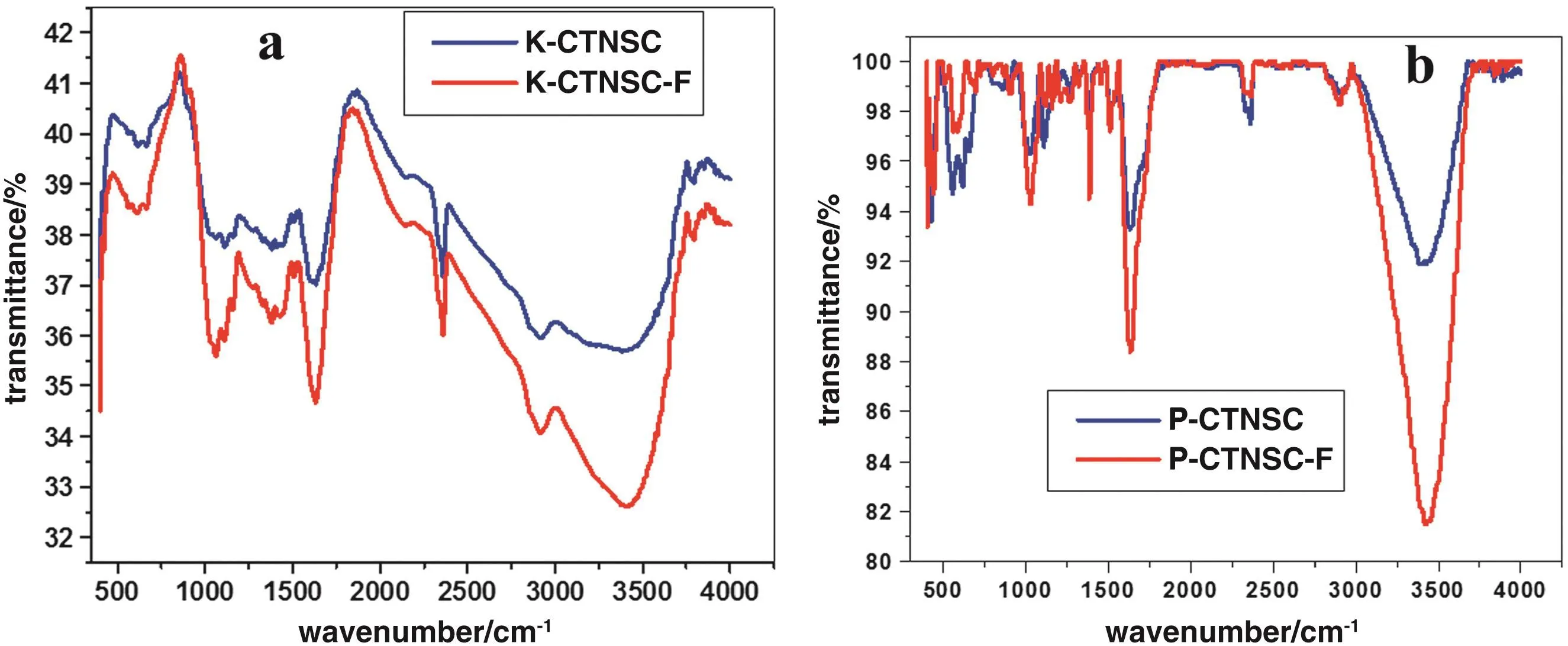
Fig.8.FTIR spectra of fluoride before and after adsorption of(a)K-CTNSC and(b)P-CTNSC.
3.2.8.Thermodynamic study
The effect of temperature has a major influence on the sorption process and hence the sorption of K-CTNSC and P-CTNSC was monitored at four different temperatures 303,313,323 and 333 K under the op tim ized condition and the therm odynam ic parameters viz.,standard free energy change(ΔG),standard en thalpy change(ΔG)and standard entropy change(ΔS)were calculated[44,45]and presentedin Table 8.The results revealed that the adsorption process is spon taneity and endothermic nature.For both of the adsorben ts,the ΔS values are positive(K-CTNSC,102.3 and P-CTNSC,13.18).This indicates the prevalence of a high degree of adsorption of fluoride on to K-CTNSC and P-CTNSC.This may be due to the fact that adsorbed water molecu les which are displaced by the adsorbate species,(F-)gain more translational entropy.Thus it show s the prevalence of random ness of the system[46].
3.2.9.FTIR and EDAX spectral studies
In order to understand the nature of fluoride sorption on K-CTNSC and P-CTNSC sam ples,the FTIR spectra of pu re and fluoride sorbed K-CTNSC and P-CTNSC were recorded and presentedin Fig.8.The FTIR spectra of the raw and fluoride treated surfaces of adsorben ts provide in form ation on the mo lecu lar groups as w ell as on the sm all changes in the environm ent of the surface groups like-OH groups,F-etc.There is a decrease in the in tensity of transm ittance in both fluoride treated sorbents at 3694 and 3620 cm-1indicating the exchange of OH from its surface[47,48].This data confirm s the fact that fluoride rem oval alsofo llow s the ion exchange mechan ism in addition to adsorption.The fluoride adsorptions change the surface morpho logy of the sorben ts which was fu rther supported by EDAX analysis[49](Fig.9).
3.2.10.Desorption and reuse potential
To make a cost effective and user-friend ly process,the adsorben t shou ld regenerate,so as to reuse for fu rther fluoride adsorption.For checking the desorption capacity of the adsorben t,the material was sub jected to an adsorption at an initial fluoride concentration of 3 mg·L-1.The exhausted K-CTNSC and P-CTNSC were regenerated using HCl and NaOH.NaOH is better regenerated than HCl.The concentrations were ranging from 0 to 10%.At 2.5%NaOH concentration,K-CTNSC and P-CTNSC had desorbed alm ost 97.0%of fluoride.To test the adsorption poten tial of regenerated K-CTNSC and P-CTNSC,two more cyclesofadsorption-desorption studieswere carried outbym aintaining the initial conditions the sam e.In the third cycle,the adsorben t capacity has show n 32.12%.How ever,in the fourth cycle,adsorption capacity was observed as 3.7%.M ore tests have to be conducted to determine the exact life cycle of the adsorben t.
4.Conclusions
The chemically activated CTNSC acts asa reasonably good adsorben t for the rem ovaloffluoride from aqueous solution.Themethodissim ple and has show n great poten tial for the rem oval of fluo ride ions.The rem oval increased with the increase in the adsorbate concentration.The83.67%salt rejection hasbeen identifiedin 3m g·L-1of100m lfluoride using 1.75 mg dosage of P-CTNSC adsorben t.The best-fitting adsorption isotherm was Freund lich model.In the adsorption kinetic modeling studies,the pseudo second order chemical reaction kinetics provided the best correlation of the experimental data for K-CTNSC and P-CTNSC.This kinetics data w ou ld be usefu l for developing an app rop riate technology in designing a treatm en t plan t for fluoride rich water.XRD studies show changes in the crystalline nature of the adsorben t due to the adsorption of fluoride on its surface.The FTIR studies indicate the participation of the surface sitesof the sorbent in theadsorben t in teraction.Com pared to the various other sorben ts reportedin the literature,the im pregnated w alnut shell in this study show s very good prom ise for practical applicability.How ever,m ore studies are needed to op tim ize the system from the regeneration poin t of view and to investigate the econom ic aspects.
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
