Isolation and structural iden tification of two bioactive phenazines from Streptom yces griseoluteus P510☆
Qin Luo,Hongbo Hu,Huasong Peng,Xuehong Zhang,W ei W ang
State Key Laboratory of Microbial Metabolism,School of Life Science and Biotechnology,Shanghai Jiao Tong University,Shanghai 200240,China
Keyw ords:Streptomyces griseoluteus P510 Phenazine antibiotic Phenazine-1-carboxylic acid 1-Hyd roxyphenazine
ABSTRACT Phenazine an tibiotics phenazine-1-carboxylic acid(PCA)and 1-hyd roxyphenazine(1-OH-PHZ)were pu rified from culture broth andm yceliaof Streptom ycesgriseoluteus P510.Both PCAand 1-OH-PHZexhibitstrong antifungal activity against six plan t pathogens,especially Fusarium oxysporium,with minim al inhibition concentrations less than 1 and 2 μg·m l-1 for PCA and 1-OH-PHZ,respectively.The presence ofPCAand 1-OH-PHZindicates that S.griseoluteus P510 can be used as a potential source of pesticides.
1.Introduction
Biosyn thesized phenazines are iso lated as secondary metabo lites from Streptom yces,Pseudom onas and other bacterial genera from soil and marine habitats.Phenazines exhibit an tiMicrobial,an tim alarial,antiparasitic,and antitum or activity[1-5].For exam ple,phenazine-1-carboxylic acid(PCA),one of the most comm on precu rsors of many biosynthesized phenazine com pounds[1-3],supp resses a severe root disease in w heat caused by Gaeum annom yces gram inis var.tritici[6]andis also high ly active against Mycobacterium tubercu losis[7].1-Hyd roxy-phenazine(1-OH-PHZ)inhibits the grow th of Candidaalbicans and Aspergillus fum igatus[8].Num erous pub lications have suggested that phenazines are promising antibiotics with broad-spectrum activity and application prospects,especially in agriculture and medicine.
Most biosyn thesized phenazine com pounds are isolated from cu lture broths[9-11]and som e phenazines are purified from mycelia[12,13].PCA can be isolated from the cu lture broths of many phenazineproducing bacteria,such as Pseudomonas fluorescens 2-79,Pseudomonas aureofaciens 30-84,and P.aureofaciens GC-B26[6,14,15].Another phenazine precursor,phenazine-1,6-dicarboxylic acid(PDC),has been isolated from the cu lture broth of P.aureofaciens strain 30-84[16].PDC has also been purified from the mycelia of Streptom yces sp.IFM 11204[12]and the Indonesian strain Strep tomyces sp.ICBB8198[13].Most phenazinep roducing bacteria biosynthesize more than one phenazine com pound.PCA,1-hydroxyphenazine,and 6-hyd roxyphenazine-1-carboxylic acid are isolated from the cu lture broth of an Indonesian Strep tom yces sp.strain[17].PCA,together with 1-phenazinecarboxam ide and 1-phenazinol,are isolated from Streptom yces sp.Ank 315[18].
Strep tom yces griseoluteus P510,which biosyn thesizes griseoluteic acid(GA),has been iso lated from soil sam ples[19].In this study,two other phenazine com pounds,PCA and 1-OH-PHZ,are purified from the cu lture broth and mycelia of strain P510 through organic so lven t extraction and co lum n ch rom atography.The an tifungal activity of these three phenazines in strain P510 is fu rther investigated.
2.Materials and Methods
2.1.Microorganism s
S.griseoluteus P510 was grow n in our lab.Specim ens of plant pathogens Alternaria solani,Fusarium oxysporium,and Fusarium gram inearum were kind ly provided by Professor Yuquan Xu(Shanghai Jiaotong University).Plan tpathogens Sclerotinia sclerotiorum and Phyricu laria grisea,and pathogen of Stevia leaf spot disease were grow n in our laboratory.
2.2.Cu lture conditions
S.griseoluteus P510 was cu lturedin liquid yeast starch agar II medium(yeast ex tract 2 g·L-1,so lub le starch 8 g·L-1,ad justed to pH 7.3)at 28°C for 4 days in an orbital incubator shaken at 180 r·m in-1,as described by W ang et al.[19].
For determination of antifungal activity,the six plant pathogens were grow n in solid potato dextrose agar medium(200 g·L-1potato extract,20 g·L-1glucose,15 g·L-1agar)at 28 °C for 3 days.
2.3.Antifungal activity assays
Antifungal activity was determined using a modified“p-iodonitrotetrazolium”method,as described by W ang et al.[20].The sam ples were dissolvedin methanol at concentrations ranging from 1 μg·m l-1to 100 μg·m l-1.Fungal spore suspensions were transferredin tofresh liquid potato dextrose agar medium and diluted to 2×103CFU·m l-1.Fungalcultures(96μl)wereaddedin to each of96w ellMicrotiter plates containing100μlofdifferent testsam ples.Then,8μlofgrow th indicator p-iodonitrotetrazo lium(1 mg·m l-1)violet(Sigm a®)(INT)dissolvedin water was added to each of the microplate w ells.The covered microplates were incubated for 3-4 days at28°C and 100%relative hum idity.M in im um inhibitory concentration(M IC)was recorded as the low est concentration of the ex tract that inhibited antifungal grow th after 24 h.
2.4.Separation and purification of active com pounds
Ferm entation suspensions(10 L)were centrifuged at 8000 r·m in-1for 40 min to separate the cu lture broth from the mycelia.
The culture filtrate(9.6 L)was extracted three times with acidified ethyl acetate(EtOAc),and the EtOAc-solub le portion was concentrated under reduced pressure.The crude ex tract(2.1 g,E1)was separated using a silica gel colum n chrom atography(3.5 cm×45 cm,CHCl3/10%M eOH)to give three fractions.Fraction II(SG2,791 mg)was fu rther separated using thin-layer chrom atography(TLC)to obtain 123.7 mg of bioactive fraction(TLC1).TLC1 was finally pu rified via reversed-phase HPLC(Agilen t,Am erica)on a C18 co lum n(Agilen t eclipse XDB-C18,5 μm,4.6 mm × 250 mm),which was eluted with a mixture of methanol and 0.1%acetic acid water(volum e ratio of 55:45)at a flow rate of 1.0 ml·m in-1.Tw o different peaks were collected and concentrated separately,and 3.2 mg of Com pound 1 and 9.1 mg of GA were obtained.
The mycelia(20.1 g)were extracted three times with acetone.The acetone-soluble portion was concentrated under reduced pressure.The crude extract(79.3 mg,E2)was purified via reversed-phase HPLC under the sam e conditions described above to obtain 6.5 mg of Com pound 2.
2.5.Structural analysis
The UV-visible absorption spectra were recorded on an 8453 UV-visible spectrophotometer(Agilent,Am erica).Mass spectra were recorded on a h igh-resolution(HR)m ass spectrometer,UPLC Acquity/QTOFMS Prem ier(waters,Am erica).Com pounds 1 and 2 were individually dissolvedin CDCl3and1HNMRspectrawas recorded on an Avance 400(Bruker,Swiss).
3.Results and Discussion
Fig.1 show s the schem atic of the iso lation and pu rification processes.An tifungal activity bioassays were used to mon itor the separation and pu rification processes:o rgan ic ex traction,silica gel co lum n ch rom atography,TLC,and HPLC pu rification.A to tal of 3.2 mg of the yellow Com pound 1 and 9.1 mg of GA were pu rified from 9.6 L of fermentation b ro th.M o reover,6.5 mg of the redorange Com pound 2 was pu rified from the mycelia through organic ex traction and HPLC.The inh ibito ry activity against the six plan t pathogens is show n in Tab le 1.Com pound 1,GA,and Com pound 2 all show strong activity against the six plan t disease pathogens,with the h ighest inh ibitory activity against F.oxysporium at M ICs of 1,2,and 2 μg·m l-1,respectively.

Tab le 1 Inhibitory activity of separation sam ples on six plant disease pathogens

Fig.1.Flow chart for extraction and purification of phenazine metabo lites from S.griseoluteus P510.
The HPLCchrom atogram ofCom pound 1 isshow n in Fig.2(1A).Natural phenazine derivatives are pigm en ts with absorption spectra that include at least two distinct peaks,and their maxim um absorption w avelengths vary according to the color and position of substituents on the heterocyclic ring[21].According to the absorption spectra of phenazines,peaks are at 207,251,and 364 nm in the absorption spectrum of Com pound 1 dissolvedin methanol[Fig.2(1B)].
The molecu lar mass of Com pound 1 on HR-ESI-MS is 224.06 as m/z 225.06,[M+H]+;m/z 207.05,[M-H2O]+;and m/z 248.05,[M+Na]+,as show n in Fig.3(1A),which is consisten t with that of PCA(C13H9N2O2,MW=224.07)[22].The UPLC-MS-MSm assspectrum of m/z 225 peak on HR-ESI-MS of Com pound 1 show s major fragm ents at m/z 181.08,193.08,and 224.06.The fragm en t at m/z 179.06 corresponds to[M-H]+of the phenazine ring(C12H8N2,MW=180.08).The differences between the fragm ents reveal the presence of-COOH group side chains[Fig.3(2A)].
The NMR chemical shifts in Com pound 1 are as fo llow s(Tab le 2):1H NMR(CDCl3,400 MHz,ppm) δ 8.57(1H,d,2H′),7.99-8.08(1H,t,3H′),9.01(1H,d,4H′),8.38(1H,d,6H′),7.99-8.08(1H,t,7H′),7.99-8.08(1H,t,8H′),8.31(1H,d,9H′),and 15.59(1H,s,-COOH).Both1H NMR spectra and the mass spectral data indicate that Com pound 1 is PCA[16,23].Based on the mass spectra,absorption spectra,and1H NMR data,Compound 1 isolated from S.griseoluteus P510 is iden tified as PCA(Fig.4).
The HPLCchrom atogram and absorption spectrum ofCom pound 2 are show n in Fig.2(2A,2B),respectively.The absorption peaks of Com pound 2 are at 259 nm and 367 nm.The molecu lar mass of Com pound 2 on HRESI-MS is 196.07 as m/z 197.07,[M+H]+,as show n in Fig.3(2A).These results are consistent with the molecular w eight of 1-hyd roxyphenazine(C12H8N2,MW=196.08)[12].The UPLC-MS-MS mass spectrum of m/z 197 peak on HR-ESI-MS of Com pound 2 also show s fragm en t at m/z 179.06 similar to that in Com pound 1.The differences between the fragm ents reveal the presence of-OH group side chains[Fig.3(2B)].
The NMR chemical sh ifts in Com pound 2 are as fo llow s:1H NMR(CDCl3,400 MHz,ppm) δ 7.27(1H,t,2H′),7.78-7.88(1H,m,3H′),8.25(2H,m,6H′),7.78-7.88(1H,m,7H′),7.78-7.88(1H,m,8H′),and 8.25(2H,m,9H′).The1H NMR spectra and mass spectral data indicate that Com pound 2 is 1-OH-PHZ[24],as show n in Tab le 3.Based on mass spectra,absorption spectra,and NMR data,Com pound 2 from S.griseoluteus P510 is identified as 1-OH-PHZ(Fig.4).
4.Conclusions
Tw o phenazine com pounds,PCA and 1-OH-PHZ,were isolated from the cu lture broth and mycelia of S.griseoluteus P510.PCA is a broadrange and poten t antifungal metabolite with an M IC of 29 μg·m l-1for Sclerotium rolfsii NCIM 1084[25],w h ich is less than 1 μg·m l-1for G.gram inis var.tritici and other fungal root pathogens,such as Bacillus cereus,Rhizoctonia solani,and Pythium aristosporium[6,26].1-OH-PHZ exhibits an M IC of 25 μg·m l-1against C.albicans[27]and 10 μg·m l-1to 100 μg·m l-1for fou ling bacteria such as 134-15,137-8,4.2,5.1,and 5.4[28].In th is study,the phenazine com pounds iso lated from S.griseoluteus P510,PCA and 1-OH-PHZ,show strong inhibitory activity against the six plan t pathogens studied.Both PCA and 1-OH-PHZ exh ibit M ICs less than 10 μg·m l-1for A.so lani,F.oxysporium,and S.sclerotiorum.1-OH-PHZ exhibits an M IC of 6 μg·m l-1against F.gram inearum,w h ich is different from those for PCA.
PCA is identified as a phenazine interm ediate in Pseudom onas[29,30],which cou ld be further modified to create more com plex phenazines via methylation,transam idation,hyd roxylation,or decarboxylation[1,2].PCA hyd roxylation produces 2-OH-PCA in P.aureofaciens 30-84[31]and 1-OH-PCA in Pseudom onasaeruginosa PAO1[22].The results of the present study im ply that PCA is synthesized as a phenazine biosyn thesis precursor in S.griseoluteus P510 and fu rther modified to produce 1-OH-PCA via hyd roxylation,as show n in P.aeruginosa PAO1[22]or GA in som e branched pathw ays.
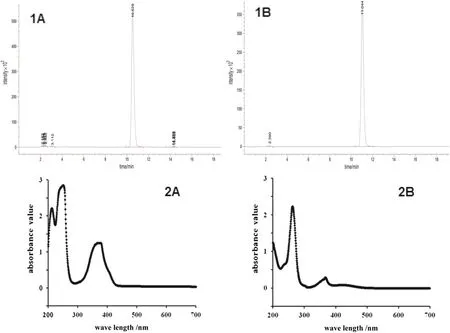
Fig.2.HPLC profile under 265 nm(1A and 2A)and UV-visible absorption spectrum(1B and 2B)of Com pounds 1 and 2 purified from S.griseoluteus P510.
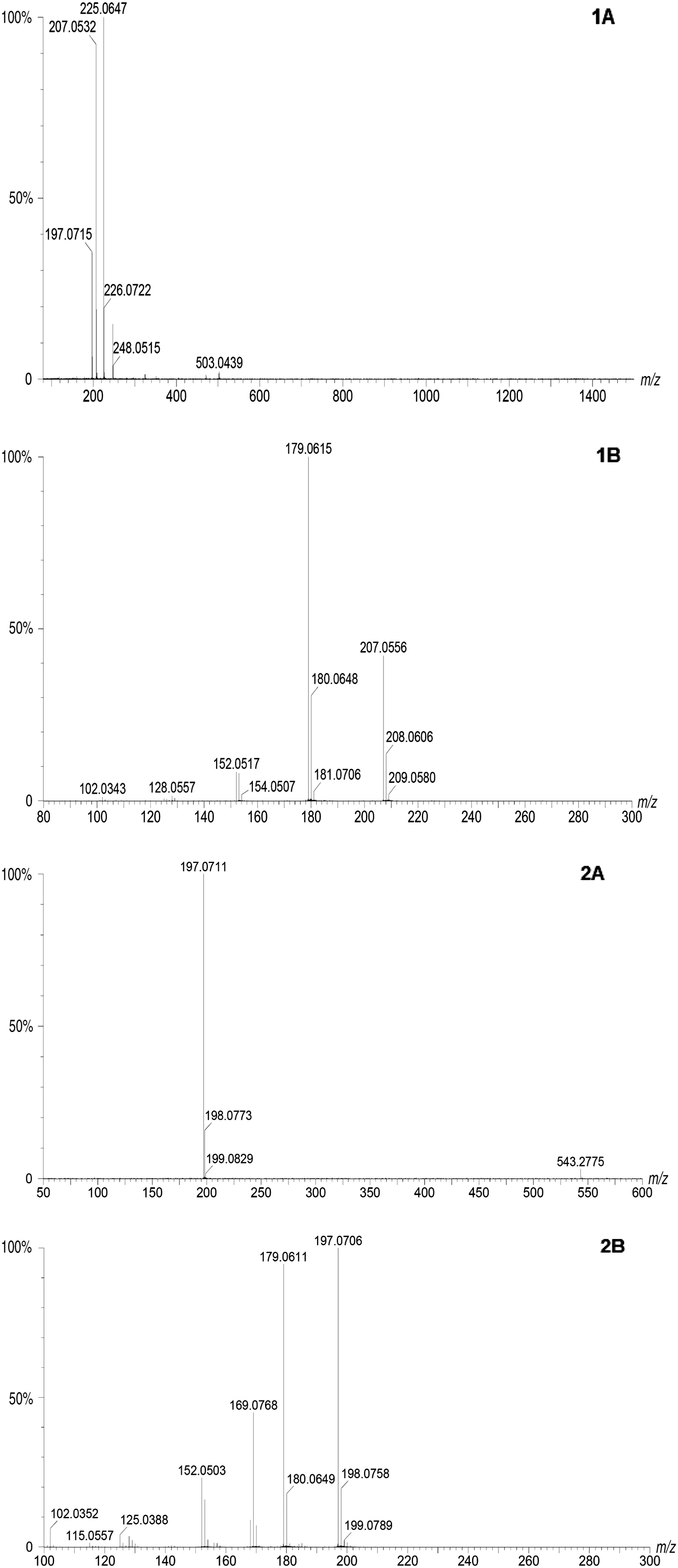
Fig.3.Spectra of Com pounds 1 and 2 from S.griseoluteus P510 on HR-ESI and UPLC-MSMS.1A:HR-ESI mass spectrum of Com pound 1;2A:HR-ESI mass spectrum of Com pound 2;1B:UPLC-MS-MS mass spectrum of m/z 225 peak on HR-ESI mass of Com pound 1;2B:UPLC-MS-MS mass spectrum of m/z 197 peak on HR-ESI mass of Com pound 2.
Streptom yces species(e.g.,Streptom yces griseolutein,Streptom yces luteogriseus,Streptomyces antibioticus,and Streptom yces prunicolor)have been u tilized as the natural source of num erous diverse and com plexphenazines,including griseo lutein,phenacein,lom ofungin,and certain biologically significant 5,10-dihydrophenazines[1].Natural products have been proven as usefu l sources of cancer therapeutic,chem op reventive agents,and new d rugs[32,33].Metabolites such as lavanducyanin[34,35]have been iso lated from Strep tom yces.Although investigations on the biosynthesis and transform ation of these three phenazines(PCA,GA,and 1-0H-PHZ)in S.griseoluteus P510 are still ongoing,the above analysis im plies that S.griseoluteus P510 has great potential for developing novel phenazine com pounds and other new phenazine derivatives,which can prom ote the developm en t and utilization of natural products,as w ell as the research and developm ent of new pollution-free biological pesticides and new drugs.

Tab le 2 1H NMR spectral data of Com pound 1 and PCA[16,23]in CDCl3

Fig.4.Chemical structure of PCA and 1-OH-PHZ isolated from S.griseoluteus P510.

Tab le 3 1H NMR spectral data of Com pound 2 and 1-OH-PHZ[24]in CDCl3
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
