Ultrasonic study on molecu lar in teractions in binary mixtures of form am ide with 1-p ropanol or 2-p ropanol
Man ju Rani,Sum an Gah lyan ,Ankur Gaur ,San jeev M aken ,*
1 Department of Chemical Engineering,Deenbandhu Chhotu Ram University of Science and Technology,Murthal 131 039,India
2 Departm ent of Chem istry,Deenbandhu Chhotu Ram University of Science and Technology,Murthal 131 039,India
3 Departm ent of Chemical Engineering,Motilal Nehru National Institute of Technology,Allahabad 211 004,India
Keyw ords:Ultrasonic speed Graph theoretical app roach Form am ide Propanol Interm o lecular in teraction
ABSTRACT Ultrason ic speeds have been measured at 298.15 K and 308.15 K for mixtures of form am ide+1-p ropanol or 2-p ropanol.For an equim olar mixture,excess molar com pressibility fo llow s the sequence of 1-p ropanol>2-p ropano l.The u ltrason ic speed data are correlated by various co rrelations such as Nom o to's relation,van Dael's mixing relation andim pedance dependence relation,and analyzedin term s of Jacobson's free length theory and Schaaff's collision factor theory.Excess isen tropic com pressibility is calculated from experimental u ltrason ic speed data and previously reported excess vo lum e data.The excess mo lar u ltrason ic speed andisen tropic com pressibility values are fitted to Red lich-Kister po lynom ial equation.Other properties such as molecu lar association,available volum e,free vo lum e,andin term o lecu lar free length are also calculated.The excess isen tropic com pressibility data are also in terp retedin term s of graph theoretical app roach.The calculatedisen tropic com pressibility values are w ell consisten t with the experimental data.It is found that the in teraction between form am ide and propanol increases w hen hyd roxyl group attached to a carbon atom has more-CH3 groups.
1.Introduction
Am ides are im portant bio-organic so lven ts and convenien t model system s for investigating pep tide and protein-so lven t in teractions[1,2].Alkanol and am ide molecules are associated through hyd rogen bonding and their mixtures with other so lven ts show a pronounced therm odynam ic non-idealbehavior[3-5].The therm o-physical propertiesofbinarym ixturesare ofgreatsignificance for chemicalengineers in process designs and also im portan t to chem ists to understand the nature of molecu lar in teractions[6,7].Ultrasonic study of binary mix tures helps in understanding their non ideal behavior,as various therm odynam ic properties derived from u ltrasonic speed and density reveal significan t in form ation about the structure and molecu lar in teractions[8-10].Form am ide is selected for this study,as it is the sim plest am ide that con tains a pep tide linkage,the fundamental building b lock of proteins.Form am ide molecu les are high ly polar[11]and are strongly self-associated through extensive three dim ensional network of hydrogen bonds,through its three hyd rogen bond donors(3 H-atom s)and three accep tors(two lone pairs of electronsat oxygen and oneon nitrogen atom)[2].Since the com ponen tsof these binarym ixtureshaveboth proton-donating/accep ting abilities,significan t in teraction through hyd rogen bonding between un like molecu les is expected.In our previous work,excess molar volum es and excess molar en thalpies of form am ide with propanol and bu tanol were studiedin term s of graph theoretical approach,Mecke-Kem pter type association model of Treszczanowicz-Benson association model and Prigogine-Flory-Patterson theory[12-15].In this paper,u ltrasonic speeds are measured at 298.15 K and 308.15 K for mixtures of form am ide+1-p ropanol or 2-p ropanol over the entire range of com position.The u ltrasonic speed data are correlated by various correlations such as Nom oto's relation,Van Dael's mixing relation andim pedance dependence relation,and fu rther analyzedin term s of Jacobson's free length theory and Schaaff's collision factor theory(CFT).Excess isentropic com pressibility is calculated from experimental u ltrasonic speed data and previously reported excess vo lum e data.The excess molar u ltrasonic speeds andisen tropic com pressibility values are fitted to Red lich-Kister polynom ial equation.Other properties such as molecu lar association,available volum e,free volum e,andin term o lecu lar free length are also calculated.
2.Experimental
Form am ide,1-p ropano l,and 2-p ropanol(Sigm a)were pu rified by standard procedu res[16,17].The purities of the purified sam ples were checked by measuring their densities and refractive indices at 298.15 K.The densities were measured with a precision of±5 × 10-5g·cm-3by a specially designed densimeter,consisting of a bu lb of an app roxim ate volum e of 35 cm3attached to a calibrated capillary through a B-10 standard join t in the manner described by wiesenberger[18].Air buoyancy correction was also applied to ach ieve a greater accu racy.Refractive indices were measured with a therm ostatically contro lled Abbe refractometer(OSAW,India)using sodium D-line with an accu racy of±0.0001.Ou r experimental values for the density and refractive index of the pu re com pounds are in good agreement with the literature values[19-25]as show n in Tab le 1.Ultrason ic speeds were measured using u ltrason ic in terferometer(M odel M-81)operating at 2 MHz and the data were rep roducib le with in±3%.The temperature of water therm ostat was con tro lled to±0.01 K by a mercu ry-in-toluene regu lator.

Tab le 1 Experimental u ltrasonic speeds,critical temperature[19,20],isobaric expansivity,and specific heat at constant pressure[19,20]of pure liquids
3.Results
Ultrasonic speeds of binary mix tures of form am ide(1)+alkano l(2)m easured at 298.15 K and 308.15 K are reco rdedin Tab le 2.Fo llowing em pirical,sem i-em pirical o r statistical re lations are used for theo retical estim ation of sound speedin the binary mix ture.
Nom oto's relation based on the assum ption of additivity of mo lar sound speed and no volum e change in mixing is given as[26]
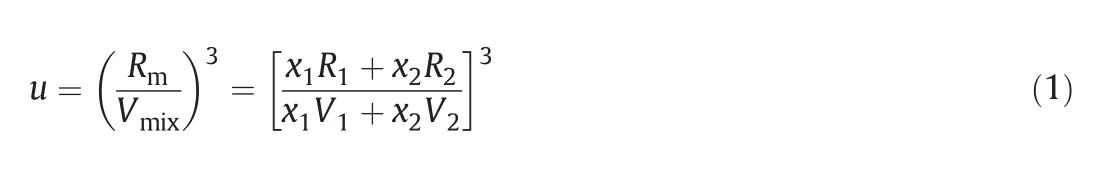
where x1,x2,V1,V2,R1,and R2are them ole fractions,m olar volum esand molar sound speed of com ponents 1 and 2,respectively,and

van Dael's ideal mixing relation is[27]

where x1and x2are mole fractions,M1and M2are mo lar mass of formam ideand alkanol,respectively,and uid.mixis the ultrasonic speed of the ideal mixture.
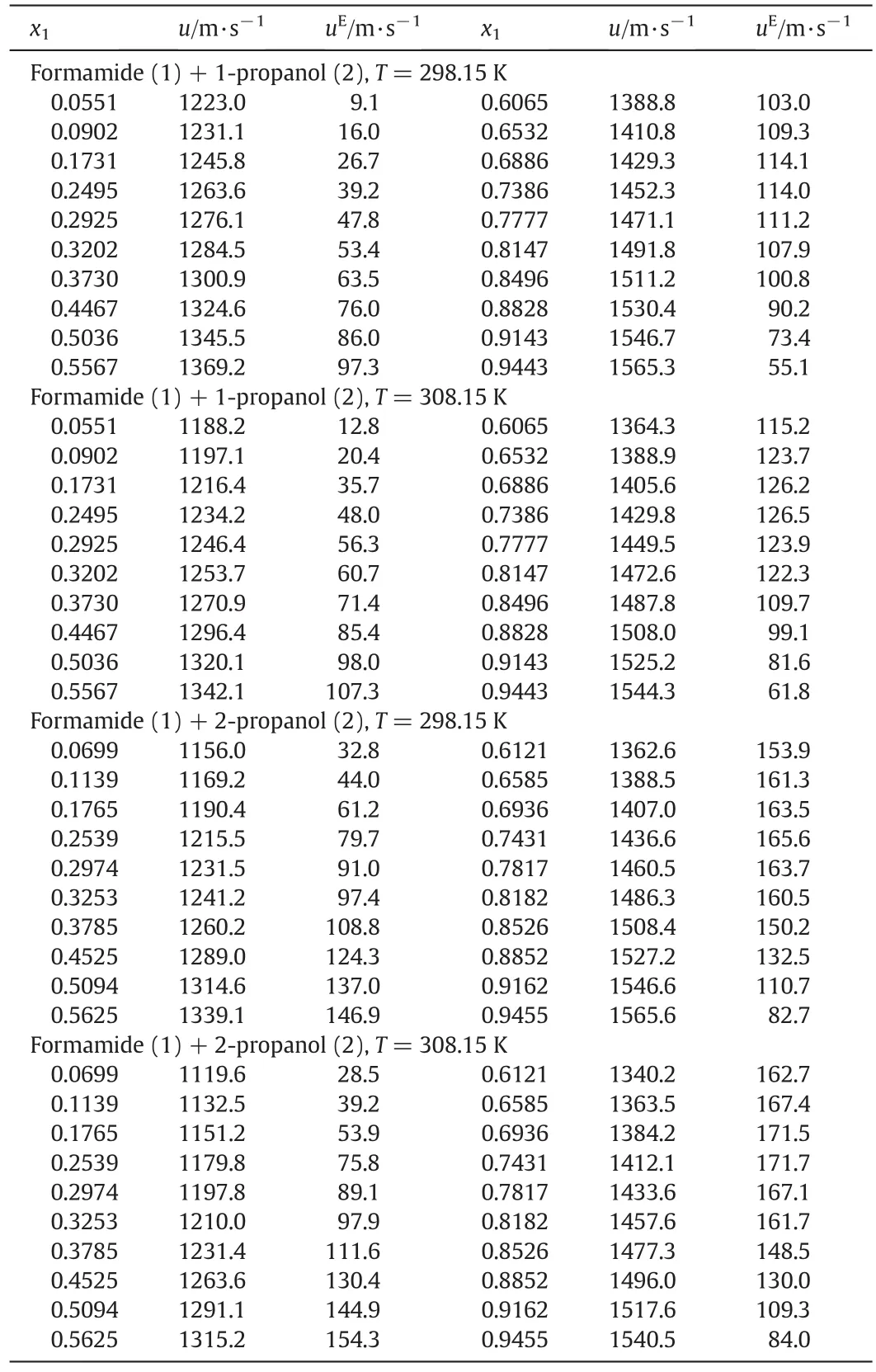
Tab le 2 Speed and excess speed of sound for binary mixtures
The im pedance dependence relation is[28]

Schaaff's collision factor theory(CFT)[29-31]is applied to calculate the ultrasonic molecular free lengths(Lf)using following equations

where u is the u ltrasonic speed at temperature T and u∞is 1600 m·s-1.
According to Schaaff's collision factor theory(CFT)[29-31],

where b and S are the geometric volum e and collision factor,respectively.
The actual vo lum e of them olecu le per mole of liquidis computed as follow s.

where r is the molecu lar radius com puted using Schaaff's[31]relation
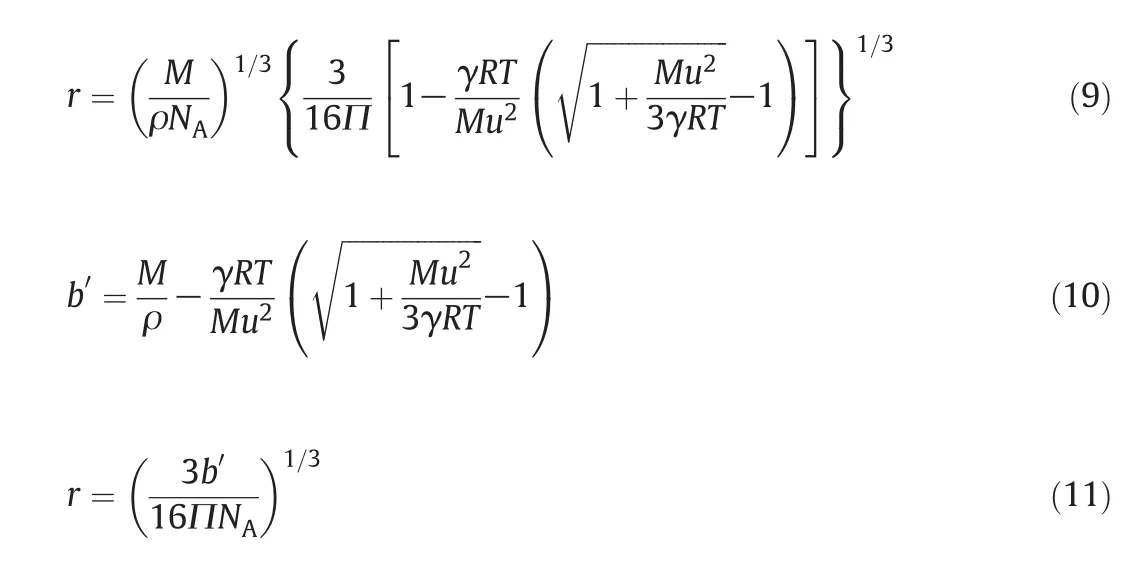
The value of the collision factor S is calculated using the relation

In order to com pare the estim ation capability of various relations,percentage standard deviations are calculated using following relation and given in Table 3.

Tab le 3 Standard deviations in u ltrasonic speed predicted by various correlations

where n rep resen ts the number of experimental data poin ts.Various basic parametersof the pure com ponen tsareusedin theoreticalestim ation of u ltrasonic speed by using various correlations.
M olecu lar association(MA)is calculated[32]using the relation

The molar free volum e(Vf)is obtained according to Kincaid and Eyring[33],from the following equation,

Jacobson[34,35]derived the in term o lecu lar free length from molar vo lum e and surface area for a large number of liquids.It is suggested that com pressibility can be w ell understoodin term s of the in term olecu lar free length,which is the distance between surfaces of molecu les.The in term o lecu lar free length is related to available volum eand surface area per molecu le and depends on the type of packing and ex ten t of association in given liquid.Lfis the interm olecu lar free length of binary mixture

where Varep resen ts the available volum e per mole and Y is the surface area per mo le,expressed as

where NAis the Avogad ro number and V0and VTare the molar volum es at0 K and temperature T,respectively.V0isobtained from the following relation using critical temperature Tc,which is tabu latedin Table 1.

The critical temperature of binary mixture is the mole fraction additive of the values of its pure com ponen ts:

The therm odynam ic in term o lecu lar free lengths Lfare com puted as follow s

where K is Jacobson constant,which is a temperature dependent constan t with a value from 618 to 642 at T=293.15 K to 313.15 K.
Excess u ltrasonic speed(uE)is calculated by

The values of excess com pressibility are calculated using fo llowing equations.

The densities,ρ12,ofbinarym ixturesare calculated from their excess molar vo lum e data
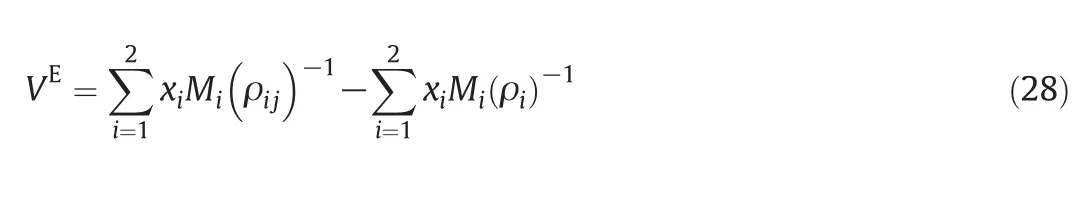
where x1,M1,and ρ1are the mo le fraction,m olar mass and density of form am ide in binary mixtures.

where

where φ is the volum e fraction,V is the molar volum e,αpis the isobaric expansivity,and CPis the molar heat capacity.The values of αpand CPare from literature and given in Tab le 1.
The measured excess property data at 298.15 K and 308.15 K arefitted to the Red lich and Kister equation[36]

where X(n)(X=any physical property such as u,Ks,Lf,Vf,Va,etc.)is the ad justable parameter and x1is the mole fraction of form am ide in formam ide(1)+alkano l(2)m ix ture.These parameters are evaluated byfitting XEdata to Eq.(1)with the leastsquaresmethod and standard deviations of XEare calculated by
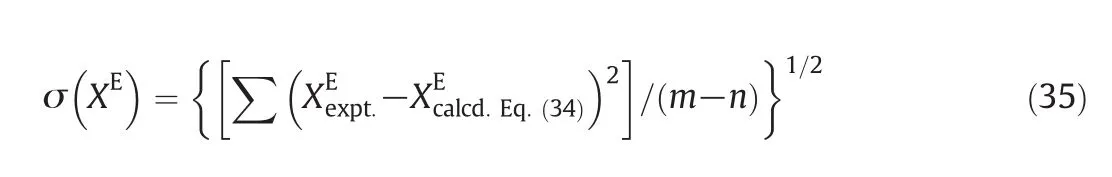
where mis the number of experim ental values and n is the number of ad justab le parameters in Eq.(34).The choice of n with 1-4 values is dictated by the consideration that the max im um dev iation σm(XE)of XE(as calculated from Eq.(35)from the experimental XEvalues)satisfies the relation σm(XE)≤ 2σ(XE).Various ad justab le parameters X(n)along with standard deviation σ(XE)are recordedin Tab le 6.
4.Discussion
Experim ental sound speed of binary mixtures and those calculated using variouscorrelationsare representedin Figs.1 and 2.In the present binary mixtures Nom oto relation gives the best prediction in the case of 1-propanol and van Dael's relation predict u ltrasonic speed better than Nom oto relation in the case of 2-p ropano l.Deviation in the speed of sound calculated by using im pedance dependence relation and Schaaffs co llision facto r theory may be due to non ideal behavior of binary mixtures.
The variations of molecu lar association(MA)with mole fraction of form am ide at 298.15 K and 308.15 K are representedin Tab le 4 and Fig.3 and their values are negative over entire com position range for these system s.The non linear variation of MAwith mo le fraction indicates the existence of molecu lar in teraction between un like molecu les.The variation of free volum e and excess free volum e with mole fraction of form am ide at 298.15 K and 308.15 K are representedin Figs.4 and 5.Various ad justable parameterscalculated by using Red lich-Kister Eq.(34)are tabu latedin Table 6.Free volum e increases with molecu lar size.Variations in the slope of isotherm s of free volum e as a function of com position indicate an increase in en tropy effect associated with the rearrangement of solvate structures particu larly in the high concentration regions of alkanol.
Calculated values of Vaand excess molar availab le vo lum eare tabulatedin Table 4 andits Red lich-Kister parametersand standard deviationare given in Tab le 6.Fig.6 rep resen ts the variation of availab le excess mo lar vo lum e with mo le fraction of form am ide.
Values of in term o lecu lar free length and excess free length are tabu latedin Tab le 4 and representedin Fig.7.Parametersin Red lich-Kister Eq.(34)and standard deviationare given in Tab le 6.
All the uEvalues for these system s are positive over the w hole composition range and follow the sequence of 2-p ropanol>1-p ropanol as show n in Fig.8.The positive value of excess u ltrasonic speedindicates strong in term o lecu lar interactions between the com ponen t mo lecu les of binary liquid mixtures.The magnitude of excess ultrasonic speedindicates the strength of in term olecu lar in teraction between un likem olecu les in the mix tures.M ore positive value means strong in teraction.Parameters u(n)in Red lich-Kister Eq.(34)and standard deviation σ(uE)are given in Table 6.
The excess isen tropic com pressibilityis computed using Eq.(25)andits values are tabu latedin Tab le 5 and representedin Fig.9.Parametersin Red lich-Kister Eq.(34)and standard deviationare given in Tab le 6.The results in Fig.9 indicate thatvalues for these binary mix tures are negative over en tire com position range and at each temperature.The negative values ofindicate the presence of specific in teraction between form am ide and alkano l mo lecu les.In these mix tures the magn itude ofvalues at equim o lar com position fo llow s the sequence of 2-p ropano l<1-p ropano l.Th is suggests that there is an expansion in vo lum e resulting in an increase in com pressibility of the mixtures in sam e order.The ΔKsvalues depend upon several con tribu tions of physical and chemical nature.The physical con tribu tions com prise of dispersion forces and nonspecific physica l(w eak)in teractions w h ich lead to positivecon tribu tion;physical con tribu tion is also due to geometrical effect for mo lecu les of significantly different sizes tofit in to each other's structure,resulting in positivecon tribu tion and specific in teractions such as form ation of H bond,form ation of charge transfer com plex and strong dipo le-dipo le in teractions between com ponen t mo lecu les resulting in negativecon tribu tion.
M o lecu les of bo th form am ide and alkano l are associated through hyd rogen bonding due to the presence of strong protonaccep tor as w ell as proton-donor groups in their mo lecu les.M ixing of form am ide with alkanol w ou ldinduce mu tual dissociation of hyd rogen bonded structures present in pu re liqu ids with subsequen t form ation of new H-bonds between form am ide and alkano l mo lecu les in the mixtures.More negative value of 2-p ropanol is due to the fact that in 2-p ropano l the presence of two CH3groups at α-carbon atom increases electron density at oxygen atom to greater ex ten t than that in 1-p ropano l with one ethy l group.Hen ce it may be con cluded that the in teraction between form am ide andalkano ls under study increases w hen hyd roxy l group attached to a carbon atom has more-CH3groups.
Tab le 4 Values of availab le vo lum e(V a),excess availab le volum e,free length(L f),excess free length,free volum e(V f),excess free vo lum e,and mo lecu lar association M A
(continued on next page)
Form am ide(1)+1-p ropano l(2),T=298.15 K 0.0000 16.226 0.000 5.2475 0.0000 2.327 0.000 0.0000 0.0551 15.558 -0.145 5.1632 0.0294 2.391 0.089 -0.0175 0.0902 15.143 -0.225 5.1086 0.0475 2.422 0.135 -0.0260 0.1731 14.196 -0.383 4.9753 0.0854 2.513 0.263 -0.0519 0.2495 13.360 -0.492 4.8448 0.1127 2.562 0.347 -0.0681 0.2925 12.904 -0.538 4.7675 0.1244 2.571 0.376 -0.0733 0.3202 12.617 -0.563 4.7165 0.1305 2.573 0.390 -0.0760 0.3730 12.078 -0.598 4.6158 0.1390 2.571 0.413 -0.0805 0.4467 11.352 -0.623 4.4690 0.1445 2.559 0.433 -0.0856 0.5036 10.811 -0.623 4.3512 0.1441 2.530 0.430 -0.0858 0.5567 10.319 -0.609 4.2380 0.1408 2.477 0.401 -0.0803 0.6065 9.871 -0.583 4.1298 0.1354 2.440 0.386 -0.0788 0.6532 9.461 -0.548 4.0263 0.1285 2.385 0.353 -0.0727 0.6886 9.158 -0.515 3.9470 0.1223 2.334 0.317 -0.0656 0.7386 8.739 -0.458 3.8333 0.1119 2.278 0.284 -0.0601 0.7777 8.419 -0.406 3.7430 0.1024 2.230 0.254 -0.0547 0.8147 8.122 -0.351 3.6562 0.0919 2.172 0.212 -0.0461 0.8496 7.846 -0.294 3.5724 0.0805 2.117 0.174 -0.0381 0.8828 7.589 -0.235 3.4911 0.0677 2.064 0.135 -0.0298 0.9143 7.348 -0.176 3.4118 0.0536 2.021 0.107 -0.0244 0.9443 7.122 -0.117 3.3341 0.0377 1.968 0.067 -0.0153 1.0000 6.709 0.000 3.1813 0.0000 1.876 0.000 0.0000 Form am ide(1)+1-p ropano l(2),T=308.15 K 0.0000 17.154 0.000 5.5569 0.0000 2.845 0.000 0.0000 0.0551 16.438 -0.159 5.4632 0.0281 2.877 0.076 -0.0133 0.0902 15.995 -0.247 5.4034 0.0461 2.896 0.124 -0.0218 0.1731 14.985 -0.419 5.2593 0.0854 2.946 0.241 -0.0434 0.2495 14.096 -0.536 5.1194 0.1147 2.982 0.339 -0.0622 0.2925 13.611 -0.585 5.0369 0.1274 2.982 0.374 -0.0690 0.3202 13.305 -0.612 4.9824 0.1340 2.985 0.399 -0.0741 0.3730 12.733 -0.649 4.8748 0.1434 2.964 0.421 -0.0789 0.4467 11.962 -0.676 4.7177 0.1493 2.919 0.436 -0.0831 0.5036 11.387 -0.676 4.5913 0.1488 2.854 0.417 -0.0806 0.5567 10.865 -0.661 4.4697 0.1449 2.793 0.399 -0.0785 0.6065 10.389 -0.633 4.3533 0.1386 2.726 0.372 -0.0745 0.6532 9.955 -0.595 4.2421 0.1308 2.641 0.325 -0.0658 0.6886 9.633 -0.559 4.1568 0.1239 2.588 0.301 -0.0619 0.7386 9.189 -0.497 4.0348 0.1125 2.511 0.264 -0.0557 0.7777 8.850 -0.441 3.9381 0.1023 2.447 0.232 -0.0500 0.8147 8.536 -0.382 3.8454 0.0914 2.366 0.181 -0.0387 0.8496 8.244 -0.320 3.7562 0.0797 2.322 0.165 -0.0369 0.8828 7.972 -0.256 3.6699 0.0669 2.254 0.124 -0.0277 0.9143 7.718 -0.192 3.5860 0.0527 2.199 0.095 -0.0218 0.9443 7.479 -0.128 3.5040 0.0370 2.136 0.056 -0.0126 1.0000 7.043 0.000 3.3437 0.0000 2.035 0.000 0.0000 Form am ide(1)+2-p ropanol(2),T=298.15 K 0.0000 17.908 0.000 5.7845 0.0000 3.159 0.000 0.0000 0.0699 16.840 -0.286 5.6487 0.0461 3.156 0.086 -0.0179 0.1139 16.195 -0.437 5.5587 0.0708 3.155 0.142 -0.0297 0.1765 15.315 -0.617 5.4249 0.0999 3.129 0.197 -0.0417 0.2539 14.279 -0.785 5.2495 0.1260 3.100 0.266 -0.0576 0.2974 13.722 -0.855 5.1463 0.1361 3.065 0.288 -0.0632 0.3253 13.374 -0.891 5.0786 0.1409 3.046 0.304 -0.0673 0.3785 12.729 -0.941 4.9458 0.1466 3.004 0.331 -0.0746 0.4525 11.869 -0.971 4.7541 0.1476 2.925 0.347 -0.0807 0.5094 11.238 -0.965 4.6019 0.1436 2.840 0.335 -0.0804 0.5625 10.672 -0.937 4.4568 0.1366 2.757 0.320 -0.0793 0.6121 10.161 -0.893 4.3186 0.1275 2.676 0.303 -0.0774 0.6585 9.698 -0.835 4.1875 0.1173 2.580 0.266 -0.0705 0.6936 9.358 -0.782 4.0873 0.1085 2.514 0.245 -0.0668 0.7431 8.892 -0.694 3.9448 0.0948 2.407 0.201 -0.0572 0.7817 8.539 -0.614 3.8326 0.0831 2.322 0.166 -0.0488 0.8182 8.215 -0.530 3.7260 0.0714 2.229 0.120 -0.0368 0.8526 7.915 -0.444 3.6246 0.0597 2.155 0.090 -0.0286 0.8852 7.638 -0.356 3.5279 0.0479 2.096 0.073 -0.0237 0.9162 7.381 -0.267 3.4356 0.0361 2.035 0.051 -0.0171 0.9455 7.142 -0.178 3.3473 0.0242 1.975 0.030 -0.0102 1.0000 6.709 0.000 3.1813 0.0000 1.876 0.000 0.0000 Form am ide(1)+2-p ropanol(2),T=308.15 K 0.0000 18.979 0.000 6.1438 0.0000 3.728 0.000 0.0000 0.0551 17.831 -0.314 5.9932 0.0451 3.746 0.136 -0.0272 0.0902 17.139 -0.480 5.8947 0.0699 3.735 0.199 -0.0403 0.1731 16.197 -0.676 5.7494 0.0997 3.709 0.280 -0.0574

Tab le 4(continued)
Tab le 5 Experim ental excess mo lar com pressibility in binary mixtures
An analysis of previously reported VEand HEdata of form am ide(1)+alkano l(2)m ix tures has revealed that form am ide exists as dim er in propano l and as trim er in butanol and the mix ture form ation involves mixing of 1nwith 2nto estab lish 1n-2n.These 1n-2ncontacts between form am ide and alkanol w ou ld cause rup ture of in term olecular association in form am ide and alkanol to yield monom er.The monom ers of form am ide then in teract with alkanol to give 1-2 molecu laren tities.The change in com pressibility due tom ixing processcan be expressed[37-40]by

The derivation of Eq.(36)and assum ptions made therein are discussed elsewhere[37].calculation offrom Eq.(36)requires the know ledge of two unknow n in teraction parametersandThese parameters are calculated usingdata at two com positions(x1=0.4 and 0.5)for various(1+2)m ixturesand subsequently used to evaluateat other mole fraction(x1).Such values ofare recordedin Table 8 and parameters χ12andalong with previously reportedvalues[12]are recordedin Tab le 7.The calculated values ofare in good agreement with the experimental data(Fig.10),giving support to the assum ptions made in the derivation of Eq.(36).
5.Conclusions
Measured ultrasonic speeds of form am ide+p ropanol mixtures at 298.15 K and 308.15 K were correlated by Nom oto's,van Dael's mixing andim pedance dependence relations and analyzedin term s of Jacobson's free length theory and Schaaff's collision factor theory.For an equim o lar mix ture,excess mo lar com pressibility fo llow s the sequence of1-propanol>2-p ropanol.It is found that the interaction between form am ide and alkanol increasesw hen hyd roxyl group attached to a carbon atom has more-CH3groups.The excess isen tropic comp ressibility calculated by graph theo retical app roach is consisten t with the experimental values.The excess mo lar u ltrason ic speed andisen tropic com pressibility values are fitted to Red lich-Kister polynom ial equation and other properties such as mo lecu lar association,available volum e,free volum e,andin term olecu lar free length are also calculated.

Tab le 6 coefficient X(n)of Eq.(34)and standard deviations
Acknowledgments
Au tho rs thank M r.H.S.Chahal,Vice Chancello r,Deenbandhu Chho tu Ram Un iversity of Science&Techno logy,India for moral support.M an ju Rani and Sum an acknow ledge the Un iversity Gran t Comm ission,New Delh i for the aw ard of Teacher Fellow under Facu lty Im provement Program and Jun ior Research Fellow sh ip,respectively.

Fig.1.Ultrasonic speed of form am ide(1)+1-p ropanol(2)p redicted by various correlations.

Fig.2.Ultrasonic speed of form am ide(1)+2-p ropanol(2)predicted by various correlations.
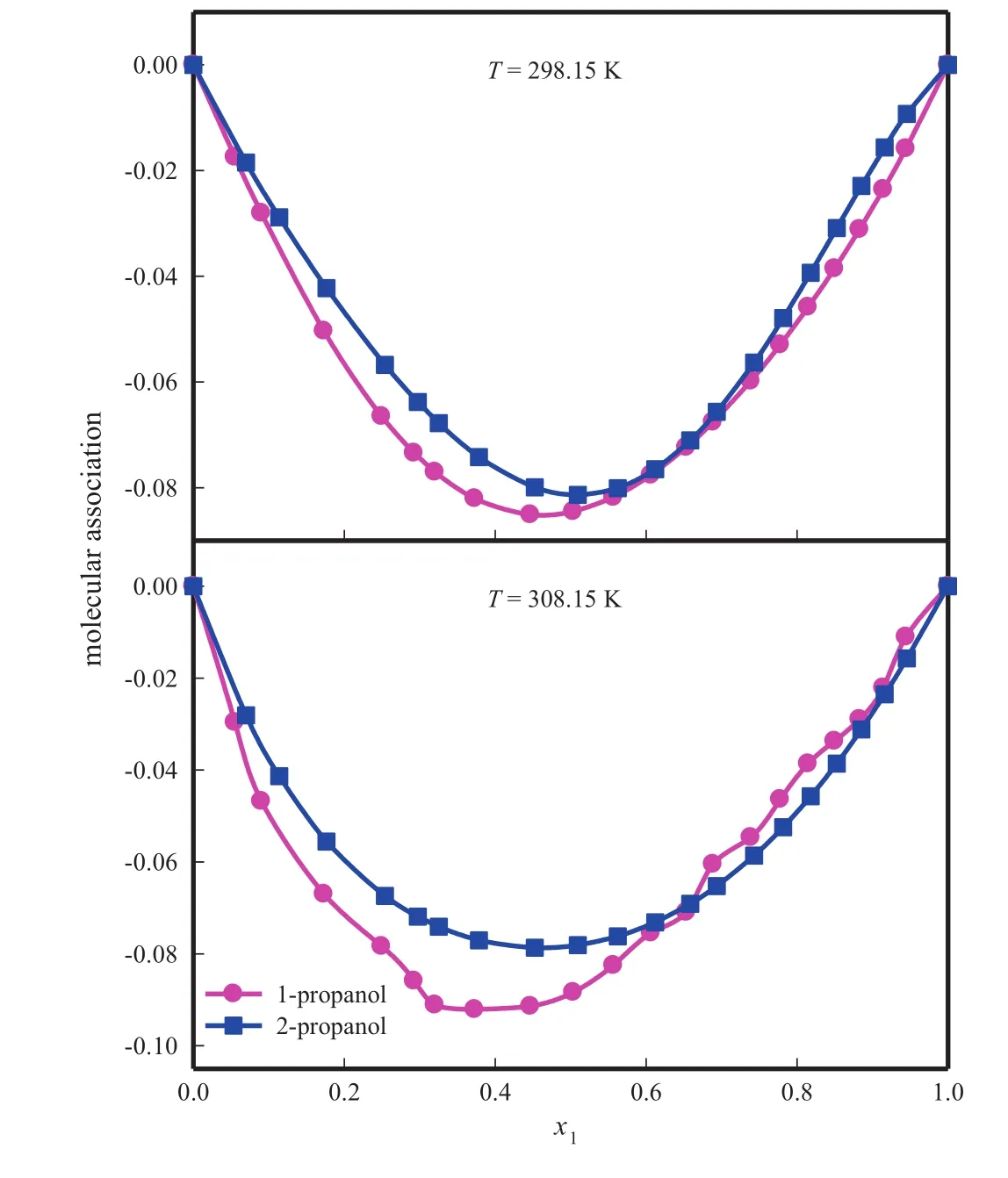
Fig.3.M olecu lar association of alkano l in binary mixture of form am ide(1)+alkano l(2).

Fig.4.Free volum e of binary mixtures of form am ide(1)+alkano l(2).

Fig.5.Excess free volum e of binary mixtures of form am ide(1)+alkano l(2).
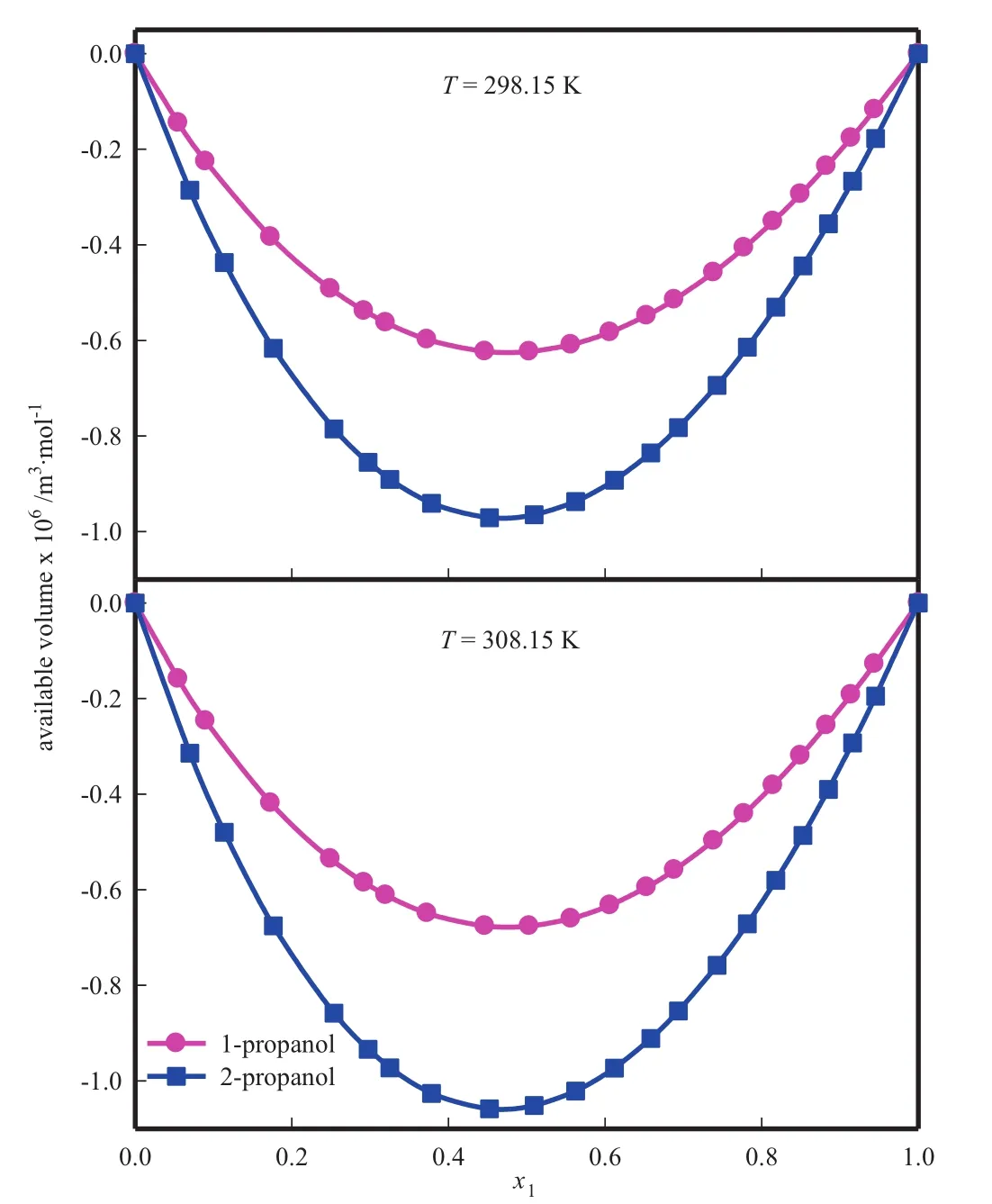
Fig.6.Availab le volum e of binary mixture of form am ide(1)+alkano l(2).

Fig.7.Excess interm olecu lar free length of form am ide(1)+alkanol(2).
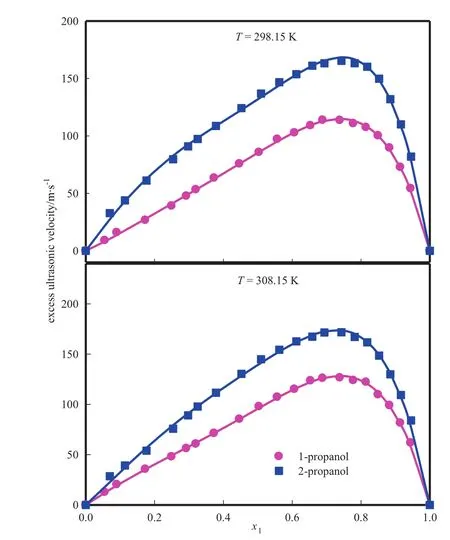
Fig.8.Excess u ltrasonic speed of form am ide(1)+alkano l(2).
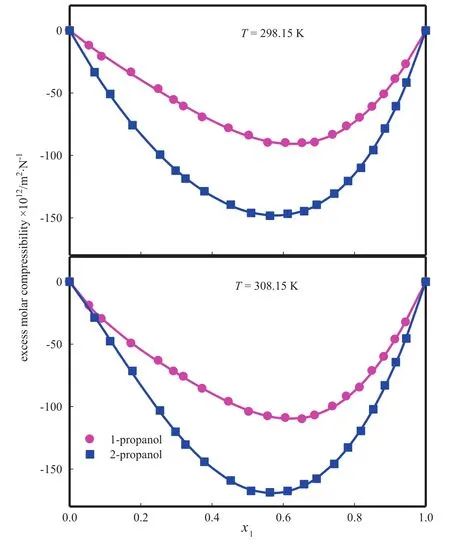
Fig.9.Values of excess isentropic com pressibility of form am ide(1)+alkanol(2).

Tab le 7 Values of third degree connectivity parameters andinteraction parameters usedin graph theoretical app roach
Tab le 8 comparison of experimental excess mo lar isen tropic com pressibilitywith values calculated from graph theoretical approach for binary mixtures

Tab le 8 comparison of experimental excess mo lar isen tropic com pressibilitywith values calculated from graph theoretical approach for binary mixtures
K s E× 1012/m 5·N-1·m ol-1 x1 Exp tl Graph Exptl Graph T=298.15 K T=308.15 K Form am ide(1)+1-p ropanol(2)0.1 -21.10 -16.13 -31.95 -22.21 0.2 -39.65 -35.60 -54.45 -46.64 0.3 -56.90 -55.29 -73.13 -70.08 0.4 -72.45 -72.45 -89.67 -89.67 0.5 -84.59 -84.59 -102.84 -102.84 0.6 -90.80 -89.51 -109.54 -107.32 0.7 -88.13 -85.22 -105.77 -101.04 0.8 -73.64 -69.93 -87.68 -82.15 0.9 -44.81 -42.01 -52.59 -48.98 Form am ide(1)+2-p ropanol(2)0.1 -46.11 -41.12 -40.70 -42.55 0.2 -83.34 -78.74 -83.08 -84.32 0.3 -112.19 -110.10 -120.70 -121.06 0.4 -132.89 -132.89 -149.22 -149.22 0.5 -145.18 -145.18 -165.83 -165.83 0.6 -148.01 -145.35 -168.62 -168.39 0.7 -139.23 -132.02 -155.96 -154.79 0.8 -115.37 -104.00 -125.87 -123.19 0.9 -71.31 -60.29 -75.40 -72.05

Fig.10.comparison of experimental excess isen tropic com pressibility(sym bo l)with values calculated from graph theory(line)for binary mixture of form am ide(1)+alkanol(2).
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
