Op tim ization of cop roduction of ethyl acetate and n-butyl acetate by reactive distillation☆
Hui Tian *,Suying Zhao ,Huidong Zheng ,Zhixian Huang
1 College of Chem istry and Chemical Engineering,Yantai University,Yantai 264005,China
2 College of Chem istry and Chemical Engineering,Fuzhou University,Fuzhou 350108,China
Keyw ords:Reactive distillation Ethyl acetate n-Bu tyl acetate Coproduction Simulation Op tim ization
ABSTRACT Based on a previous investigation,a sim ulation model wasused for optim ization ofcop roduction of ethyl acetate and n-buty lacetate by reactive distillation.An experimentalsetup wasestablished to verify thesim ulated results.The effects of various operating variables,such as ethanol feed location,acetic acid feed location,feed stage of reaction mixture of acetic acid and n-bu tanol,reflux ratio of ethyl acetate reactive distillation colum n,and distillate tofeed ratio of n-butyl acetate colum n,on the ethanol/n-butanol conversions,ethyl acetate/n-bu tyl acetate pu rity,and energy consum ption were investigated.The optim al results in the sim ulation study are as follow s:ethano l feed location,15th stage;acetic acid feed location,eighth stage;feed location of reactionm ix ture ofacetic acid and n-butanol,eigh th stage;reflux ratio of ethy l acetate reactive distillation co lum n,2.0;and distillate tofeed ratio of n-bu tyl acetate,0.6.
1.Introduction
Ethyl acetate(EtAc)and n-butyl acetate(n-BuAc)are comm on solven ts.They are widely usedin areas such as lacquering and coating,and many reports on their production have been pub lished[1-8].Hanika et al.[7]studied n-BuAc syn thesis experim entally using reactive distillation(RD)in a packed colum n and perform ed com puter sim ulations to evaluate the experimental data.three co lum ns were used to obtain high-purity(>99%)n-BuAc and the reactive distillation colum n had 50 theoretical stages.n-BuAc(>96%)was obtained using only one reactive distillation co lum n with 26 theoretical stages;a high-pu rity product(>99%)was obtained by sim ple separation from the raw product,significan tly reducing the energy used.Gangadw ala et al.[9]explored various reactive distillation configu rations for the production of n-BuAc to eliminate dibutyl ether byp roduct,bu t did not validate the model results experimentally.Jim enez and Costa-Lopez[10]used methyl acetate and n-butanol(n-BuOH)to produce n-BuAc and methanol;three colum ns were used to obtain high-purity n-BuAc and the reactive distillation colum n had 43 theoreticalstages.Calvarand Gonzalez[11]investigated the reaction kinetics for theesterification ofacetic acid(HAc)with ethano l(EtOH),catalyzed hom ogeneously by HAc and heterogeneously by Am berlyst 15.A packed-bed reactive distillation colum n filled with Am berlyst 15 was used to obtain EtAc.The influence of feed com position and reflux ratio was analyzed.Sm ejkal and Kolena[12]used a com plex two-co lum n system consisting of one reactive distillation co lum n(10 trays for reaction plus structured packing for separation)and a dow nstream stripper,with a decanter in between,to produce high-purity EtAc.
In industry,EtAc and n-BuAc are usually produced by the reaction of EtOH and n-BuOH with HAc,
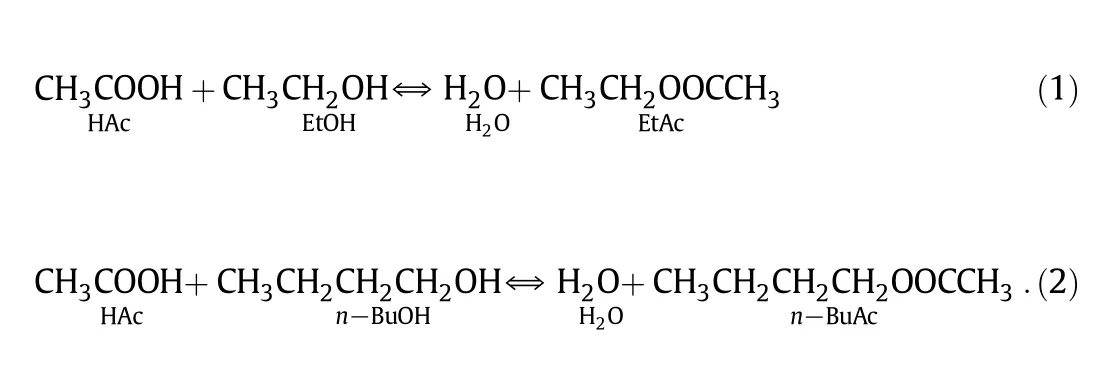
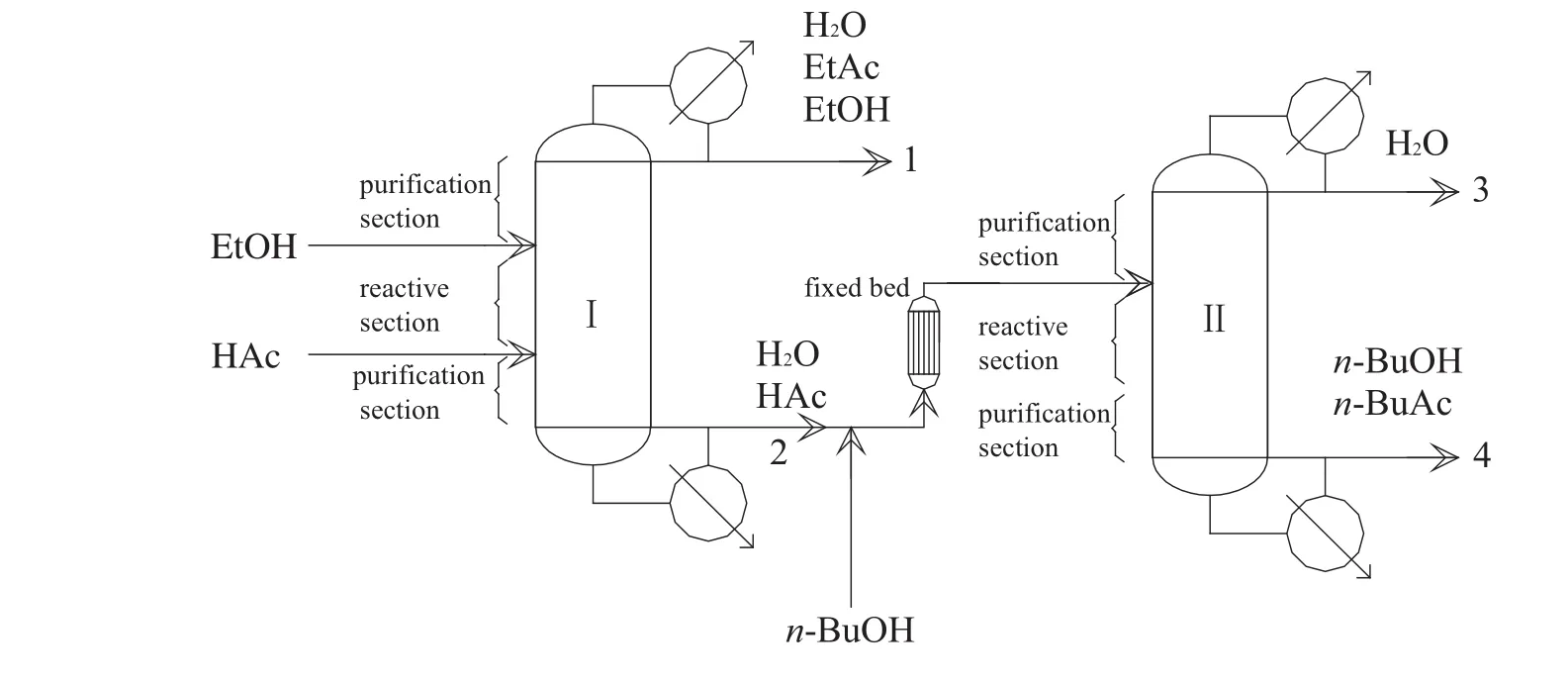
Fig.1.Flow diagram of EtAc and n-BuAc cop roduction.I:EtAc reactive distillation colum n;II:n-BuAc reactive distillation co lum n.
In our preliminary study[13],the feasibility of cop roduction of EtAc and n-BuAc by reactive distillation was investigated and the system as show n in Fig.1 was proposed.Reactive distillation colum n I was used to syn thesize EtAc.The overhead vapor products were azeotropic mixture of EtAc-H2O-EtOH,EtAc-H2O,and H2O-EtOH,while excess HAc,the high boiling-poin t product,was the bottom product.The bottom product in co lum n I was mixed with n-BuOH andin troduced to the fixed bed.The water form ed by the reaction and brought by HAc was removed from the top product and the organ ic phase was refluxed back to colum n II.EtAc and n-BuAc outpu ts were ad justed by changing the molar feed ratio of EtOH to n-BuOH in the coproduction procedu re,and the HAc pu rification co lum n usedin the separate production process was eliminated.At the feed mo lar ratio of EtOH to n-BuOH of 0.5,20.4%energy-saving was achieved com pared with the separate procedures.The total annual costs of the individual and cop roduction procedu res were calculated.The total cost of cop roduction is 3795912 USD per year less than that of separate processes,5190921 USD per year,for production of 10000 t of EtAc and 26363 t of n-BuAc.
Based on the preliminary study,w e perform experim ental study for cop roduction of EtAc and n-BuAc by reactive distillation to verify the model.Em phasis is on using the sim ulationm odel to analyze theeffects of key parameters such as EtOH feed location,HAc feed location,feed stage of reaction mix ture of HAc and n-BuOH,reflux ratio of EtAc reactive distillation colum n,and distillate tofeed ratio of n-BuAc colum n on conversions of EtOH/n-BuOH,EtAc/n-BuAc pu rity,and energy consum ption.
2.Experimental
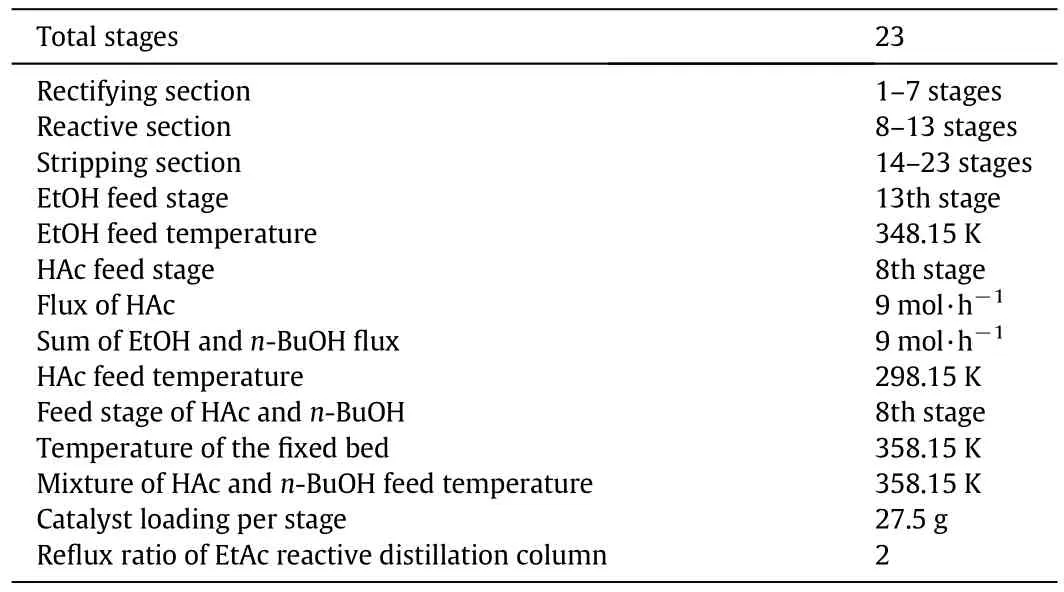
The cop roduction experimentalsetupisshow n in Fig.1.The reactive distillation colum ns I andiI had the sam e configurations,with inner diameters of 32 mm,a strong cation-exchange resin(Am berlyst 36W et)as the catalyst,and parameters given in Tab le 1.The temperature in the fixed-bed was main tained at 358.15 K.The effluent from thefixed-bed reactor,which alm ost ach ieved the chemical equilibrium,entered the reactive distillation colum n II above the reactive zone.Thereboiler of the reactive distillation colum n was heated using an electric heating stick and the heating power was measured directly.From the top of the co lum n to the reaction section was the rectifying section,and the stripping section was from the reboiler to the reaction section.The rectifying and stripping sections of the reactive distillation colum n were packed with bu lk θ packing(Ф 2.5 mm × 2.5 mm),and catalyst bund les[14,15]con taining cation-exchange resin were packedin the midd le of the colum n asshow n in Fig.2,which was the reaction section.The heigh ts of the rectifying,reaction,and stripping sections were 500,1050,and 650mm,respectively.To describe the separation efficiency of reactive distillation colum n quantitatively,the number of theoretical stages of reactive distillation co lum n was estimated,using the Fenske equation,by separating a mixture of EtOH andisop ropyl alcohol[16].The results indicated that the separation efficiencies of the rectifying,reaction,and stripping sections were equivalen t to seven,six,and nine theoretical stages,respectively.The theoretical stages were numbered from the top to the bottom in the co lum n.The temperature was measured at eigh t points in the reactive colum n while the sam ples were taken from the top,bottom and along the colum n(as show n in Fig.3).The relationship between sam pling and number of theoretical stages is show n in Table 2.The temperature was measured by therm ocouple and the vapor phase was extracted from the co lum n by a pipe and collected by a sm all vessel.

Tab le 1 Physical properties of Am berlyst 36W et

Fig.2.The schem atic diagram of catalyst bund le(1—sm all bag;2—ripple silk screen).

Fig.3.The sam pling and temperature measurement location in the reactive colum n.
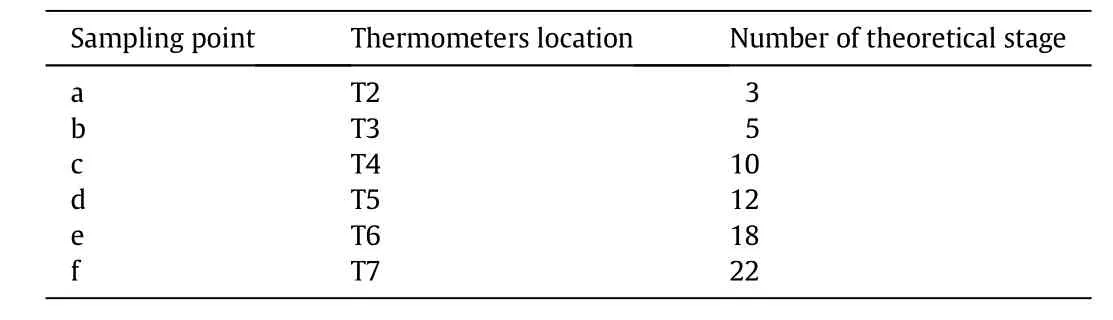
Tab le 2 The relationship of sam pling and therm ometer location with the number of theoretical stage
Experim ents were conducted to evaluate the cop roduction technology.The product purity and energy consum ption were determined.The temperature at eigh t poin ts along the colum n was recorded every half an hour and the com position of sam ple taken from the top and bottom of the reactive distillation colum n wasanalyzed.W hen the com position of sam ple taken from the top of the reactive distillation co lum n main tained constantatconsecutive 3 times,the system cou ld be considered to reach steady state.Sam ple from the reactive distillation colum n wascollected du ring thisperiod for analysis.Both titration and gas chrom atography were used for the analysis of reaction mixtures.The water in organic phase was measured by means of the Karl-Fischer instrum en t.The concentration of free HAc was determined through titrating with standard 0.1 mol·L-1sodium hyd roxide solution and phenolph thalein was used as the indicator.The gas ch rom atograph(VARIAN CP-3900,USA)equipped with a hyd rogen flam e detector was used to measure other organics.A CP-8907 colum n was used with nitrogen as the carrier gas at a flow rate of 1 ml·m in-1.In jector and detector temperatures were 523.15 K and 573.15 K,respectively.The heating power of the reboilerwasalsom easured.Detailsof the reactive distillation colum n specification are listedin Table 3.
Tab le 3 Specification of reactive distillation colum n
3.Model Validation
The simulation model was estab lishedin ou r preliminary study[13]for the sam e experimental setup.Reactions(1)and(2)catalyzed by Am berlyst 36W et ion-exchange resin were previously studied.A pseudo-hom ogeneous model was proposed for esterification[7,17]and the parameters were all obtained from Refs.[18,19].The equilibrium stage model has been successfully used for reactive distillation co lum n simulation[20-23],so a rigo rous equ ilib rium stage mode l con tain ing the mass balance,phase equilib rium,summ ation,and energy balance equations is used to describe the mu ltistage vapor-liquid separation in the reactive distillation.In the simulation,the un iversal quasi-chemical activity coefficient model is used for the n-BuAc system and the non-random twoliquid activity coefficient model is used for the EtAc system[9].The Hayden-O'Connell second virial coefficient model with association parameters is used to accoun t for the dim erization and trim erization of HAc in the vapor phase[24].
To checkw hether the model issuitable for sim ulating the cop roduction process,the model is used to predict the temperature distribution and vapor mass com position in the two reactive distillation co lum ns,and the predictions are com pared with the experimental data obtainedin this study.Fig.4 show s the experimental and simulation results for temperature and concentration profiles.The experim ental values are the average values of three experiments and the relative errors are less than 2.0%.The results of the numerical simulation agree very w ell with the experimental data,so the model describes the cop roduction process w ell.M eanwhile,the temperature variation trendin the EtAc reactive distillation co lum n is unusual.The temperature decreases as the stage number increases,because the reaction is an exothermic reaction and the reaction occurs at the top of the reactive zone mostly,where HAc has higher concentration.The temperature variation trendin the n-BuAc reactive distillation co lum n is norm al,because the feed molar ratio of n-BuOH and HAc is 1.0,and they are pre-reactedin thefixed bed.
4.Discussion
Som e im portant parameters could not be examined experim entally,because of the constrain ts and lim itations in the experimental setup.The im portant design variables consideredinclude EtOH and HAc feed locations,feed stage of reaction mix ture of HAc and n-BuOH,reflux ratio in the EtAc reactive distillation co lum n,and distillate tofeed ratio in the n-BuAc colum n.A series of analyses are conducted to determine theeffectsofdesign variableson the conversionsofEtOH/n-BuOH,EtAc/n-BuAc purity,and energy consum ption.The conditions for the base case simulation study are similar to the specification given in Tab le 3.From the base case,a series of sensitivity studies are perform ed to investigate the cop roduction.
4.1.Feed stage location of EtOH
The effects of EtOH feed location on the conversions of EtOH and n-BuOH,purities of EtAc and n-BuAc,heat duties of reboilers,and the total energy consum ption of the system are show n in Fig.5,with the feed rate of HAc at 9 mol·h-1,feed mo lar ratios of HAc to EtOH and n-BuOH being 1,feed mo lar ratio of EtOH to n-BuOH being 0.5,reflux ratio of 2.0 in the EtAc reactive distillation colum n,distillate tofeed ratio of 0.6 in the n-BuAc reactive distillation co lum n,feed location of HAc at the nin th stage,feed location of HAc and n-BuOH reaction mixture at the nin th stage,and other parameters as show n in Tab le 3.The EtOH and n-BuOH conversions present opposite trends with the increase of EtOH feed location,because the mo lar feed ratios of HAc to EtOH and n-BuOH are 1.W hen the EtOH conversion decreases,m ore HAc enters the n-BuAc reactive distillation colum n and the molar feed ratio of HAc to n-BuOH is higher,so that the conversion of n-BuOH increases.In con trast,w hen the EtOH conversion increases,lessHAc enters the n-BuAc reactive distillation colum n,so the n-BuOH conversion decreases.W hen the feed location of EtOH is at the 15th stage,EtAc and n-BuAc purities are the maxim um,while reboiler heat duties of EtAc and n-BuAc reactive distillation colum ns and the total energy consum ption are them inim um.The op tim alEtOH feed location is therefore the 15th stage.

Fig.4.Experimental and simulated profiles.

Fig.5.Effects of EtOH feed location on cop roduction.
4.2.Feed stage location of HAc
The effects of HAc feed location on the conversions of EtOH and n-BuOH,pu rities of EtAc and n-BuAc,reboiler heat du ties,and to tal energy consum ption are show n in Fig.6,with the feed rate of HAc at 9 mo l·h-1,feed mo lar ratios of HAc to EtOH and n-BuOH being 1,feed mo lar ratio of EtOH to n-BuOH being 0.5,reflux ratio of 2.0 in the EtAc reactive distillation co lum n,distillate tofeed ratio of 0.6 in the n-BuAc reactive distillation co lum n,feed location of EtOH at the 15th stage,feed location of HAc and n-BuOH mixture at the n in th stage,and o ther parameters as show n in Tab le 3.with the HAc feed location at the bottom of the rectifying section,EtOH and n-BuOH conversions are unchanged.with the HAc feed location in the reaction section,EtOH conversion decreases sign ifican tly.Th is is because w hen HAc is fedin to the reaction section,its residence time in th is section decreases and the length of reaction section also reduces.EtAc and n-BuAc pu rities are the max im um with the HAc feed location at the eigh th stage.The reboiler heat du ties of EtAc and n-BuAc reactive distillation co lum ns and the total energy consum ption,respectively,are the minim um w h ile feeding EtOH at the eigh th and nin th stages.The op tim al HAc feed location is therefore the eigh th stage.
4.3.Feed stage of reaction mixture of HAc and n-BuOH
The effects of feed location of HAc and n-BuOH reaction mix ture on EtOH and n-BuOH conversions,EtAc and n-BuAc purities,reboiler heat du ties,and to tal energy consum ption are show n in Fig.7,with HAc feed rate at 9 mo l·h-1,feed mo lar ratios of HAc to EtOH and n-BuOH being 1,feed molar ratio of EtOH to n-BuOH being 0.5,reflux ratio of 2.0 in the EtAc reactive distillation co lum n,distillate tofeed ratio of 0.6 in the n-BuAc reactive distillation colum n,EtOH feed location at the 15th stage,HAc feed location at the nin th stage,and other parameters as show n in Tab le 3.The EtAc reactive distillation colum n is unaffected by the feed location,so the EtOH conversion,EtAc product pu rity,and heat du ty of EtAc reactive distillation co lum n reboiler do not change.n-BuOH conversion and EtAc pu rity decrease sligh tly as the feed location of reaction mixture increases,because the residence time of HAc and n-BuOH in the reaction section decreases w hen the feed location of reaction mixture is in the reaction section.The total energy consum ption is the min im um with the feed location of reaction mixture at the eighth stage.The op tim al feed location of reaction mixture of HAc and n-BuOH is therefore at the eigh th stage.
4.4.Reflux ratio in EtAc reactive distillation column
The effects of reflux ratio in the EtAc reactive distillation colum n on EtOH and n-BuOH conversions,EtAc and n-BuAc purities,reboiler heat du ties,and total energy consum ption of the system are show n in Fig.8,with the HAc feed rate at 9 mo l·h-1,feed molar ratios of HAc to EtOH and n-BuOH being 1,feed mo lar ratio of EtOH to n-BuOH being 0.5,distillate tofeed ratio of 0.6 in the n-BuAc reactive distillation colum n,EtOH feed location at the 15th stage,HAc feed location at the nin th stage,feed location ofHAc and n-BuOHm ixtureat thenin th stage,and other parameters as show n in Tab le 3.The conversionsof EtOH and n-BuOH do not increase significan tly after the reflux ratio increases to 2.0,and neither do the EtAc and n-BuAc pu rities,while the reboiler heat du ty in the EtAc reactive distillation colum n increases.The op tim al reflux ratio in the EtAc reactive distillation colum n is therefore 2.0.

Fig.6.Effects of HAc feed location on cop roduction.
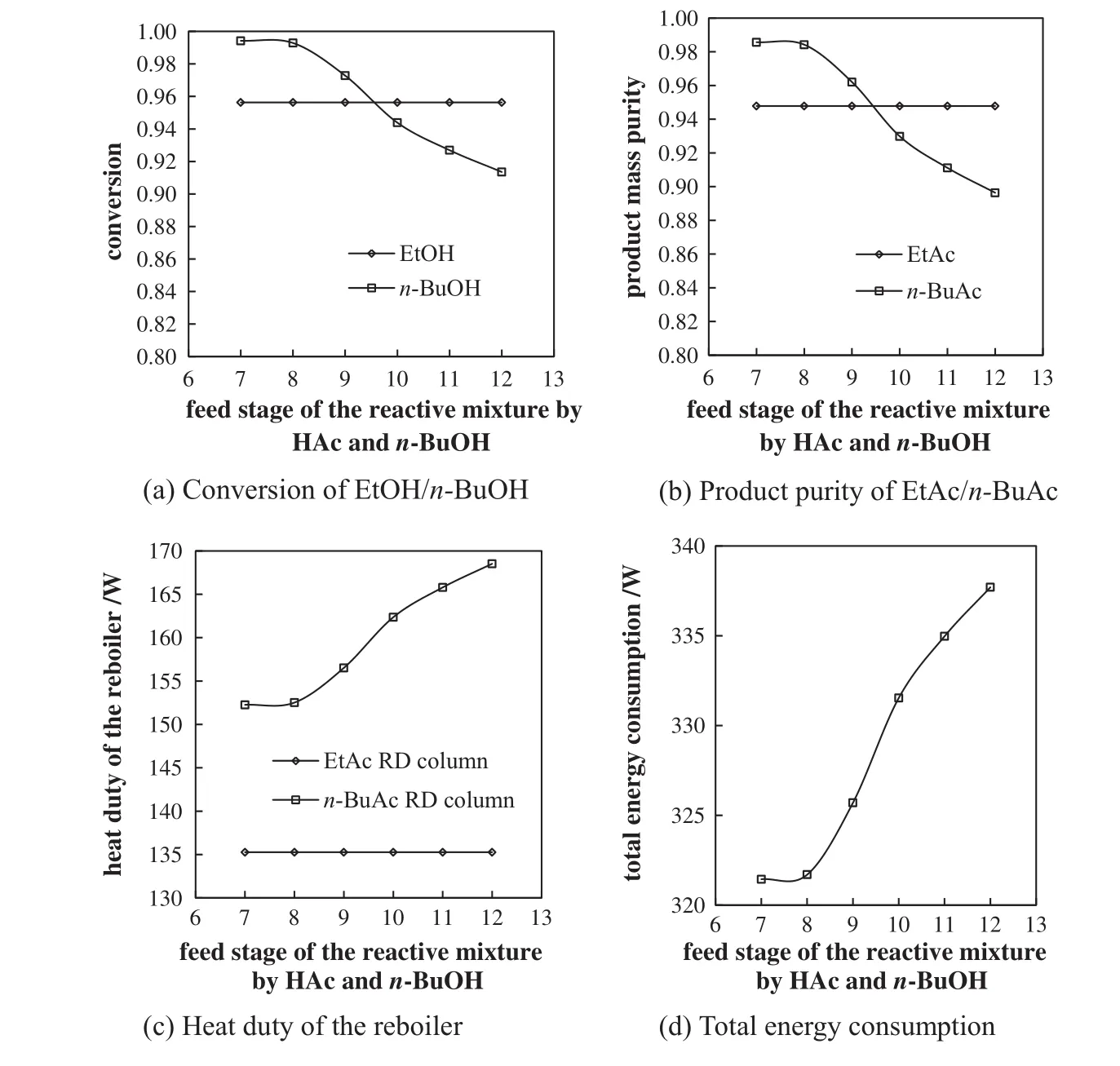
Fig.7.Effects of feed stage of reaction mixture of HAc and n-BuOH on cop roduction.

Fig.8.Effects of reflux ratio on the co-production in the EtAc reactive distillation colum n.
4.5.Distillate tofeed ratio in n-BuAc reactive distillation colum n
The effects of distillate tofeed ratio of n-BuAc reactive distillation colum n on EtOH and n-BuOH conversions,EtAc and n-BuAc purities,reboiler heat duties,and total energy consum ption are show n in Fig.9,with the HAc feed rate at 9 mol·h-1,feed molar ratios of HAc to EtOH and n-BuOH being 1,feed molar ratio of EtOH to n-BuOH being 0.5,reflux ratio of 2.0 in the EtAc reactive distillation colum n,EtOH feed location at the 15th stage,HAc feed location at the ninth stage,feed location of HAc and n-BuOH mixture at the ninth stage,and other parameters as show n in Table 3.The distillate tofeed ratio has little effect on the EtAc reactive distillation colum n.The conversion of n-BuOH and n-BuAc purity does not increase significan tly after the ratio reaches 0.6,while the reboiler heat du ty increases.The op tim al distillate tofeed ratio in the n-BuAc reactive distillation colum n is therefore 0.6.
4.6.The optim al results
The design variab les above are analyzed separately to obtain their op tim al values.How ever,com bination of separate op tim ization may not lead to a global op tim al so lution,so the simulation is made to see w hether the op tim al design variab les are the global op tim al so lution.In this simulation,HAc feed rate is 9 mo l·h-1,feed mo lar ratios of HAc to EtOH and n-BuOH are 1,feed mo lar ratio of EtOH to n-BuOH is 0.5,reflux ratio in the EtAc reactive distillation colum n is 2.0,EtOH feed location is at the 15th stage,HAc feed location is at the 8th stage,feed location of HAc and n-BuOH reaction mixture is at the 8th stage,distillate tofeed ratio in the n-BuAc reactive distillation co lum n is 0.6,and other parameters are show n in Tab le 3.The results are as follow s:conversions of EtOH and n-BuOH are 96.55%and 99.56%,respectively,EtAc and n-BuAc purities are 96.01%and 98.99%,respectively,and the heat duty of the system is 324.78 W.The results are better than those with the design variables op tim izedindividually,so the op tim al results are the global op tim al solution.
5.Conclusions
Based on the superior water-including ability of n-BuAc com pared with that of EtAc,a new procedu re for the cop roduction of EtAc and n-BuAc by reactive distillation was investigatedin ou r previous study,and the simulation model was used for op tim ization in this paper.Tw o reactive distillation colum ns with inner diameters of 32 mm,containing a solid catalyst(Am berlyst 36W et),were used to produce EtAc and n-BuAc.In the new procedure,EtAc and n-BuAc outputs were adjusted by changing them olar feed ratio ofEtOH and n-BuOH.The effects of operating variables such as EtOH feed location,HAc feed location,feed stage of reaction mixture of HAc and n-BuOH,reflux ratio in the EtAc reactive distillation colum n,and distillate tofeed ratio of n-BuAc colum n on EtOH/n-BuOH conversions,EtAc/n-BuAc purities,and energy consum ption were investigated.The op tim al results obtained by sim ulation are as follow s:EtOH feed location,15th stage;HAc feed location,eigh th stage;feed location of reaction mix ture of HAc and n-BuOH,eighth stage;reflux ratio of EtAc reactive distillation colum n,2.0;and distillate tofeed ratio of n-BuAc colum n,0.6.Under the op tim al condition,EtOH/n-BuOH conversions and EtAc/n-BuAc pu rities were all greater than 95%,and the energy consum ption decreased.

Fig.9.Effects of distillate tofeed ratio of n-BuAc reactive distillation colum n on coproduction.
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
