Adsorption of zinc onto anionic ion-exchange resin from cyanide barren solution☆
Yali Zhang ,Xian jin Yu ,*,Qinglong W ang ,Zhijun Jiang ,Tao Fang College of Chemical Engineering,Shandong University of Technology,Zibo 55049,China
2 Zibo Guoli New Pow er Source Technology Co.,Ltd.,Zibo,Shandong 255086,China
Keyw ords:Anion-exchange resin Adsorption isotherm s Adsorption kinetics
ABSTRACT Rem oval of zinc from cyanide barren solution isobligatory for its reuse in leach process.Batch experim ents were carried out to evaluate the adsorption capacity of resins under different experim ental conditions,including concentration,resin am oun t,initial pH,con tact time and temperature.More than 99%of adsorption was achieved under the op tim al condition.High adsorption rates on the resin were observed at the beginning and plateau values were obtainedin 60 min.The therm odynam ic parameters(free energy change ΔG,en thalpy change ΔS and en tropy change ΔH)for the adsorption were evaluated.The adsorption kinetic mechanism was studied with four models.The experimental results show that the adsorption fo llow s the Langm uir isotherm and the kinetics follow s the pseudo-second-order model.
1.Introduction
In metallu rgy operations,cyanidation is a predom inant method to extract gold from ores.Goldis dissolvedin aqueous cyanide solution,which is rich in precious metal[1].The precious metals are removed by zinc pow der precipitation.After separation,the rem aining “barren”solution is rich in cyanide and contains heavy metals such as copper,zinc and low levels of precious metals.Barren solutions are usually reusedin leach process or treated to destroy and/or remove cyanide and heavy metals[2].In the recycled barren solution,the concentration of heavy metalcyanide ishigh enough to causea deleterious effecton the leach ef ficiency.Therefore,theheavymetalionsm ustbe removed from the solution.
Various techniques have been u tilized to recovery cyanide and heavy metals such as adsorption[3-5],p recipitation with sulfuric acid[6],ion-exchange[7,8],so lven t-extraction[9],m em brane separation[10]and biological processes[11,12].How ever,these techniques excep t ion exchange have considerab le disadvan tages,including special requirement for expensive corrosion-resistan t equipm en t,high energy requirement and the difficu lty to implement in developing coun tries.The ion exchange method has been widely usedin industry for separation[13,14].Its main advantages are high chemical and mechanical stability,and high ion exchange capacity.It is hopefu l for the method to be more efficien t than other treatm en t methods.
The pu rpose of this work is to investigate som easpects for the use of strong alkali anion exchange resin D201×7.Effects of agitation time,pH,resin am oun t,and temperature are investigated at 25°C.The equilibrium and kinetic parameters are analyzed.
2.Experimental
2.1.Materials
The sam ples of cyanide barren solution were obtained from a plan t in Shandong Province in China.The concentration of total cyanide and Zn(CN)42-is 1625 mg·L-1and 636.5 mg·L-1,respectively.Ionexchange resin(IER)D201×7 wasused.The specificationsof this comm ercially availab le resin provided by DongDa Chemical Industry Company are given in Tab le 1.Its functional groupis-N(CH3)3,so it is strongly alkaline.This isclose to thepH valueofcyanidebarren solution.It can release ch lorine ions and anion exchange occu rs in the solu tion.The resin is suitab le for industrial implementation.Doub le distilled water was filtered through a mem brane filter and used through all the experiments.All chemicals were pu rchased as of analytical purity and used without further purification.Solutions of 0.01 mol·L-1NaOH and HCl were used for pH ad justm en t and to pretreat the resins.
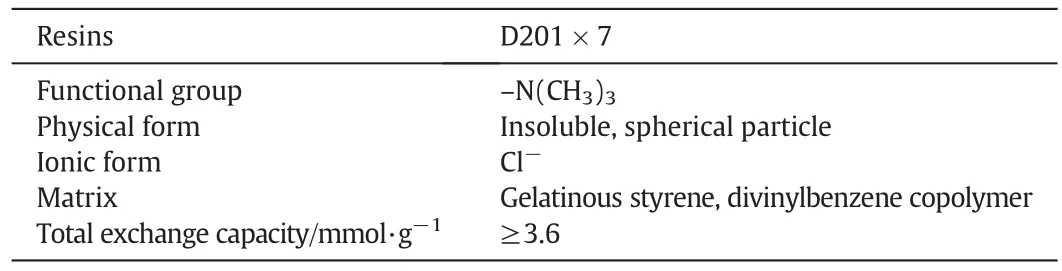
Tab le 1 Physical and chemical properties of D201×7
2.2.Experim ental procedure
An AA9000 flam e atom ic absorption spectrophotometer(China)was used for metal analysis.A PHS-3C pH meter was used to determine the pH of solutions.A therm ostatically con tro lled oil bath of±0.5 K accu racy was used for adsorption experiments.The resin was washed severaltimeswith doubly distilled water and treated with a certain volum e of 1 mol·L-1NaOH and 1 mol·L-1HCl solutions,then left for 24 h and washed with distilled water until the pH value was close to 7.The batch adsorption experiments were typically perform edin po lyethylene tubes.The pH was ad justed to the desired value by using a sm all am oun t of HCl or NaOH solution.The contents were shaken thorough ly using a shaker rotating at 400 r·m in-1.After a preset reaction period,the solution was filtered and the ion concentration was measured.The effects of con tact time for maxim um adsorption,ion dosage,pH of medium and temperature were investigated.The am oun t of ion adsorbed on IER was calculated from the difference between initial concentration and equilibrium one.The adsorption percent(%)is calculated as fo llow s

where C0is the initial concentration and Ceis the final concentration offilter liquor.All experimental data are the averages of duplicate determinations.The relative errors of the data are about 3%.
3.Results and Discussion
3.1.Effect of pH value
In order to explain the binding mechanism of zinc by ion exchange materials,it is necessary to understand the solu tion chem istry of zinc.ion is the main form in the solution in the experimental concentration range.Sm allam oun tsof Zn(OH)2andalsoform atdifferent pH values.The sites for ion exchange are due to the-N(CH3)3groups in the resin matrix.An anion-exchange resin will adsorb zincaccording tofollowing reactions.

Fig.1.Adsorption of zinc from cyanide barren solution to IER as a function of pH value at different temperatures.Resin am oun t:6 ml;concentration:636.5 mg·L-1;time:60 min.
The influence of initial pH on the adsorption was investigated by using a 180 ml cyanide barren solution and D201×7 with a dose of 6 ml in the w et state at room temperature.The effect of pH value on theadsorption ofzinc cyanide isshow n in Fig.1.Theadsorption changes sligh tly with the variation of pH value,which may suggest that the adsorption of zinc cyanide is main ly dom inated by ion exchange rather than surface com plexation[15].As pH increases from 9 to 10,the adsorption rate rem ains constant;at pH above 10,a little precipitate of Zn(OH)2appears,w h ich cannot be adsorbed,so that the adsorption rate of zinc decreases.As pH increases,Zn(OH)2transform s toThe adsorption ofon the resin is governed by hyd rogen bonding and the adsorption ofis less than that ofdue to the absence of hyd rogen bonding.
3.2.Effect of resin amount
Theam oun tof ion-exchange resin in the adsorption is critical for the application of resin in comm ercial processes.The effect of resin dose with different adsorption times is show n in Fig.2.The adsorption percentage increases with the resin am oun t,while ion exchange density decreases.M ore resin provides greater surface area,bu t for the sam e quan tity of target ions,som e of the resin rem ains unsaturated.It is a waste of resin,so determination of the am oun t of resin usedis needed.W hen the resin am ount ism ore than 6 ml,theadsorption changes little.with the increase of adsorption time,m ore target ions are adsorbed on the resin.

Fig.2.Adsorption ofzinc from cyanide barren so lution to IERasa function of resin am oun t for different adsorption times.concentration:636.5 mg·L-1;temperature:298 K;pH:10.
3.3.Effect of Zn(CN)4 2-concentration and adsorption isotherm s
Fig.3 show s the effect of initial concentration on the adsorption.As the initial am oun t ofincreases,the adsorption capacities ofincrease while the percentage of adsorption decreases.At the sam e resin content,the com petition am ongions increases with the concentration,decreasing adsorption percentage because of the electronic repu lsion.
For the adsorption of resin,the two most comm only usedisotherm s(Freund lich and Langm uir)are adopted.The Langm uir model can be rep resented as[16]

where Q0is the am oun t of adsorbate at com plete monolayer coverage(m g·g-1),w h ich gives the maxim um adsorption capacity of the sorbent,and b(L·m g-1)is the Langm uir isotherm constant related to the energy of adsorption.

Fig.3.Adsorption of zinc from cyanide barren solution to IER as a function of Zn(CN)4 2-concentration.temperature:298K;adsorption time:60m in;pH value:10;resin am ount:6 ml.
The Langm uir isotherm is show n in Fig.4.The Langm uir isotherm constan t is calculated to be 0.2335.The essential characteristics of the Langm uir isotherm can be expressedin term s of a dim ension less constant,separation factor or equilibrium parameter RL[17,18].


Fig.4.Langm uir isotherm of the resin.
The RLvalue is calculated to be between 0 and 1,which indicates favorable adsorption for all the initial concentrations.
The linear form of the Freund lich isotherm[19,20]is represented as

where qeis the am oun t of adsorption per unit mass of sorben t(m g·g-1),Ceis the equilibrium concentration ofin the solution(m g·L-1),kfis a measure of adsorption capacity,and 1/n is the adsorption in tensity.The Freund lich isotherm is show n in Fig.5.The Freund lich isotherm constants are calculated and presentedin Table 2.The values of correlation coefficient R2indicate that the Langm uir model is more app rop riate than the Freund lich model,indicating monolayer coverage on the resin.The resin has a lim ited adsorption capacity,so the adsorption is better described by the Langm uir model.
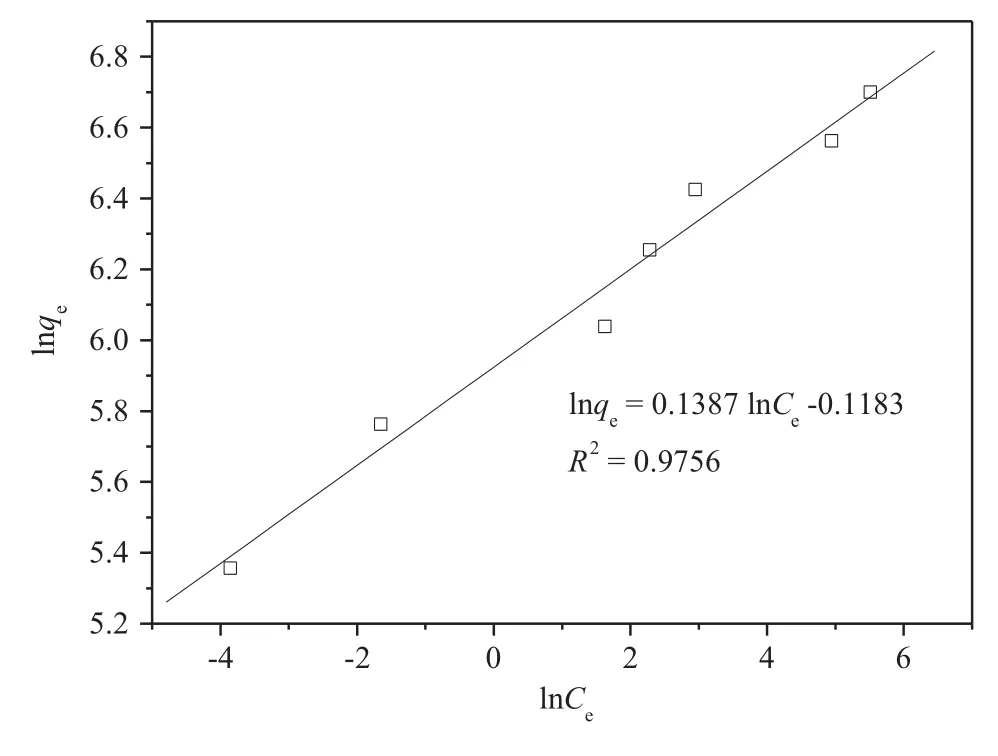
Fig.5.Freund lich isotherm of the resin.

Tab le 2 Parameters in Langm uir and Freund lich isotherm s
3.4.Effect of temperature and thermodynam ic study
The effect of temperature on the adsorption of Zn(CN)42-by D201×7 is show n in Fig.6.Higher temperature is advan tageous for Zn(CN)42-adsorption onto the resin,yet the rem oval rate increased very slightly.And at 298 K the rem oval rate is satisfactory,so an app ropriate adsorption temperature shou ld be room temperature(298 K),considering the implementation in the factory.

Fig.6.Adsorption ofzinc from cyanide barren solution to IER as a function of temperature.Adsorption time:60 min;pH value:10;resin am oun t:6 ml;concentration:636.5 mg·L-1.
Therm odynam ic parameters associated with the adsorption are calculated by[21]


where ΔG is the free energy of adsorption(kJ·m ol-1),T is the temperature in Kelvin,R is the universalgasconstant(8.314 J·m ol-1·K-1),ΔH is the heat of adsorption(kJ·m ol-1),and ΔS is the standard entropy change(kJ·m ol-1).The values of ΔH and ΔS can be obtained from the slope andin tercep t of a plot of ln Kcagainst 1/T[22].
The calculated therm odynam icparametersare show n in Table3 and Fig.7.ΔG values are negative at all temperatures used,confirm ing the spon taneousnatureofsorption.The valueofΔG becom esm orenegative as temperature increases,indicating more efficien t adsorption at higher temperature.The positive values of ΔS suggest thatis not restrictedin the resin.The positive value of ΔH confirm s the endothermic nature of the adsorption process.The low er values of ΔH suggest that the adsorption of cyanide on the resins is governed by hyd rogen bonding.
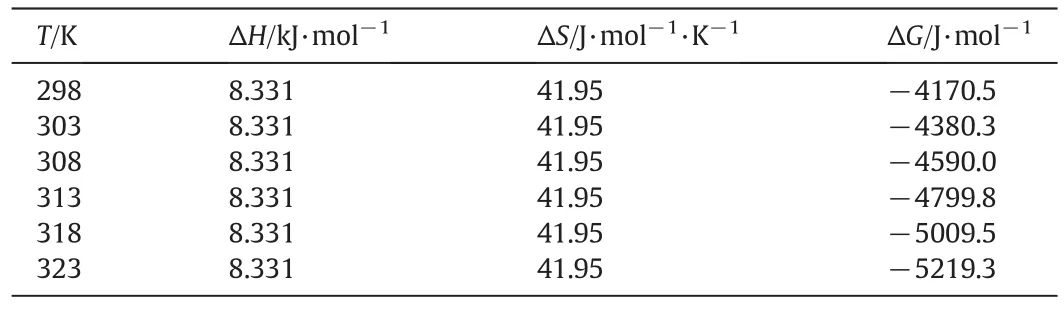
Tab le 3 Values of therm odynam ic parameters for the adsorption of zinc cyanide

Fig.7.Plot of ln K c against 1/T.
3.5.Effect of adsorption time and kinetic model of adsorption
Fig.8 show s the effect of contact time on the adsorption ofby the resin.The adsorption rate increases with time and reaches 95%at 20 min.High adsorption rates ofare observed at the onset and then it rem ains constant after 60 min.
Experim ents were also directed to understand the kinetics ofrem oval by resin D201×7.The adsorption offrom aqueous solution to the resin may be considered as a reversible adsorption for a single species on a heterogeneous surface.Kinetics of adsorption is one of the im portan t characteristics defining the ef ficiency.The adsorption offrom liquid to solid phase may be expressed as[23].

Fig.8.Adsorption of zinc from cyanide barren solution to IER as a function of adsorption time.Concentration:636.5 mg·L-1;temperature:298 K;pH:10;resin am oun t:6 ml.
The first-order reversib le model is used[24,25]

where k is the overall rate constan t.
According to the work of Nam asivayam and Kardivelu[26],a first order rate expression is

where qtis theam ountadsorbed on the surfaceofsorbent resin at time t(m g·g-1)and kadis the equilibrium rate constant of first-order adsorption(m in-1).The straigh t-line plotsof lg(qe-qt)against t under different experim ental conditions give the values of rate constants.
Ho and M cKay[27,28]p resented a pseudo-second order rate expression,relating the rate to the adsorption capacity.The pseudo-second order model describes the adsorption kinetics as

where k is the pseudo-second-order rate constan t(g·m g-1·m in-1).The initial adsorption rate is h=,where the values of qe(1/slope),k(slope2/intercept),and h(1/intercept)can be obtained by plotting t/qtagainst t.
The pore diffusion model refers to the theory proposed by W eber and Morris[29].W hen the rate lim iting stepis the diffusion in particles,w e can use the intraparticle diffusion equation[29,30]

where kiis the in traparticle diffusion coefficient(m g·g-1·m in-1).The slope of the plot of qtagainst t1/2gives the value of the in traparticle diffusion coefficient.
The kinetic adsorption data are correlated with the first-order reversib le model,first order model,pseudo-second-order model,andin traparticle diffusion model.Fig.9 show s the plots of the left-hand side of Eqs.(7)-(10)against t or t1/2.The results are listedin Tab le 4.From the values of R2,the pseudo-second-order model fits the experimental data best am ong the four models,so the adsorption mechanism is due to chem isorptions[31],which is in accordance with the conclusion in Subsection 3.1.The adsorption capacity indicates that 100%of Zn(CN)42-can be adsorbed to resin D201×7 after a con tact time of 60 min at an initial concentration of 636.5 mg·L-1at pH 10 and a resin dose of 6m l.Thus resin D201×7 isan efficientm aterial for the rem oval of Zn(CN)42-from aqueous solu tions.

Fig.9.Plots with different kinetic models.(a—first-order reversible model;b—first-order model;c—pseudo-second-order model;d—pore diffusion model.)

Tab le 4 Constants for adsorption kinetics of zinc cyanide to resins using different adsorption models
3.6.SEM-EDS analysis of resins before and after adsorption
A typicalim ageof resin D201×7 obtained by SEM,with EDSanalysis of selected particles,is presentedin Fig.10,with a relatively large am oun t of ch lorine detected.The SEM/EDS analysis suggests that the resin is of chlorine type.
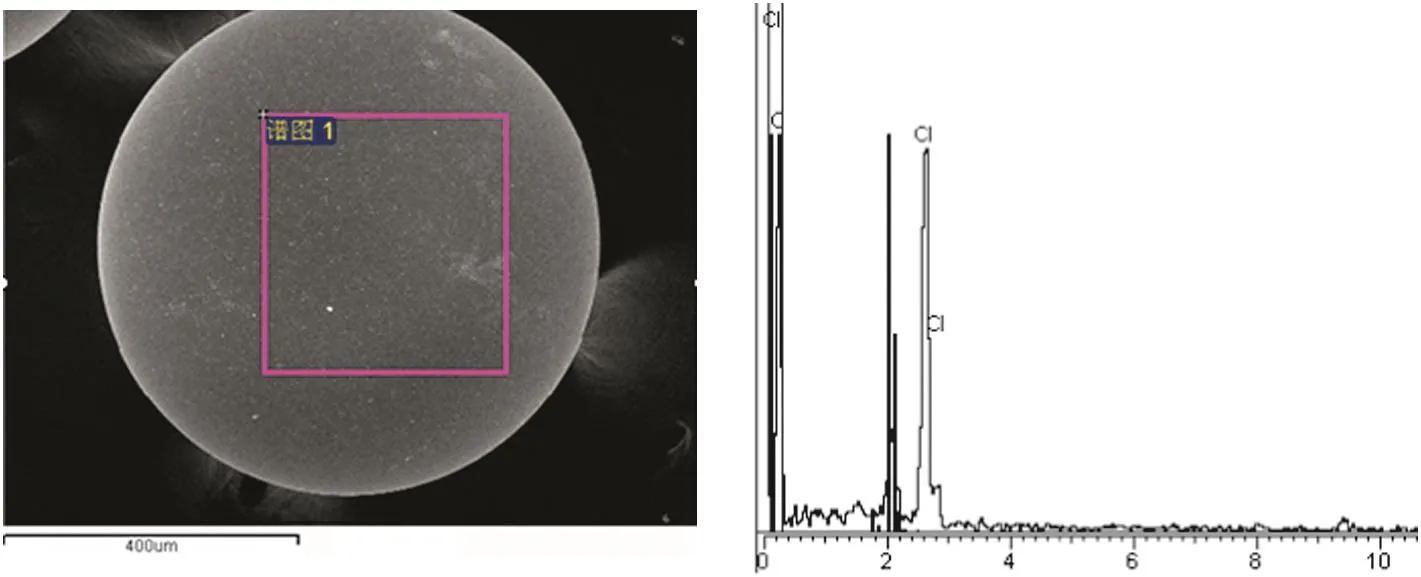
Fig.10.SEM/EDS pattern of resin before adsorption.

Fig.11.SEM/EDS pattern of resin after adsorption.
Fig.11 show s the resin after adsorption for 60m in at298 Kwith 6m l resin analyzed by SEM/EDS.The spherical resin is bigger com pared to that presentedin Fig.10.Chorine element was no longer detected by the EDS,and the peaks of the elements such as zinc and nitrogen becam e clearer,indicating that chorine ion was substituted by Zn(CN)42-.The resin after adsorption is excellen t without dam age,suggesting that the resin has a large exchange capacity.
4.Conclusions
This work assesses the performance of anion-exchange resin D201×7 in absorbing zinc from cyanide barren solution.The experim ental results indicate that a high adsorption is at a pH value of 9-10.The rate for adsorption was rapidin the first 30 min and equilibrium attainedin 60 min at room temperature.M ore than 99%adsorption was achieved under op tim al conditions.The equilibrium data agree w ell with the Langm uir isotherm.The kinetics of adsorption fo llow s the pseudo-second-order model.The anion exchange resin is thus an app rop riate sorben t for the treatm en t of cyanide barren solution.
 Chinese Journal of Chemical Engineering2015年4期
Chinese Journal of Chemical Engineering2015年4期
- Chinese Journal of Chemical Engineering的其它文章
- Accurate level set method for simulations of liquid atom ization☆
- Heat transfer augmentation in a circular tube with winglet vortex generators☆
- Influence of im peller diameter on local gas dispersion properties in a sparged mu lti-im peller stirred tank☆
- Pow er dem and and mixing performance of coaxial mixers in a stirred tank with CMC solution
- CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator☆
- Em u lsion liquid mem brane for selective extraction of Bi(III)
