Degradation of acephate using combined ultrasonic and ozonation method
Bin Wng,Chng-ping Zhu,*,Run-hng Gong,Jin Zhu,Bo Hung,Fei Xu,b,Qing-gong Ren,Qing-bng Hn,Zhen-bing He
Degradation of acephate using combined ultrasonic and ozonation method
Bin Wanga,Chang-ping Zhua,*,Run-hang Gonga,Jin Zhua,Bo Huanga,Fei Xua,b,Qing-gong Renc,Qing-bang Hana,Zhen-bing Hed
aChangzhou Key Laboratory of Sensor Networks and Environmental Sensing,Hohai University,Changzhou 213022,PR China
bNanjing Institute of Electronic Technology,Nanjing 210039,PR China
cCollege of Petrochemical Engineering,Changzhou University,Changzhou 213164,PR China
dChangzhou Xinlinian Ultrasonic Equipment Co.,Ltd.,Changzhou 213104,PR China
Available online 17 August 2015
Abstract
The degradation of acephate in aqueous solutions was investigated with the ultrasonic and ozonation methods,as well as a combination of both.An experimental facility was designed and operation parameters such as the ultrasonic power,temperature,and gas flow rate were strictly controlled at constant levels.The frequency of the ultrasonic wave was 160 kHz.The ultraviolet-visible(UV-Vis)spectroscopic and Raman spectroscopic techniques were used in the experiment.The UV-Vis spectroscopic results show that ultrasonication and ozonation have a synergistic effect in the combined system.The degradation efficiency of acephate increases from 60.6%to 87.6%after the solution is irradiated by a 160 kHz ultrasonic wave for 60 min in the ozonation process,and it is higher with the combined method than the sum of the separated ultrasonic and ozonation methods.Raman spectra studies show that degradation via the combined ultrasonic/ozonation method is more thorough than photocatalysis.The oxidability of nitrogen atoms is promoted under ultrasonic waves.Changes of the inorganic ions and degradation pathway during the degradation process were investigated in this study.Most final products are innocuous to the environment.
©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Acephate;Ultrasonic wave;Ozonation;Degradation pathway
1.Introduction
Acephate is a low-toxicity pesticide.Due to its wide applicability,pesticide wastewaterdischarge may cause poisoning in humans.Many studies have investigated effective methods for degrading acephate,most of which involve the biodegradation method,photocatalytic decomposition,and electrolyzed water treatment(Phugare et al.,2012;Hao et al.,2011;Han et al.,2009).In addition,there are other mainstream pesticide wastewater degradation methods called advanced oxidation processes(AOPs).In these methods,the oxidant breaks down organic compounds by dissolving them in water(Chen et al.,2013).These processes have attracted great interest in the pesticide wastewater treatment field.
Ozonation is an AOP,and ozone(O3)is widely used in wastewater pretreatment as a strong oxidant.It generates a single atom of oxygen(O)and a hydroxyl radical(·OH)with a strong oxidation capacity,which can decompose the organic compounds in water instantly(Esplugas et al.,2002;Yang et al.,2012).Because the oxidability of hydroxyl radicals is as strong as fluorine,O3water can not only break the acephate's carbon chain in the molecular structure,but also oxidize the nitro or amino group and completely change themolecular structure of organics.Thus,the toxicity of pesticides can be degraded(Li et al.,2012;Xu et al.,2002). Moreover,excess O3can be safely decomposed into oxygen,and most products can be discharged into the environment directly,because they are water-soluble.The degradation efficiency of pesticides during ozonation increases when the ultrasonic wave is involved(Naddeo et al.,2009).First,the degradation process is feasible when the ultrasonic wave is used alone.Instantaneous negative pressure is generated in liquid when there is sufficient ultrasonic power.When the medium molecular spacing exceeds the critical molecular spacing,cavitation bubbles are formed.At the moment that bubbles explode,local high-temperature and high-pressure environments are produced.The water molecules crack and become strong oxidants such as·OH,HO2,and·O(Shriwas and Gogate,2011;Matouq et al.,2008;Xiong et al.,2012;Golash and Gogate,2012).Zhang et al.(2010)investigated degradation behavior of ultrasonic waves,and found that productsof malathion and chlorpyrifos in applejuice increased significantly with ultrasonic treatment.The maximum degradation efficiencies were achieved for malathion(41.7%)and chlorpyrifos(82.0%)after ultrasonic treatment at 500 W for 120 min.Second,when the ultrasonic and ozonation methods are combined,the ultrasonic wave breaks O3into micro-bubbles and improves the solubility of O3in solution.Meanwhile,micro-bubbles enhance the intensity of ultrasonic cavitation(Sivakumar and Pandit,2001;Wang et al.,2006).An appropriate frequency value of ultrasonic wave can be chosen to create a sonochemical reaction,which provides its maximum yield according to the distribution of bubbles in liquid(Zhu et al.,2011).A Gaussian-shape distribution of gas bubble radii is to be expected in water:

where N(R)is the number of bubbles with a radius of R in a unit volume of liquid;R0is the bubbles'most probable value of radii in a certain volume of liquid,and also the center value of the distribution curve;δ is the scale factor of this Gaussian function;and A is a coefficient.N(R)is at its highest value when R=R0.The frequency effect of the low-frequency ultrasound using the electrical detection method was investigated by Huang et al.(1995).The experimental data showed that the optimum frequency should be about 159.54 kHz and the bubbles'most probable value of radii was 17.88 μm.
It is difficult to use the traditional treatment methods to effectively solve the secondary pollution problem,especially in chemical water treatment.Sonochemical degradation is a purely physical wastewater treatment method,and O3is much safer compared to other chemical reagents.The ultrasonic method combined with ozonation(the combined ultrasonic/ ozonation method)is still a recommended method in the wastewater treatment field.In the past few years,many studies(Liu et al.,2008;Naddeo et al.,2009;Krishnamoorthya et al.,2013)have been performed using the ultrasonic method to degrade different organic compounds,but investigation of acephate degradation using the sonochemical method has rarely been carried out.This study examined the performance of the combined ultrasonic/ozonation method in degrading the acephate solution.We observed the variation of degradation efficiency with time using only the ultrasonic method,only the ozonation method,and the combined ultrasonic/ozonation method under a 160 kHz ultrasonic wave,in order to find the relationship between the promotion effect and the degradation efficiency throughout the processes.The products of inorganic ions were measured separately for comparison with the results presented in Han et al.(2009)using photocatalytic decomposition.
2.Materials and methods
2.1.Pesticide solution
The raw material of the pesticide solution was the waterdispersible granules of acephate,with a purity of 97%,which is produced by India United Phosphide Co.,Ltd.The granules were dissolved in water,with a concentration of 100 mg/L.The solution was stored in a brown glass bottle and the pH value was stable at 7.9 after the solution became motionless.
2.2.Experimental setup
The experimental facility of the combined ultrasonic/ozonation process is illustrated in Fig.1.The reactor was a cylindrical steel structure with a diameter of 90 mm,a height of 120 mm,and a maximum capacity of 500 mL.An ultrasonic transducerwithafrequencyof160kHz wasgluedtothebottom of the reactor,with a maximum power of 50 W.The lateral wall of the reactor was filled with cooling water in order to keep the solution temperature at(25±1)°C.An ultrasonic wave generator(developed by the authors)was used to drive the 160 kHz ultrasonic transducer.An air-fed ozonator(YL-G3500,from Beijing Yilang Technology Co.,Ltd.,in China)was used,with an O3amount of 3 500 mg/h.The gas was transmitted into the reactor via a porous diffuser and the gas flow rate was maintained by a rotameter(LZB-3WB,from Changzhou Ruiming Instrument Co.,Ltd.,in China)at 2 L/min.An ultravioletvisible(UV-Vis)spectrophotometer(T6S,from Beijing Persee Instrument Co.,Ltd.,in China)was used to detect thedegradation effect.A Raman spectrometer(R-3000-532,from Ocean Optics Company,in the USA),a high-performance liquid chromatograph(5000 A,from Varian Technology Company,in the USA),and a gas chromatography-mass spectrometer(GC-MS)system(HP6890GC-5972MSD,withanHP-5columnof0.25mm×0.1μm×30m)wereusedtodetermine quantitative and qualitative data,and identify degradation products.

Fig.1.Schematic diagram of combined ultrasonic/ozonation process.
2.3.Treatment of pesticide wastewater
Different experiments using the ultrasonic,ozonation,and combined ultrasonic/ozonation methods were performed to examinethedegradationofacephateinanaqueoussolution.An acephate solution of 400 mL was added into the reactor.The worktimeoftheultrasonicgeneratorandozonatorwerebothset at 60 min,and they were started at the same time in the combined process.The gas flow rate was limited to 2 L/min.All of the experiments were carried out at a temperature of(25±1)°C. In general,it was not necessary to control the pH value in practical wastewater treatment.The initial pH value of the solution was 7.0.In the experiment using only the ultrasonic method,the electro-discharge function of the ozonator was disabled in order to generate air only.Samples taken every 10 min were detected with the UV-Vis spectrophotometer and Raman spectrometer,in order to obtain the absorbance curve and Raman spectrogram respectively.
2.4.Analytical methods

Fig.2.Spectrum curve of untreated acephate solution.
In the three groups of experiments(ultrasonic,ozonation,and combined ultrasonic/ozonation),we strictly controlled the environmental conditions,such as the ultrasonic power,gas flow rate,and environmental temperature.The UV-Vis spectrophotometer was used to determine the degradation effect. The spectrum curve of untreated acephate solution with a concentration of 100 mg/L is shown in Fig.2.There are two peaks in the curve.One is at a wavelength of 198 nm,and the corresponding absorbance value is 1.621.The other is at a wavelength of 212 nm,and the corresponding absorbance value is 0.395.We observed and investigated the variation of the absorbance value at 198 nm with the solution concentration.It should be noted that both acephate and its intermediate products during degradation had peaks at 198 nm.The declines of the absorbance value at 198 nm not only meant a decrease of the acephate concentration,but also the decomposition of intermediate products.Variations of the absorbance value indicated the complete degradation of the mixed solution.Mixed gas was added into the solution continuously throughout the process.The gas dissolved in water,and new chemical substances were introduced,so curves of new substances were stacked on the curve of acephate.This leads to the problem of the absorbance value at 198 nm rising when the solution curves of every 10 min were directly contrasted with one another.In order to observe the degradation of the mixed solution,we treated the same volume of deionized water under the same condition.The processed water was also scanned as a base line every 10 min.There was a linear relationship between the solution concentration and absorbance value(Mirmohseni and Houjaghan,2013),so the degradation efficiency of the mixed solution was calculated using the absorbance value.High-performance liquid chromatography(HPLC)analyses utilized a Spectra 100 UV-Vis detector and a stainless ODS C18 column(with an inside diameter of 4.6 mm and a height of 250 mm).The mobile phase was a mixture of acetonitrile and water(with a volume ratio of 95:5).The eluent was delivered at a rate of 1 mL/min and the wavelength of detection was 220 nm.The injection volume was 10 μL. The concentrations of acephate during the process were measured directly with HPLC.
A surface-enhanced Raman spectroscope(SERS)was used to detect products and determine quantitative and qualitative data.It was operated at a wavelength of 532 nm and at a low power level of 20 mW in order to avoid any heating effect due to laser irradiation.The SERS substrates,Q-SERS™ substrates,were obtained from Nanova Inc.(Columbia,MO,USA).Q-SERS™ substrates are gold-based nanostructures fabricated on a silicon wafer.A volume of 0.5 μL of the solution from the previous step was dropped on the surface of the substrate using a micropipette.The laser beam was focused on the sample surface with an optical microscope.The spectrum included peak characteristics for all degradation products(Liu et al.,2013).The products of acephate destruction in the aqueous solutions were detected with the GC-MS system with electron impacts.The chromatographic temperature program for GC-MS was as follows:it started at 60°C,and increased to 280°C at a gradient of 10°C/min.The temperature of the injector and transfer line were set to 200°C and 250°C,respectively.
3.Results and discussion
3.1.Degradation efficiency
All of the experimental data were the average values over four repetitions of the same experiments.This was meant to reduce the error of results.The experimental conditions for the reaction were as follows:the volume of solution was 400 mL,the concentration of acephate in the solution was 100 mg/L,the ultrasonic frequency was 160 kHz,the ultrasonic power was 50 W,the gas flow rate was 2 L/min,the O3amount was3 500 mg/h,and the reaction temperature was(25±1)°C.For the three groups of experiments(with ultrasonic,ozonation,and combined ultrasonic/ozonation methods),the variation tendency of the degradation efficiency over 80 min is shown in Fig.3.The experimental results show that all of these methods had degradation effects.The degradation with only the ultrasonic method was slowest,the degradation with only the ozonation method was faster,and the degradation with the combined ultrasonic/ozonation method was fastest.After 60 min of processing,the degradation efficiencies tended to be stable.As Fig.3 shows,22.9%of the acephate was removed with only the ultrasonic method,60.6%with only the ozonation method,and 87.6%with the combined ultrasonic/ozonation method.
3.2.Promotion effect of ultrasonic method
The ultrasonic method played a significant role in promoting the degradation when the ultrasonic and ozonation methods were combined.The degradation efficiency of the combined method was 27%greater than the degradation efficiency when only the ozonation method was used after 60 min.The promotion effect was stronger than the sum of the two separate methods.This means that the degradation of the combined method was not a simple superposition of the two separate methods.The ultrasonic wave in the combined process played a role in promoting the degradation,and increased the degradation efficiency.It broke O3into micro-bubbles and increased the contact area of O3with water.The solubility of O3in the solution was improved(Song et al.,2007;Zhang et al.,2007).
The degradation efficiency every 10 min is shown in Fig.4. With a constant ultrasonic power and frequency,the degradation efficiency was almost stable in the experiment using only the ultrasonic method during the first 60 min.In the experiment using the combined ultrasonic/ozonation method,O3played the main role in the degradation reaction.This led to the degradation efficiencies using only the ozonation method and the combined method having the same variation trend.The effect of promotion of ultrasonication in the combined method was twice the effect of the ultrasonic method after 10 min.Cavitation bubbles breaking down could cause an extremely high temperature(5 000 K)and high pressure(50 000 kPa)environment in the local area.This extreme physical environment accelerated the reaction speed greatly,and interrupted the chemical bond of acephate.The acephate lost its toxicity and became innocuous inorganic matter.
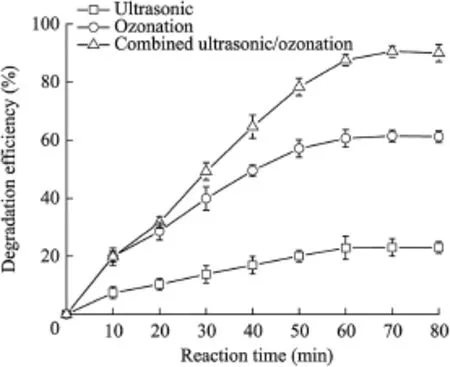
Fig.3.Variation of degradation efficiency with reaction time.

Fig.4.Degradation efficiency every 10 min.
3.3.Identification of degradation products
The SERS spectra of degraded acephate solution after 60 min of treatment are shown in Fig.5.The peaks in spectra reflected different kinds of ions dissolved in water.Characteristic peaks were exhibited of(at 892 cm-1),(at 957 cm-1),(at 1 066 cm-1),and(at 1 100 cm-1). The intensities at Raman shift values of 613 cm-1(-OCH3),670 cm-1(CH3CONH-)and 1 308 cm-1(-SCH3)decreased when the concentration of acephate decreased.All of these peaks were correctly identified by experiments before they were used for quantification analysis in the experiments.The concentrations of each ion were detected by SERS and GCMS and are shown in Fig.6.The initial concentration of acephate was 0.52 mmol/L.

Fig.5.SERS spectra of degraded acephate solution.

Fig.6.Changes of concentrations of inorganic ions during degradation process at initial acephate concentration of 0.52 mmol/L.
According to the measurement results,changes of the intensity of each SERS spectra peak are listed in Table 1.The degradation pathway is shown in Fig.7.The degradation products of acephate were divided into three classes:primary products(such as CH3O(CH3S)P(O)NH2and CH3COOH),intermediate products(such as CH3O(CH3S)P(O)OH,CH3O(HO)P(O)OH,and CH3S(O)2SCH3),and final products(such asand H3PO4).The CH3CONH-(at 670 cm-1)concentration of acephate quickly decreased in the first 20 min.This indicated that the C-N bond in acephate was broken by the·OH radicals first under the condition of local extremely high temperature and high pressure.One of the primary products,methamidophos(CH3O(CH3S)P(O)NH2),was a kind of deadly pesticide and usually cannot be degraded easily in typical ways,so the degradation should be complete,as far as possible.The other primary product was acetic acid,which was rapidly hydrolyzed to CO2and H2O.After 20 min,the increasing speed of(at 892 cm-1)increased.The N-P bond in the methamidophos was broken by a hydrolysis reaction and transformed into CH3O(CH3S)P(O)OH and some ionic groups.CH3O(CH3S)P(O)OH waseasilyoxidized into CH3O(HO)P(O)OH and CH3S(O)2SCH3,and CH3S(O)2SCH3was oxidized by O3.Finally,these two products broke down into,CO2,and H3PO4.All of the main final products were low-toxicity or nontoxic and easily dissolved in water. Han et al.(2009)presented an experimental investigation of photocatalytic decomposition of acephate in irradiated TiO2suspensions.It can be noted that the acephate degradation pathway created by photocatalysis is similar to the pathway created by the combined ultrasonic/ozonation method.However,variations of the concentrations of inorganic ions are much different.In the study of Han et al.(2009),only 3%of the nitrogen atoms and 2%of the phosphorus atoms were transformed into inorganic ions,indicating that the oxidation of nitrogen and phosphorus atoms was inhibited.In contrast,Fig.6 shows that the concentration ofincreases gradually,but the concentration ofwas much greater than that of.The formation ofwas due to the further oxidation of methamidophos.Almost all of the nitrogen atoms were transformed intoin 60 min.Thus,the oxidation of nitrogen atoms was not inhibited.This result implies that the acephate degradation by the combined ultrasonic/ozonation method was sufficiently complete and methamidophos was destroyed during the process.The concentration ofwas always higher than that of.This indicates that the C-O bond in intermediate products was more susceptible to being broken than the C-S bond under ultrasonic irradiation.In addition,CO2from air was added to the solution continuously and was the main reason for the continuous rise of.In addition,the CO2in final products made only a small contribution.

Table 1Changes of intensity of each SERS spectra peak.
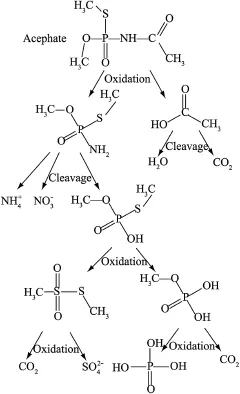
Fig.7.Degradation pathway of acephate.
4.Conclusions
The degradation of acephate through a combined ultrasonic/ozonation method was measured under various conditions,and compared to degradation of acephate through only the ultrasonic and ozonation methods.The degradation was slowest with only the ultrasonic method,faster with only the ozonation method,and fastest with the combined ultrasonic/ ozonation method.The degradation efficiency of acephate increases from 60.6%to 87.6%after the solution is irradiated by a 160 kHz ultrasonic wave for 60 min in the ozonation process.The role of the 160 kHz ultrasonic wave included both stacking and promoting.One of the primary products in degradation,methamidophos,is a highly toxic substance. Compared with photocatalysis,the degradation of acephatewith the combined method was complete enough that methamidophos was decomposed thoroughly,and most final products were innocuous to the environment.The synergy of a 160 kHz ultrasonic wave and O3was significant,and the combined method is worth further study as an effective way to improve degradation efficiency in the pesticide wastewater treatment field.
Acknowledgements
We gratefully acknowledge Professor Cai-hua Ni and Dr. Bing-yan Chen for their technical support and also Wen Wen,Ying Gao,and Cheng Yin for their advice and discussion.
References
Chen,J.Y.,Lin,Y.J.,Kuo,W.C.,2013.Pesticide residue removal from vegetables by ozonation.J.Food Eng.114(3),404-411.http://dx.doi.org/ 10.1016/j.jfoodeng.2012.08.033.
Esplugas,S.,Gimenez,J.,Contreras,S.,Pascual,E.,Rodriguez,M.,2002. Comparison of different advanced oxidation processes for phenol degradation.Water Res.36(4),1034-1042.http://dx.doi.org/10.1016/S0043-1354(01)00301-3.
Golash,N.,Gogate,P.R.,2012.Degradation of dichlorvos containing wastewaters using sonochemicalreactors.Ultrason.Sonochem.19(5),1051-1060.http://dx.doi.org/10.1016/j.ultsonch.2012.02.011.
Hao,J.,Wuyundalai Liu,H.J.,Chen,T.P.,Zhou,Y.X.,Su,Y.C.,Li,L.T.,2011. Reduction of pesticide residues on fresh vegetables with electrolyzed water treatment.J.Food Sci.76(4),C520-C524.http://dx.doi.org/10.1111/ j.1750-3841.2011.02154.x.
Han,S.T.,Li,J.,Xi,X.L.,2009.Photocatalytic decomposition of acephate in irradiated TiO2suspensions.J.Hazard.Mater.163(2-3),1165-1172. http://dx.doi.org/10.1016/j.jhazmat.2008.07.077.
Huang,J.L.,Feng,R.,Zhu,C.P.,Chen,Z.H.,1995.Low-MHz frequency effect on a sonochemical reaction determined by an electrical method.Ultrason. Sonochem.2(2),S93-S97.http://dx.doi.org/10.1016/1350-4177(95)00024-Z.
Krishnamoorthya,K.,Kima,G.S.,Kim,S.J.,2013.Graphene nanosheets: Ultrasound assisted synthesis and characterization.Ultrason.Sonochem. 20(2),644-649.http://dx.doi.org/10.1016/j.ultsonch.2012.09.007.
Li,L.,Xie,M.N.,Liang,Y.M.,He,Y.Q.,Chan,G.Y.S.,Luan,T.G.,2012. Degradation of cypermethrin,malathion and dichlorovos in water and on tea leaves with O3/UV/TiO2treatment.Food Control 28(2),374-379. http://dx.doi.org/10.1016/j.foodcont.2012.05.009.
Liu,Y.N.,Jin,D.,Lu,X.P.,Han,P.F.,2008.Study on degradation of dimethoate solution in ultrasonic airlift loop reactor.Ultrason.Sonochem. 15(5),755-760.http://dx.doi.org/10.1016/j.ultsonch.2007.12.004.
Liu,B.,Zhou,P.,Liu,X.M.,Sun,X.,Li,H.,Lin,M.S.,2013.Detection of pesticides in fruits by surface-enhanced Raman spectroscopy coupled with gold nanostructures.Food Bioprocess Technol.6(3),710-718.http:// dx.doi.org/10.1007/s11947-011-0774-5.
Matouq,M.A.,Al-Anber,Z.A.,Aljbour,T.S.,Al-Shannag,M.,2008.Degradation of dissolved diazinon pesticide in water using the high frequency of ultrasound wave.Ultrason.Sonochem.15(5),869-874.http://dx.doi.org/ 10.1016/j.ultsonch.2007.10.012.
Mirmohseni,A.,Houjaghan,M.R.,2013.Measurement of the pesticide methomyl by modified quartz crystal nanobalance with molecularly imprinted polymer.J.Environ.Sci.Health,Part B:Pestic.Food Contam.Agric. Wastes 48(4),278-284.http://dx.doi.org/10.1080/03601234.2013.743779.
Naddeo,V.,Belgiorno,V.,Ricco,D.,Kassinos,D.,2009.Degradation of diclofenac during sonolysis,ozonation and their simultaneous application. Ultrason.Sonochem.16(6), 790-794.http://dx.doi.org/10.1016/ j.ultsonch.2009.03.003.
Phugare,S.S.,Gaikwad,Y.B.,Jadhav,J.P.,2012.Biodegradation of acephate using a developed bacterial consortium and toxicological analysis using earthworms(Lumbricus terrestris)as a model animal.Int.Biodeterior. Biodegrad.69,1-9.http://dx.doi.org/10.1016/j.ibiod.2011.11.013.
Shriwas,A.K.,Gogate,P.R.,2011.Ultrasonic degradation of methyl Parathion in aqueous solutions:Intensification using additives and scale up aspects. Sep.Purif.Technol.79(1),1-7.http://dx.doi.org/10.1016/j.seppur.2011. 02.034.
Sivakumar,M.,Pandit,A.B.,2001.Ultrasound enhanced degradation of Rhodamine B:Optimization with power density.Ultrason.Sonochem. 8(3),233-240.http://dx.doi.org/10.1016/S1350-4177(01)00082-7.
Song,S.,He,Z.Q.,Chen,J.M.,2007.US/O3combination degradation of aniline in aqueous solution.Ultrason.Sonochem.14(1),84-88.http:// dx.doi.org/10.1016/j.ultsonch.2005.11.010.
Wang,S.,Huang,B.B.,Wang,Y.S.,Liao,L.,2006.Comparison of enhancement of pentachlorophenol sonolysis at 20 kHz by dual-frequency sonication.Ultrason.Sonochem.13(6),506-510.http://dx.doi.org/10.1016/ j.ultsonch.2005.10.004.
Xiong,S.F.,Yin,Z.L.,Yuan,Z.F.,Yana,W.B.,Yang,W.Y.,Liu,J.J.,Zhang,F.,2012.Dual-frequency(20/40 kHz)ultrasonic assisted photocatalysis for degradation of methylene blue effluent:Synergistic effect and kinetic study.Ultrason.Sonochem.19(4),756-761.http://dx.doi.org/10.1016/ j.ultsonch.2012.01.007.
Xu,P.,Janex,M.L.,Savoye,P.,Cockx,A.,Lazarova,V.,2002.Wastewater disinfection by ozone:Main parameters for process design.Water Res. 36(4),1043-1055.http://dx.doi.org/10.1016/S0043-1354(01)00298-6.
Yang,D.M.,Wang,B.,Ren,H.Y.,Yuan,J.M.,2012.Effects and mechanism of ozonation for degradation of sodium acetate in aqueous solution.Water Sci. Eng.5(2),155-163.http://dx.doi.org/10.3882/j.issn.1674-2370.2012.02.004.
Zhang,H.,Duan,L.J.,Zhang,D.B.,2007.Absorption kinetics of ozone in water with ultrasonic radiation.Ultrason.Sonochem.14(5),552-556. http://dx.doi.org/10.1016/j.ultsonch.20i06.09.005.
Zhang,Y.Y.,Xiao,Z.Y.,Chen,F.,Ge,Y.Q.,Wu,J.H.,Hu,X.S.,2010. Degradation behavior and products of malathion and chlorpyrifos spiked in apple juice by ultrasonic treatment.Ultrason.Sonochem.17(1),72-77. http://dx.doi.org/10.1016/j.ultsonch.2009.06.003.
Zhu,C.P.,Huang,B.,Han,Q.B.,Ni,C.H.,Zhu,G.J.,Liu,M.H.,Shan,M.L.,2011.Frequency effect on p-nitrophenol degradation under conditions of strict acoustic and electric control.Water Sci.Eng.4(1),74-82.http:// dx.doi.org/10.3882/j.issn.1674-2370.2011.01.007.
7 July 2014;accepted 20 March 2015
This work was supported by the National Natural Science Foundation of China(Grants No.11274092,11274091,and 11304026),the Jiangsu Graduate Education Reform Research and Practice Project in 2009(Grant No.22),and the Fundamental Research Fund for the Central Universities(Grant No. 14B10128).
*Corresponding author.
E-mail address:cpzhu5126081@163.com(Chang-ping Zhu).
Peer review under responsibility of Hohai University.
http://dx.doi.org/10.1016/j.wse.2015.03.002
1674-2370/©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Water Science and Engineering2015年3期
Water Science and Engineering2015年3期
- Water Science and Engineering的其它文章
- Uniqueness,scale,and resolution issues in groundwater model parameter identification
- Flash flood hazard mapping:A pilot case study in Xiapu River Basin,China
- Variable fuzzy assessment of water use efficiency and benefits in irrigation district
- Effects of thermodynamics parameters on mass transfer of volatile pollutants at air-water interface
- Analysis of soluble chemical transfer from soil to surface runoff and incomplete mixing parameter identification
- Adsorption of Cr(VI)in wastewater using magnetic multi-wall carbon nanotubes
