Analysis of soluble chemical transfer from soil to surface runoff and incomplete mixing parameter identification
Ju-xiu Tong*,Jin-zhong Yng,Bill X.Hu
Analysis of soluble chemical transfer from soil to surface runoff and incomplete mixing parameter identification
Ju-xiu Tonga,b,c,*,Jin-zhong Yanga,Bill X.Hub,c
aState Key Laboratory of Water Resources and Hydropower Engineering Science,Wuhan University,Wuhan 430072,PR China
bKey Laboratory of Groundwater Cycle and Environment Evolution,Ministry of Education,China University of Geosciences,Beijing 100083,PR China
cSchool of Water Resources and Environment,China University of Geosciences,Beijing 100083,PR China
Available online 17 August 2015
Abstract
A two-layer mathematical model proposed by Tong et al.(2010)was used to predict soluble chemical transfer from soil into surface runoff with ponded water on the soil surface.Infiltration-related incomplete mixing parameter γ and runoff-related incomplete mixing parameter α in the analytical solution of the Tong et al.(2010)model were assumed to be constant.In this study,different laboratory experimental data of soluble chemical concentration in surface runoff from initially unsaturated and saturated soils were used to identify the variables γ and α based on the analytical solution of the model.The values of γ and α without occurrence of surface runoff were constant and equal to their values at the moment when the surface runoff started.It was determined from the results that γ decreases with the increase of the ponded water depth,and when the initial volumetric water content is closer to the saturated water content,there is less variation of parameter γ after the occurrence of surface runoff.As infiltration increases,the soluble chemical concentration in surface runoff decreases.The values of parameter α range from 0 to 1 for the fine loam and sand under the controlled infiltration conditions,while it can increase to a very large value,greater than 1,for the sand under the restrained infiltration conditions,and the analytical solution of the model is not valid for experimental soil without any infiltration if α is expected to be less than or equal to 1.The soluble chemical concentrations predicted from the model with variable incomplete mixing parameters γ and α are more accurate than those from the model with constant γ and α values.
©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Surface runoff;Incomplete mixing parameter;Analytical solution;Soluble chemical
1.Introduction
Chemical transfer from soil to surface runoff during rainfall has become a serious agronomic and environmental problem(Wang et al.,1999;Tian et al.,2011;Yu et al.,2011). Agronomists focus on the loss of soil productivity,while environmental scientists focus on the deterioration of water quality(Wang et al.,2014).Therefore,it is necessary to study the chemical transfer from soil to surface runoff and identify the important factors helping to reduce chemical loss in surface runoff and subsequent pollution.
One of the most popular theories used in the study of soluble chemical transfer from soil to surface runoff is the mixing zone(or mixing layer)theory(Ahuja et al.,1981b).The mixing zone theory assumes that there is a region below the soil surface where the soil solution,surface water,and infiltrating water mix instantaneously;that the soil below will not supply chemicals to that region;and that the mixing zone depth is constant.The theory has been applied to experimentalsoil with ponded water on the soil surface by Gao et al.(2004). However,Zhang et al.(1997)have found that the mixing zone depth in the model for predicting soluble chemical transfer from soil into surface runoff is much less than in reality. Therefore,some researchers have developed an incomplete mixing theory(Ahuja and Lehman,1983).Wang et al.(1999)have applied the incomplete mixing theory in the northern part of China without ponded water.The process of ponded water increasing prior to the occurrence of surface runoff has not been studied,although ponded water is very common in the southern part of China.
Using the incomplete mixing theory,Tong et al.(2010)established a two-layer model to predict the concentration of soluble chemicals,which come from soil,in surface runoff,considering an increase in ponded water.They derived an analytical solution under the assumption that the incomplete mixing parameters related to surface runoff and infiltration are constant throughout the rainfall process,and applied their model to initially unsaturated and saturated soils.Their experimental and modeled results clearly showed differences between the incomplete mixing parameters of the two soils. However,they did not analyze the experimental and modeled results with variable incomplete mixing parameters,perhaps leading to an inaccurate prediction of the soluble chemical concentration in surface runoff.
Therefore,the main objective of this study was to analyze soluble chemical transfer from soil to surface runoff and to find a way to identify and analyze the incomplete mixing parameters for the two-layer model of Tong et al.(2010),which will help provide more accurate practical predictions of the soluble chemical concentration in surface runoff from soil in the future.The mathematical model for predicting soluble chemical concentration in surface runoff from soil and the method of determining incomplete mixing parameters after surface runoff occurs is presented.An experimental study was performed of soluble chemical transfer from soil to surface runoff and the identified incomplete mixing parameters were analyzed.The results are described below.
2.Mathematical model and identification method for incomplete mixing parameters
2.1.Mathematical model and its analytical solution
The simple two-layer model from Tong et al.(2010)was used in this study(Fig.1).The upper layer,called the whole mixing layer,includes the ponding-runoff zone and the soil mixing zone(Ahuja et al.,1981b).The lower layer is the underlying soil layer.In accordance with the assumptions of Govindaraju et al.(1996)and Ahuja et al.(1981a),chemicals in the soil mixing zone are the only source of chemicals for runoff and infiltrated water,and the chemicals are only considered to be transported vertically(Steenhuis and Walter,1980).The chemicals in the soil mixing zone can move to the underlying soil layer with the infiltrated water.Meanwhile,the chemicals in the underlying soil layer can move to the soil mixing zone through the mass diffusion process because the chemical concentration in the underlying soil layer is higher than that in the mixing soil zone.The net chemical flux from the soil mixing zone to the underlying soil layer is expressed as iγCw,where i is the soil infiltration rate,γ is the infiltrationrelated incomplete mixing parameter,and Cwis the chemical concentration in the soil mixing zone.Here,it should be noted that Cwis a function of time because the soil infiltration rate changes with different rainfall periods,which will be shown in the following section.

Fig.1.Sketch of two-layer model.
In order to describe the incomplete solute mixing in the ponding-runoff zone,a runoff-related incomplete mixing parameter α is introduced.The chemical concentration in the ponding-runoff zone is αCw.In order to simplify the complicated chemical transport process near the soil surface,it is assumed that the chemical concentration is uniform in the ponding-runoff and soil mixing zones.
The mass of soluble chemicals in the whole mixing layer is written as

where Mwis the mass of soluble chemicals per unit area in the water phase(μg/cm2),hpis the depth of ponded water on the soil surface(cm),hmixis the soil mixing zone depth(cm),θsis the saturated volumetric water content in the soil mixing zone,and t is time(min).
If the chemical concentration in the rainfall is assumed to be zero,the following equation can be obtained based on mass conservation:

where q is the specific discharge rate of the surface flow(cm/min).
Eqs.(1)and(2)can create a mass conservation model in the kinetic and static conditions in the whole mixing layer.The rainfall event is divided into four different periods,including the periods from the beginning of rainfall to the occurrence of ponded water on the soil surface,from the occurrence of ponded water to the generation of runoff,from the generation of runoff to the formation of steady runoff,and from the formation of steady runoff to the end of rainfall.The solutions to Eqs.(1)and(2)in the four periods can be obtained.
During the period from the beginning of rainfall to the occurrence of ponded water,the infiltration rate is equal to therainfall intensity p(cm/min)for initially saturated and unsaturated experimental soils,soil solute in the mixing zone is leached by the infiltrated water,and Eq.(2)can be solved as follows:

where C0is the initial saturated chemical concentration(mg/L),and tsais the time when soil is saturated in the soil mixing zone(min).
During the period from the occurrence of ponded water to the generation of runoff,the depth of ponded water at time t(min)is hp(t)=(p-i1p)(t-tp),where i1pis the average soil infiltration rate during this period(cm/min),and tpis the time when the ponded water occurs(min).Soil solute in the mixing zone is lost into the infiltrated water and ponded water,and the chemical concentration in the soil mixing zone is as follows:

where Cw(tp)can be obtained from Eq.(3).
During the period from the generation of runoff to the formation of steady runoff,the ponded water depth is maximum and constant at hp(tr)(cm),soil solute in the mixing layer moves into the infiltrated water and surface runoff,and the chemical concentration in runoff αCw(t)is as follows:

where tris the time when runoff is generated(min),i2pis the average soil infiltration rate during the period from the generation of runoff to the formation of steady runoff(cm/min),and Cw(tr)can be obtained by substituting trinto Eq.(4).
During the period from the formation of steady runoff to the end of rainfall,the ponded water depth is constant and maximum at hp(tr)(cm),soil solute in the mixing layer is transported into the stable infiltrated water and surface runoff,and the solute concentration in runoff αCw(t)is obtained as follows:
where tsis the time when steady runoff forms(min),isis the average infiltration rate during the period from the formation of steady runoff to the end of rainfall,and Cw(ts)can be obtained according to Eq.(5).
2.2.Identificationmethodforincompletemixingparameters
Before the occurrence of surface runoff,α and γ are constant and equal to their values at the moment when surface runoff starts,which can be obtained through the best fit of the calculated soluble chemical concentrations in surface runoff to the observations when surface runoff begins.Meanwhile,the soil mixing zone depth is constant all the time.If γ and α are still constant after surface runoff occurs,the soluble chemical concentration in surface runoff C(mg/L)at any time t(min)can be expressed through Eqs.(5)and(6).For the sake of the simplification,the soluble chemical concentration in surface runoff after the occurrence of surface runoff can be rewritten as follows:

where ir=i2pduring the period from the generation of runoff to the formation of steady runoff,and ir=isduring the period from the formation of steady runoff to the end of rainfall.
After the occurrence of surface runoff,γ and α are assumed to be constant between two successive sampling times,but they vary at different sampling times.The time period from the generation of surface runoff to the last sampling moment is divided into m time steps:t1,t2,…,tm,according to the sampling time.For the time step tj(j=1,2,…,m),the infiltrationrelated and runoff-related incomplete mixing parameters are denoted as γjand αj,respectively,and the infiltration rate is denoted as irj.Thus,for time step t1,the chemical concentration in surface runoff is

For time step t2,the solute concentration in surface runoff can be expressed as
For time step tm,the solute concentration in surface runoff is


The times tr,tr+t1,tr+t1+t2,…,tr+t1+t2+…+tmare the experimental sampling moments.Thus,the incomplete mixing parameter values of γjand αj(j=1,2,…,m)can be obtained through the best fit of the numerical results to the experimental data.
3.Experimental conditions

Fig.2.Sketch of experimental frame in Tong et al.(2010).
The experimental frame is presented in Fig.2.Different layers were arranged from top to bottom,including the surface runoff,ponded water,experimental soil,and a filter layer.Two different kinds of soil were used:fine loam sieved through a 2-mm sieve in cases 1 through 3,and even sand in cases 4 through 10.The surface runoff was at a height of 25 cm above the experimental box bottom.Gravel with a diameter of 5-10 mm below the experimental soil was used as a filter layer,with a height hfof 5 cm.Two drainage holes were placed at the box bottom,and they were connected with a tee pipe.The parameters for different experiments are presented in Table 1,where θ0is the initial volumetric water content,heis the depth of the experimental soil,hdrainis the drainage outlet height from the experimental box bottom,and teis the time when rainfall ends.The bulk density ρsand saturated volumetric water content θsfor the fine loam in cases 1 through 3 were 1.4 g/cm3and 0.476,respectively,but 1.47 g/cm3and 0.443 for the even sand,respectively,in cases 4 through 10.All experiment parameters in cases 9 and 10 were the same as those in Tong et al.(2010).
In fine loam experiments in cases 1 through 3,the infiltrated water from the bottom was obtained with free drainage,which means that hdrainwas 0(Table 1).For sand experiments in cases 4 through 10,the drainage outlet height was set above the experimental box bottom to obtain ponded water and surface runoff,which means that hdrainwas greater than 0(Table 1).The value of irwas obtained based on the water balance between the collected water,ponded water,and the total simulated rainfall.The soluble chemical was KCl in our experiments,and its concentration was measured in surface runoff.To avoid the presence of other chemicals,distilled water was used as rain water,and the soils were flushed with distilled water before the experiments.
4.Identification results and discussion
4.1.Results for fine loam experiments
The results for fine loam experiments in cases 1,2,and 3 are shown in Figs.3-5.From Figs.3(b),4(b),and 5(b),we can see that the parameter γ decreases with time for all of three cases,and even decreases to less than 0.This is because γ is the net infiltration of a soluble chemical considering both advection(downward due to the leached infiltration)and diffusion(upward due to the concentration gradient)processes.When infiltration plays a more important role than diffusion in soluble chemical transfer early in the surface runoff process,γ is positive.Then,the infiltration decreases gradually when the initially unsaturated soil becomes saturated.With the soluble chemicals in the soil mixing zone moving into the soil below,the chemical concentration in the soil mixing zone decreases while it increases in the soil below. Thus,the upward concentration gradient gradually increases,leading to larger upward diffusion.When the upward diffusion plays a more important role than the downward infiltration,γ is negative.The stronger the upward diffusion is,the larger the absolute value of negative γ.
It can be seen from Figs.3,4,and 5 that the value of γ in case 1 is a little less than in case 2 on average,and the soluble chemical concentration in surface runoff in case 1 is also less than in cases 2 and 3.This is because the experimental soil is initially unsaturated and the maximum ponded water depth hpwith a value of hp=2.0 cm in case 1 is much greater than in cases 2 and 3,with values of hp=0.5 cm,as shown in Table 1. More water is needed for the experimental soil to reach saturation and to store maximum ponded water on the soil surface in case 1 than in cases 2 and 3,which causes the surface runoff occurrence time in case 1 to be later than incases 2 and 3 and the soluble chemical concentration in the soil mixing zone in case 1 to be much less than in cases 2 and 3 when surface runoff occurs.

Table 1Experimental parameters for different cases.

Fig.3.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 1.

Fig.4.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 2.

Fig.5.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 3.
The value of γ in case 3 is much less than in cases 2 and 1,as demonstrated in Figs.5(b),4(b),and 3(b),and the soluble chemical concentration in surface runoff in case 3 is higher than in cases 2 and 1 on average.This is because the initial volumetric water content of the experimental soil in case 3,with a value of θ0=0.420,is close to the saturated water content,with a value of θs=0.476,and there is almost no infiltration during the experiment as the value of iris so small,leading to less downwardly leached chemicals from the soil mixing layer and a much greater soluble chemical content in the soil mixing layer in case 3 than in cases 1 and 2.For the initially unsaturated experimental soil in case 1,γ varies all the time after the occurrence of surface runoff;for the initially saturated experimental soil in case 2,γ has only three different constant values;and γ varies continuously early in the surface runoff process in case 3,gradually reaching a constant value later in the surface runoff process.These results are due to the small variation of the infiltration rate in the saturated soil and constant average infiltration rates after the occurrence of surface runoff.It is concluded that when the initial volumetric water content is closer to the saturated water content,there is less variation of parameter γ after the occurrence of surface runoff.
Parameter α decreases early in the surface runoff process and then remains at a constant value of 0 later in the surface runoff process in case 1,as shown in Fig.3(a).This is because the soluble chemical in the soil mixing zone in the initially unsaturated experimental soil in case 1 has been leached into the soil below,and the chemical concentration in the soilmixing zone decreases,leading to a decrease in the chemical concentration gradient between the surface runoff and the soil mixing layer,which in turn causes the chemical diffusion from the soil mixing layer into the surface runoff to decrease with time when the depth of the ponded water on the soil surface remains unchanged.In contrast,α increases early in the surface runoff process in cases 2 and 3,then gradually reaches a constant value of 1.0(Figs.4(a)and 5(a)).This is because a larger infiltration rate is needed for the initially unsaturated soil to reach saturation in case 1 than that for the initially saturated soil in case 2 throughout simulated rainfall experiments,so infiltration plays a major role in chemical transport during the surface runoff process for the initially unsaturated soil with θ0=0.100 in case 1,while diffusion plays a more important role in case 2,with θ0=0.476,and case 3,with θ0=0.420,resulting in the increase of α early in the surface runoff process in cases 2 and 3.In addition,the chemical concentration in the soilmixing zone demonstratesa decreasing trend during the surface runoff process,and finally reaches zero,which causes the chemicals in the soil mixing zone to mix completely with the surface runoff.Thus,α values in cases 2 and 3 reach a constant value of 1.0 later in the surface runoff process.
Furthermore,as shown in Figs.3(a)-5(a),α values in the fine loam experiments of cases 1 through 3 are all less than or equal to 1,which means that the soluble chemical concentration in the surface runoff is less than or equal to that in the soil mixing zone.
4.2.Results for sand experiments

Fig.6.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 4.

Fig.7.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 5.

Fig.8.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 6.

Fig.9.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 7.

Fig.10.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 8.
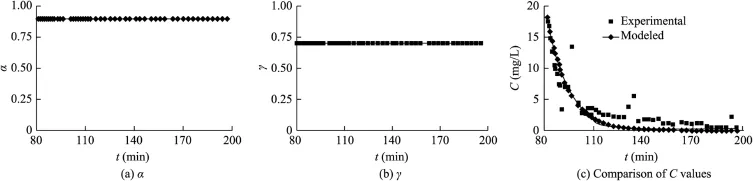
Fig.11.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 9.

Fig.12.Identified incomplete mixing parameters and comparison of experimental and modeled KCl concentrations in surface runoff in case 10.
The results for the sand experiments in cases 4 through 10 are shown in Figs.6-12.The results show that infiltrated water can only be measured at the drainage outlet in cases 4,5, 9,and 10 when 0<hdrain<25 cm,conditions referred to as controlled infiltration hereafter,while there is no drainage water at all in cases 6,7,and 8 when hdrain>25 cm,conditions referred to as restrained infiltration hereafter.
As in the loam experiments,the parameter γ is variable for the initially unsaturated soil in case 4 with θ0=0.046(Fig.6),while γ remains almost unchanged for initially saturated soils in cases 6,7,and 10 with θ0=0.443(Figs.8,9,and 12).In theinitially unsaturated soil experiment in case 8 with θ0=0.280,γ is also constant,which is quite different from the results in case 4.These results can be explained by the fact that the initial volumetric water content in case 8 is much closer to the saturated water content than in case 4.Under restrained infiltration conditions in cases 6,7,and 8,the infiltration rate is 0,indicating that the infiltration process does not play a role in soluble chemical transport from the soil mixing zone to the underlying soil layer.Thus,the parameter γ can take an arbitrary value.The constant values of γ before the occurrence of surface runoff are displayed in Figs.8(b),9(b),and 10(b).
The parameter α in cases 4 and 5 decreases first and then remains at a constant value close to 0 under the controlled infiltration conditions.This is because the decrease of the soluble chemical concentration in the soil mixing zone causes the chemical concentration gradient between the soil mixing layer and the surface runoff to decrease.
However,α increases with time during the surface runoff process in case 7 under the restrained infiltration condition with a very large initial volumetric water content(θ0=0.443),and its value becomes much larger than 1.0 soon after the early stages of the surface runoff process.These results can be explained by the fact that the rainwater infiltrates into the soil surface at an early time with the increase of the ponded water,and the chemical concentration in the soil mixing zone decreases with the increase of the infiltrated water.At the same time,the soluble chemical concentration in the soil below the soil mixing layer increases.Since there is no infiltrated water at the drainage outlet when hdrain>25 cm,there will be no water to infiltrate into the soil surface after a certain period of time,except the water that is exchanged between the whole mixing layer and the underlying soil layer. Without infiltrated water,the soluble chemical concentration gradient between the soil mixing layer and underlying soil layer is the major driving force for chemical transport,so the soluble chemical in the underlying layer will diffuse into the soil mixing zone.Under the assumption that soluble chemicals in the soil mixing zone are the only source of chemicals in infiltration and surface runoff,and there will be no soluble chemical that is transferred into the soil mixing zone,α increases with time and even reaches a value greater than 1. However,α is considered to be less than or equal to 1 in the proposed analytical model by Tong et al.(2010),so it can be concluded that the model is only valid for the case with infiltrated water.
In cases 6 and 8,α slightly decreases over a very short period early in the surface runoff process and increase at later times,which differs from the variation of α in case 7.This is probably because the ponded water depth in cases 6 and 8 is greater than in case 7,and more rainwater infiltrates into the soil after the surface runoff starts in cases 6 and 8,resulting in more downward infiltration water on the soil surface and less solute transfer upward from the soil mixing zone to the ponding-runoff zone.Thus,α decreases.After a certain period of time,the soluble chemical concentration in the soil mixing zone decreases with time,and some soluble chemicals are transferred from the underlying soil layer into the soil mixing layer through the diffusion process,leading to an increase in parameter α.
Comparing the experimental and simulated results in cases 4,5,and 9 under the conditions of controlled infiltration with those in cases 6,7,and 8 under the conditions of restrained infiltration,one can see that the soluble chemical concentrations in the surface runoff in cases 4,5,and 9 are much lesser than those in cases 6,7,and 8,so the infiltration or drainage conditions are very important factors that affect the soluble chemical transfer from the soil into the surface runoff.
However,under the controlled infiltration conditions,the soluble chemical concentration in the surface runoff in case 10 is much greater than in cases 4,5,and 9,with the same order of magnitude as in cases 6,7,and 8 under the restrained infiltration conditions.This phenomenon can be attributed to the shallower ponded water in case 10 than in cases 4,5,and 9.From these results it is can be concluded that the depth of ponded water on the soil surface significantly affects the soluble chemical transfer from the soil into the surface runoff.
The results in cases 9 and 10 shown in Figs.11 and 12 are obtained from Tong et al.(2010),where the parameters α and γ are constant.It can be seen from Fig.8 to Fig.11 that there is a better agreement between the modeled and experimental KCl concentrations in surface runoff in cases 6,7,and 8 than in case 9,indicating that the chemical concentration predicted from the model with variable incomplete mixing parameters γ and α are more accurate than those with constant γ and α values.
4.3.Comparison of results between fine loam and sand experiments
Using the results for the fine loam and sand experiments,we will make a comparison between these two different types of experimental soil.Both γ and α vary with time during the surface runoff process for the initially unsaturated experimental fine loam in case 1 and initially unsaturated experimental sand in case 4.γ takes three constant values for three stages in case 2 for the initially saturated experimental fine loam and also in case 5 for the initially saturated experimental sand.α increases with time first in case 2 for the fine loam experiment,but decreases with time first in case 5 for the sand experiment.The values of α for both soils range from 0 to 1 in the two cases.The saturated hydraulic conductivity in the sand is much greater than in the fine loam,so the infiltration rate in the sand is higher than that in the fine loam even though the sand is kept in the conditions of controlled infiltration.As discussed above,with a higher infiltration rate during the surface runoff process,the proposed model based on the assumption that there is no chemical source for the soil mixing zone becomes more accurate.Moreover,the higher infiltration rate leads to more soluble chemical loss downward,so the upward transfer of chemicals from the mixing soil zone to the surface runoff is less in case 5 than in case 2.
5.Conclusions
In this paper,a method is introduced to determine the variable infiltration-related incomplete mixing parameter γ and runoff-related incomplete mixing parameter α on the basis of the analytical solution to the model proposed by Tong et al.(2010)and the observed fine loam and sand experimental data. The simulations fit the observed data.The value of hmixwas constant across all experiments,and the values of γ and α remained unchanged before surface runoff occurred.According to analysis of the results,some conclusions can be drawn:
(1)The parameter γ decreases with the increase of the ponded water depth.When the initial volumetric water content is closer to the saturated water content,there is less variation of parameter γ after the occurrence of surface runoff.The chemical concentration in the surface runoff decreases with the increase of infiltration.
(2)The values of parameter α range from 0 to 1 for the fine loamandsandunderthecontrolledinfiltrationconditions,while it can increase to a very large value,greater than 1,for the sand under the restrained infiltration conditions.It was determined that the analytical solution of the model is not valid for conditions without any infiltration if α is still expected to be less than or equal to 1,as shown in Tong et al.(2010).
(3)Though the soluble chemical concentrations predicted from the model with variable incomplete mixing parameters γ and α are more accurate than with from constant γ and α values,there are still some problems that need to be studied in future.We plan to expand our model under the restricted infiltration conditions.This solution for two incomplete mixing parameters may not be unique,and requires our further study to obtain unique parameters for the model.Moreover,this study is based on laboratory experiments with only one soluble chemical,and its application to the real field scale with different adsorptive chemicals in more complex processes is another issue that requires deeper thought and investigation.
References
Ahuja,L.R.,Ross,J.D.,Lehman,O.R.,1981a.A theoretical analysis of interflow of water through surface soil horizons with implications for movement of chemicals in field runoff.Water Resour.Res.17(1),65-72. http://dx.doi.org/10.1029/WR017i001p00065.
Ahuja,L.R.,Sharpley,A.N.,Yamamoto,M.,Menzel,R.G.,1981b.The depth of rainfall-runoff-soil interactions as determined by32p.Water Resour. Res.17(4),969-974.http://dx.doi.org/10.1029/WR017i004p00969.
Ahuja,L.R.,Lehman,O.R.,1983.The extent and nature of rainfall-soil interaction in the release of soluble chemicals to runoff.J.Environ.Qual.12(1),34-40.http://dx.doi.org/10.2134/jeq1983.00472425001200010005x.
Gao,B.,Walter,M.T.,Steenhuis,T.S.,William,L.H.,Parlange,J.-Y.,2004.Rainfall induced chemical transport from soil to runoff:Theory and experiments. J.Hydrol.295(1-4),291-304.http://dx.doi.org/10.1016/j.jhydrol.2004.03.026.
Govindaraju,R.S.,Kavvas,M.L.,Jones,S.E.,Rolston,D.E.,1996.Use of Green-Ampt model for analyzing one-dimensional convective transport in unsaturated soils.J.Hydrol.178(1-4),337-350.http://dx.doi.org/ 10.1016/0022-1694(95)02796-3.
Steenhuis,T.S.,Walter,M.F.,1980.Closed form solution for pesticide loss in runoff water.Trans.Am.Soc.Agric.Eng.23(3),615-620.http:// dx.doi.org/10.13031/2013.34634.
Tian,K.,Huang,C.H.,Wang,G.Q.,Tian,P.,Fu,X.D.,Guo,H.D.,2011. Movement of soil solute under simulated rainfall runoff.Trans.Chin.Soc. Agric.Eng.27(4),81-87(in Chinese).
Tong,J.X.,Yang,J.Z.,Hu,B.X.,Bao,R.C.,2010.Experimental study and mathematical modeling of soluble chemical transfer from unsaturatedsaturated soil to surface runoff.Hydrol.Process.J.24(21),3065-3073. http://dx.doi.org/10.1002/hyp.7722.
Wang,Q.J.,Shao,M.A.,Li,Z.B.,Lei,T.W.,Lu¨,D.Q.,1999.Analysis of simulating methods for soil solute transport with runoff in loess plateau. Res.Soil Water Conserv.6(2),67-71,104(in Chinese).
Wang,S.B.,Ma,X.X.,Fan,Z.Q.,Zhang,W.Q.,Qian,X.Y.,2014.Impact of nutrient losses from agricultural lands on nutrient stocks in Dianshan Lake in Shanghai,China.Water Sci.Eng.7(4),373-383.http://dx.doi.org/ 10.3882/j.issn.1674-2370.2014.04.003.
Yu,C.R.,Gao,B.,Mu~noz-Carpena,R.,Tian,Y.,Wu,L.,Perez-Ovilla,O.,2011.A laboratory study of colloid and solute transport in surface runoff on saturated soil.J.Hydrol.402(1-2),159-164.http://dx.doi.org/ 10.1016/j.jhydrol.2011.03.011.
Zhang,X.C.,Norton,D.,Nearing,M.A.,1997.Chemical transfer from soil solution to surface runoff.Water Resour.Res.33(4),809-815.http:// dx.doi.org/10.1029/96WR03908.
14 May 2014;accepted 20 April 2015
This work was supported by the Open Foundation of the State Key Laboratory of Water Resources and Hydropower Engineering Science,Wuhan University(Grant No.2013B108),the National Natural Science Foundation of China(Grant No.51209187),the Fundamental Research Fund for the Central Universities(Grant No.2652011286),and the Beijing Higher Education Young Elite Teacher Project(Grant No.YETP0653).
*Corresponding author.
E-mail address:juxiu.tong@cugb.edu.cn(Ju-xiu Tong).
Peer review under responsibility of Hohai University.
http://dx.doi.org/10.1016/j.wse.2015.04.011
1674-2370/©2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Water Science and Engineering2015年3期
Water Science and Engineering2015年3期
- Water Science and Engineering的其它文章
- Uniqueness,scale,and resolution issues in groundwater model parameter identification
- Flash flood hazard mapping:A pilot case study in Xiapu River Basin,China
- Variable fuzzy assessment of water use efficiency and benefits in irrigation district
- Effects of thermodynamics parameters on mass transfer of volatile pollutants at air-water interface
- Adsorption of Cr(VI)in wastewater using magnetic multi-wall carbon nanotubes
- Degradation of acephate using combined ultrasonic and ozonation method
