贝伐单抗动脉灌注联合化疗栓塞术治疗原发性肝癌的血管造影变化及初步评价
朱帝文,张海潇,顾俊鹏,纪卫政,曹耿飞,任伟新
·全科医生技能发展·
贝伐单抗动脉灌注联合化疗栓塞术治疗原发性肝癌的血管造影变化及初步评价
朱帝文,张海潇,顾俊鹏,纪卫政,曹耿飞,任伟新
目的 分析贝伐单抗动脉灌注联合化疗栓塞术(TACE)治疗原发性肝癌患者的血管造影变化,评价其治疗的有效性和安全性。方法 选取2012年10月—2013年8月于新疆医科大学第一附属医院至少经过1次TACE治疗ART评分≥2.5分的13例原发性肝癌患者为研究对象,给予其贝伐单抗动脉灌注联合TACE治疗。治疗后于2012 年11月—2014年3月进行随访,患者每隔4~8周入院复查,评价治疗效果及不良反应,并观察患者血管造影表现。结果 第1次贝伐单抗动脉灌注联合TACE治疗后,3例患者疾病进展(PD),未再次行贝伐单抗动脉灌注联合TACE治疗;其余10例患者接受第2次贝伐单抗动脉灌注联合TACE治疗,动脉造影可见肿瘤血管明显减少,而剩余的血管出现正常化表现,部分未沉积碘油颗粒的病灶在延迟期未见显影;正常肝动脉血管管径变细,肿瘤组织血流量降低。在随访过程中,1例患者随访1次后因更改联系方式失访。随访的12例患者中,4例死亡,8例存活。无疾病进展生存时间为356.5 d(220~452 d),中位生存时间为410.0 d(246~585 d)。13例患者术后评价疗效完全缓解(CR)0例,部分缓解(PR)2例,疾病稳定(SD)8例和PD 3例。未观察到其他严重药物及治疗相关性不良反应发生。结论 贝伐单抗动脉灌注联合TACE用于治疗肝细胞癌患者,动脉造影可见肿瘤血管减少,肿瘤血管正常化,安全、有效,对TACE治疗后ART评分≥2.5分的患者不失为一个有效的替代策略。
肝肿瘤;血管生成抑制剂;化学栓塞,治疗性;血管造影术
朱帝文,张海潇,顾俊鹏,等.贝伐单抗动脉灌注联合化疗栓塞术治疗原发性肝癌的血管造影变化及初步评价[J].中国全科医学,2015,18(3):350-354.[www.chinagp.net]
Zhu DW,Zhang HX,Gu JP,et al.Arterial infusion with bevacizwmab carcinoma with Anti-angiogenesis therapy combined with transcatheter arterial chemoembolization:the angiographic appearance and preliminary evaluation of efficacy[J]. Chinese General Practice,2015,18(3):350-354.
原发性肝癌是临床常见恶性肿瘤之一,发病隐匿,肿瘤血管形成活跃是其突出特征,仅有不到20%的患者能够早期发现并进行根治性治疗[1-2],但预后不佳。目前经导管肝动脉化疗栓塞术(transcatheterarterialchemoembolization,TACE)已成为不可切除的中晚期肝癌的标准治疗方案[3]。但TACE后病灶内血管再生常是肿瘤复发进而导致进展的重要原因[4]。抗血管生成药物贝伐单抗是重组的人源化抗血管内皮生长因子(VEGF)的单克隆抗体,可结合VEGF并防止其与内皮细胞表面的受体结合,抑制肿瘤新生血管生成,使现有血管退化,并促使异常肿瘤血管正常化[5]。本研究通过贝伐单抗动脉灌注联合TACE治疗原发性肝癌患者,观察治疗后其动脉血管造影变化,初步评价治疗的有效性与安全性。
1 资料与方法
1.1纳入与排除标准 纳入标准:(1)至少经过1次TACE治疗后,14~90 d行ART评分[6]≥2.5分的原发性肝癌患者;(2)不需要治疗的胸、腹腔积液和肝性脑病者;(3)体力状况美国东部肿瘤协作组(ECOG)评分为0~2分者; (4)预期寿命至少12周者。排除标准为TACE治疗后有以下之一者:(1)肿瘤侵犯胆管致胆管梗阻者;(2)明确的肝动脉-门静脉瘘,而术中无法封堵瘘口者;(3)门静脉广泛癌栓且肝功能Child-Pugh评分为C级者;(4)脑转移者。
1.2一般资料 选取2012年10月—2013年8月新疆医科大学第一附属医院符合纳入与排除标准的13例原发性肝癌患者为研究对象,其中男12例,女1例;年龄34~73岁,中位年龄55岁;体力状况ECOG评分:3分4例,2分9例;肝功能Child-Pugh评分,A级 (5~6分)1例,B级(7~9分)9例,C级(10~15分)3例;肝癌巴塞罗那分期(BCLC):C期10例,D期3例;肝病史:乙肝12例,丙肝1例;实验室检查:甲胎蛋白4.7~1 000.0 μg/L,总胆红素192~5 162 μmol/L,清蛋白21~24 g/L;肿瘤特征:平均肿瘤直径7.6 cm,多发肿瘤结节10例,血管侵犯5例。
1.3研究方法 患者再次治疗时给予贝伐单抗动脉灌注联合TACE治疗。具体操作步骤:在数字减影血管造影(DSA)设备监视下,按Seldinger穿刺技术规范[7]行股动脉穿刺,同时行肝动脉造影,观察肝脏内病灶的供血状况,然后进超选插管至肿瘤供血动脉,经导管动脉灌注贝伐单抗注射液 (5 mg/kg),再根据肿瘤的供血状况、肝功能分级及肿瘤大小等,给予适量的碘化油与奥沙利铂(40 mg/m2)配置成的混悬液行TACE。患者治疗4~8周后行血常规、肝肾功能、凝血功能、腹部增强CT、甲胎蛋白等检查,评价后再次行贝伐单抗动脉灌注联合TACE治疗,共2次。术中肝动脉造影观察动脉血管变化。
1.4随访及预后 治疗后于2012年11月—2014年3月进行随访,患者每隔4~8周入院复查,记录患者疾病进展(PD)时间、生存时间、体力状况ECOG评分等指标。按照改良版实体肿瘤疗效评价标准(mRECIST)[8],治疗4~8周后根据腹部增强CT评价疗效,分为完全缓解(CR),部分缓解(PR),疾病稳定(SD)和PD。
1.5不良反应 以美国国家癌症研究所通用毒性标准4.0(CTCAE v4.0)[9]评价术后不良反应,主要评价指标有:血常规、肝肾功能、血压、出血倾向、胃肠道反应等。
2 结果
2.1一般结果 第1次贝伐单抗动脉灌注联合TACE治疗后,3例患者PD,未再次行贝伐单抗动脉灌注联合TACE治疗;其余10例患者接受第2次贝伐单抗动脉灌注联合TACE治疗。
2.2血管造影变化 肝内多发肝癌结节患者7例,于动脉期可见肝动脉血管较治疗前变细,血流灌注量减少;毛细血管期可见前次栓塞后的碘油颗粒沉积影,部分碘油颗粒沉积良好,肿瘤体积缩小,而形态扭曲、管径扩大、大小不一、相互交联的肿瘤血管明显减少;延迟期可见异常结节状浓染的肿瘤染色灶较治疗前减少(见图1)。巨块型肝癌患者2例,其中1例患者碘油颗粒呈完全填充型,肿瘤较治疗前缩小,未见明显造影剂浓染;1例患者病灶表现为部分填充型,见肿瘤内残存血管显影,远端肿瘤血管内碘油颗粒沉积良好,肿瘤体积较治疗前无明显变化,同常规TACE治疗后的造影表现相同。弥漫性肝癌并肝动脉-门静脉瘘及门静脉癌栓患者1例,术前血管造影可见肝内多发、满布的小结节状异常造影剂浓染,并有多支肝动脉-门静脉瘘;术中栓塞肝动脉-门静脉主要瘘口,于肝固有动脉给予奥沙利铂与碘油混悬颗粒行栓塞术,再给予动脉灌注贝伐单抗;再次行贝伐单抗动脉灌注联合TACE治疗后肝动脉造影可见肝内散在的碘油颗粒沉积影,肝内弥漫性的小结节状异常染色灶较治疗前减少(见图2)。
同行评议:
(1)ART评分系统是一种简单、快捷评价TACE治疗后肿瘤是否进展的方法,该方法尚未广泛应用。
(2)对于肝癌患者行肝动脉化疗栓塞术后的进一步治疗方案,指南中未给予明确答案。
(3)贝伐单抗应用于肝癌治疗,相关文献为静脉滴注,本研究应用介入技术,将导管置于肝动脉,提高肝脏局部药物浓度,能够提高治疗效果。
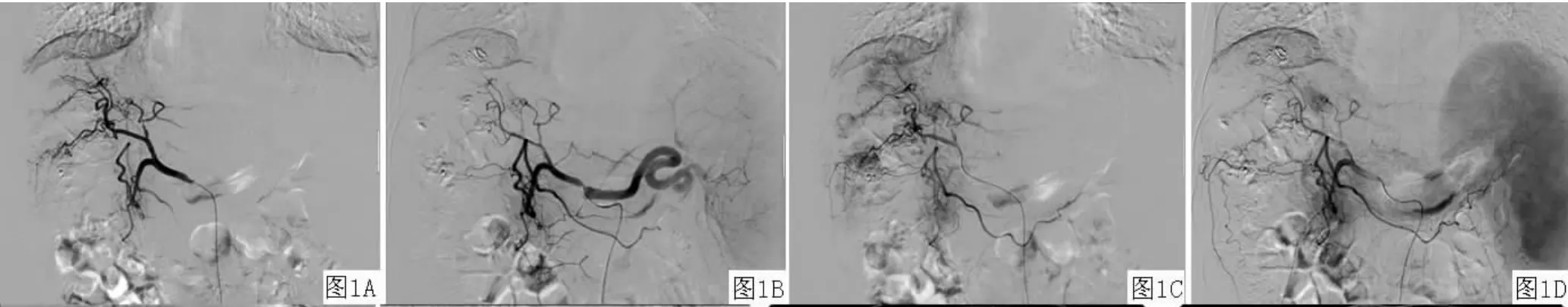
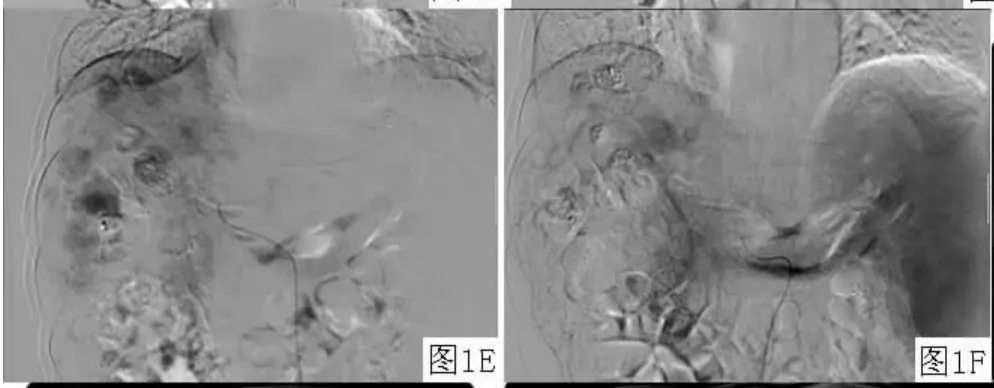
图1 血管造影表现Figure 1 Angiographic appearance

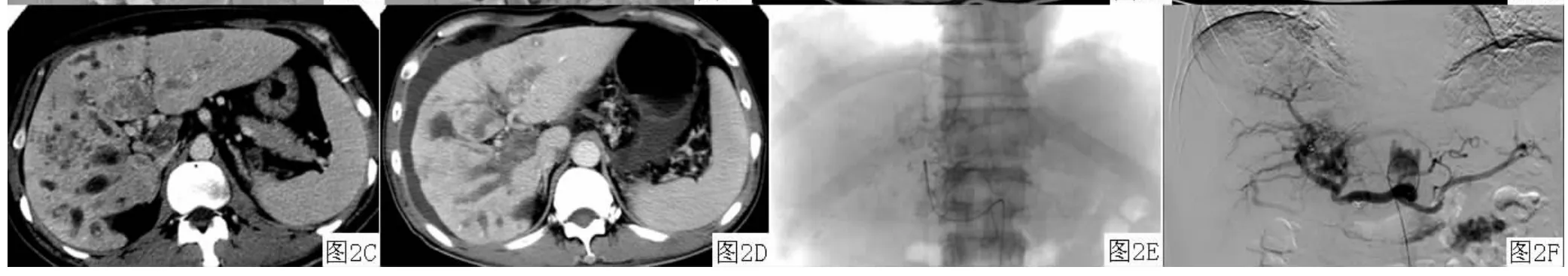
图2 增强 CT和血管造影表现Figure 2 Findings of enhanced CT and angiography
2.3随访及预后 在随访过程中,1例患者随访1次后因更改联系方式失访。随访的12例患者中,4例死亡,8例存活。无疾病进展生存期为356.5 d(220~452 d),中位总生存期为410.0 d(246~585 d)。13例患者4~8周入院复查后再次评价,6例患者肝功能Child-Pugh评分等级上升,6例患者保持稳定,1例患者下降;6例体力状况ECOG评分较前好转,7例保持原有水平,未有体力状况ECOG评分下降患者;评价疗效CR 0例,PR 2例,SD 8例和PD 3例。
2.4不良反应 主要不良反应包括栓塞后综合征(发热及腹痛,6例);肝功能方面:转氨酶升高(11例),胆红素升高(2例);胃肠道反应(恶心及呕吐,5例);2例出现过敏反应,表现为颜面及手部出现皮肤红疹,给予地塞米松注射液抗过敏治疗后恢复;2例出现黑便,出血量较少,出血自行停止,未给予特殊处理。血常规未观察到骨髓抑制表现,肾功能、凝血时间未有明显异常。无其他严重药物及治疗相关性不良反应发生。
3 讨论
肿瘤血管靶向治疗(tumor vasculartargeted therapy)以肿瘤供养血管网为靶点,是提高肿瘤治愈率的一种新方法。根据作用机制不同,血管靶向治疗分为抗血管生成疗法 (anti-angiogenesis therapy)和血管阻断疗法(vascular-disrupting therapy)。前者旨在抑制血管新生,而后者则旨在迅速毁灭性关闭实体瘤中已经形成的血管网。TACE属于血管阻断疗法,将化疗药物与碘化油混合后使其沉积于肿瘤血管,可使化疗药物长时间聚集于肿瘤细胞周围,并且能阻断肿瘤滋养血管,使肿瘤细胞死亡。
对于行TACE治疗的患者,影响其生存的因素主要包括肿瘤负荷、肝功能评分、胆红素水平、门静脉癌栓、肿瘤血供及边缘情况等,而在TACE治疗后可因VEGF呈高表达状态使得肿瘤血管新生、再通、血管代偿以及肿瘤血管的通透性增加等而导致疾病进展[10]。
ART评分系统是一种简单、快捷评价TACE治疗后肿瘤是否进展的方法[11]。对原发性肝癌患者初次行TACE治疗后,根据患者不同病情在14~90 d内运用ART评分系统进行评估。对于ART评分≥2.5分的患者不能从TACE治疗中长期获益,因而不建议再次行TACE治疗,而应当考虑替代策略[12]。从ART评分系统进行分析,这部分患者肿瘤进展的原因一部分是肿瘤无应答,另一部分为肝癌患者行TACE治疗后,对正常肝组织造成不可逆的肝功能损害。因此,如何提高肿瘤应答率,同时最大程度上保护肝功能成为肝癌患者介入治疗能否成功的关键。
抗血管生成药物贝伐单抗能封闭VEGF,阻止其在血管内皮细胞中发挥作用,可被动地截除未成熟和渗漏的脉管,主动重塑剩余脉管结构,使之与正常脉管结构更相似;还可减小血管直径和长度、降低血管通透性[13-15]。这种正常的脉管结构表现为渗漏少、管壁扩张少、扭曲度减少,而且基底膜趋于正常,周细胞覆盖范围更广。形态学改变的同时伴随着功能改变,肿瘤组织间质压力降低、肿瘤氧供增加、药物向肿瘤细胞的渗透性增强[16-17]。这些形态和功能上的变化可以增加肿瘤化疗和放疗的敏感性。而对于肝硬化肝癌患者,血管通透性的减少可以减少腹腔积液[18-20]。此外,贝伐单抗结合VEGF后可作用于肝窦内皮细胞(SEC)降低在窦周基质降解及肝窦扩张中发挥重要作用的基质金属蛋白酶9(MMP-9)的表达[21],进而改善患者的肝功能[22]。
本研究将抗血管生成疗法和血管阻断疗法有机结合。通过贝伐单抗动脉灌注联合TACE治疗13例患者,2例为PR,8例为SD,仅有3例为PD。13例患者中位总生存时间为410.0 d,而相关文献进展期原发性肝癌患者总生存时间为321 d[23-24]。在肝功能评价方面,6例肝功能Child-Pugh评分等级上升,6例保持稳定,仅有1例下降。提示贝伐单抗动脉灌注联合TACE治疗能够使患者在生存时间方面获益,同时提高了肿瘤应答率,而对于肝功能能够起到保护作用。但是,1例患者失访,对于小样本的观察性研究造成一定的信息量损失。由于失访率<10%,本研究还是具有可信度的。
本研究中,通过对再次行贝伐单抗动脉灌注联合TACE治疗患者的肝动脉造影可以观察到形态扭曲、管径扩大、大小不一、相互交联的肿瘤血管明显减少,而剩余的血管出现正常化表现,部分未沉积碘油颗粒的病灶在延迟期未见显影;正常肝动脉血管管径变细,肿瘤组织血流量降低。
综上所述,肝动脉肿瘤供血血管灌注贝伐单抗,可以有效降低药物使用量,降低不良反应发生率,抑制栓塞后高表达状态的VEGF,促进肿瘤血管正常化,增加局部化疗药物的敏感性,同时能够减少腹腔积液、保护肝功能。对于BCLC B期、TACE治疗后ART评分≥2.5分的肝癌患者,行贝伐单抗动脉灌注联合TACE治疗不失为一个有效的替代TACE治疗的策略。但本研究纳入样本量较少,随访时间较短,未获得总生存时间,需要继续完善大样本分析,不断探索对于个体患者更好的治疗剂量和治疗时间,进一步完善这一治疗方案。
[1]OsakiY,NishikawaH.Treatmentfor hepatocellular carcinoma in Japan over the last three decades:Our experience and published work review[J].Hepatol Res,2014.doi:10.1111/hepr.12378.[Epub ahead of print].
[2]Chen X,Liu HP,Li M,et al.Advances in non-surgical management of primary liver cancer[J].World J Gastroenterol,2014,20(44):16630-16638.
[3]Forner A,Gilabert M,Bruix J,et al. Treatmentofintermediate-stage hepatocellular carcinoma[J].Nat Rev Clin Oncol,2014,11(9):525-535.
[4]Seki A,Hori S.Switching the loaded agent fromepirubicintocisplatin: salvage transcatheter arterial chemoembolization with drug-eluting microspheres for unresectable hepatocellular carcinoma[J].Cardiovasc Intervent Radiol,2012,35(3):555-562.
[5]Poon RT,Ho JW,Tong CS,et al.Prognostic significance of serum vascular endothelial growth factorandendostatininpatientswith hepatocellular carcinoma[J].Br J Surg,2004,91(10):1354-1360.
[6]Sieghart W,Hucke F,Pinter M,et al. The ART of decision making:retreatment withtransarterialchemoembolizationin patients with hepatocellular carcinoma[J]. Hepatology,2013,57(6):2261-2273.
[7]Fang P,Hu JH,Cheng ZG,et al.Efficacy and safety of bevacizumab for the treatment of advancedhepatocellularcarcinoma:a systematic review of phaseⅡtrials[J].PLoS One,2012,7(12):e49717.
[8]Lencioni R,Llovet JM.Modified RECIST (mRECIST)assessment for hepatocellular carcinoma[J].Semin Liver Dis,2010,30(1):52-60.
[9]AnnSetser,CBIIT,NCI.CTCAEv4.0 Common Terminology Criteria for Adverse Events[DB/OL].(2009-06-08)[2014-05-26].http://www.calgb.org/ Public/meetings/presentations/2009/summer _group/cra_cont_ed/06a_CTCAESetser_062009.pdf.
[10]WangB,XuH,GaoZQ,et al. Increasedexpressionofvascular endothelial growth factor in hepatocellular carcinomaaftertranscatheterarterial chemoembolization[J].Acta Radiol,2008,49(5):523-529.
[11]Hucke F,Sieghart W,Pinter M,et al. The ART-strategy:sequential assessment of theARTscorepredictsoutcomeof patients with hepatocellular carcinoma retreatedwithTACE[J].JHepatol,2014,60(1):118-126.
[12]Sieghart W,Hucke F,Pinter M,et al. The ART of decision making:retreatment withtransarterialchemoembolizationin patientswithhepatocellularcarcinoma [J].Hepatology,2013,57(6):2261 -2273.
[13]Tong RT,Boucher Y,Kozin SV,et al. Vascularnormalizationbyvascular endothelialgrowthfactorreceptor2 blockade induces a pressure gradient across thevasculatureandimprovesdrug penetration in tumors[J].Cancer Res,2004,64(11):3731-3736.
[14]Winkler F,Kozin SV,Tong RT,et al. Kineticsofvascularnormalizationby VEGFR2blockadegovernsbraintumor response to radiation:role of oxygenation,angiopoietin-1,andmatrix metalloproteinases[J].CancerCell,2004,6(6):553-563.
[15]Trédan O,Lacroix-Triki M,Guiu S,et al. Angiogenesis and tumor microenvironment:bevacizumab in the breast cancer model [J].Target Oncol,2014.[Epub ahead of print].
[16]Jain RK,Tong RT,Munn LL.Effect of vascularnormalizationbyantiangiogenic therapyoninterstitialhypertension,peritumoredema,andlymphatic metastasis:insights from a mathematical model[J].CancerRes,2007,67 (6):2729-2735.
[17]Arjaans M,Oude Munnink TH,Oosting SF, et al.Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake[J].Cancer Res,2013,73(11):3347-3355.
[18]Willett CG,Boucher Y,di Tomaso E,et al.Direct evidence that the VEGF-specificantibodybevacizumabhas antivascular effects in human rectal cancer [J].Nat Med,2004,10(2):145-147.
[19]O′Connor JP,Carano RA,Clamp AR,et al. Quantifying antivascular effects of monoclonal antibodiestovascularendothelialgrowth factor:insights from imaging[J].Clin Cancer Res,2009,15(21):6674-6682.
[20] NinomiyaS,InomataM,TajimaM,et al.Effectofbevacizumab,a humanized monoclonal antibody to vascular endothelial growth factor,on peritoneal metastasis of MNK-45P human gastric cancer in mice[J].J Surg Res,2009,154(2):196-202.
[21]Karroum A,Mirshahi P,Faussat AM,et al.Tubularnetworkformationby adriamycin-resistant MCF-7 breast cancer cells is closely linked to MMP-9 and VEGFR-2/VEGFR-3overexpressions[J].EurJPharmacol,2012,685(1/2/3):1-7.
[22]Rubbia-Brandt L,Lauwers GY,Wang H,et al.Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis [J].Histopathology,2010,56(4):430-439.
[23]Wang Z,Wu XL,Zeng WZ,et al.Meta -analysis of the efficacy of sorafenib for hepatocellular carcinoma[J].Asian Pac J Cancer Prev,2013,14(2):691-694.
[24] CaoJF,LyuJ,ZhangHY,et al. ResearchontheEffectof MicroenvironmentofTumorBlood PerfusionunderAnti-angiogenesis Therapy[J].ShanghaiJournalof BiomedicalEngineering, 2010, 31 (3):125-130.(in Chinese)曹金凤,吕杰,张洪一,等.抗血管生成治疗实体肿瘤正常化期间微环境对血液灌注的影响[J].生物医学工程学进展,2010,31(3):125-130.
(
(本文编辑:崔丽红)
Arterial Infusion with Bevacizwmab Carcinoma with Anti-angiogenesis Therapy Combined with Transcatheter Arterial Chemoembolization:the Angiographic Appearance and Preliminary Evaluation of Efficacy
ZHU Di-wen,ZHANG Hai-xiao,GU Jun-peng,et al.Department of Interventional Radiology,the First Affiliated Hospital of Xinjiang Medical University,Urumqi 830054,China
Objective To analyse the angiographic appearance of patients with primary hepatocellular carcinoma who are treated with arterial infusion with Bevacizumab combined with transcatheter arterial chemoembolization(TACE),and to evaluate the efficacy and safety of the treatment.Methods 13 patients with primary hepatocellular carcinoma who underwent TACE at least 1 time in the First Affiliated Hospital of Xinjiang Medical University from October 2012 to August 2013,were selected as study subjects,ART score of each case was≥2.5 points after treatment,all cases were treated with arterial infusion with Bevacizumab combined with TACE.After combination treatment,all cases were followed up from November 2012 to March 2014,all cases received reexamination every 4-8 weeks,the therapeutic effects and adverse reactions were evaluated,and the angiographic appearance was observed.Results After the first treatment with arterial infusion with Bevacizumab combined with TACE,3 patients did not undergo the second combination treatment because of progression disease(PD),the other 10 patients received the second combination treatment.The angiographic appearance showed the number of tumor vascular decreased significantly,the remaining vessels appeared normal,and some lesions without iodine oil particles deposition were not visualized in the delayed phase;the diameter of normal hepatic artery decreased,and blood flow volume of tumor tissues decreased.During follow-up,1 patient lost contact with research team due to ways of contact changed.Among the remaining 12 patients,4patients died,8 patients survived.The mean progression free survival was 356.5 d(220-452 d),the median overall survival time was 410.0 d(246-585 d).According to results of postoperative efficacy evaluation,nobody experienced a complete response(CR),a partial response(PR)was observed in two patients,and eight showed a stable disease(SD),the other three cases showed a PD.The drug-related and treatment-related adverse effects were not observed.Conclusion When treating hepatocellular carcinoma patients with arterial infusion with Bevacizumab combined with TACE,the decreased number of tumor vascular and tumor vascular normalization can be seen through arteriography,the combination treatment is safe and effective,and is a valid alternative strategy for the patients whose ART score are≥2.5 points after TACE treatment.
Liver neoplasms;Angiogenesis inhibitors;Chemoembolization,therapeutic;Angiography
R 735.7
B
10.3969/j.issn.1007-9572.2015.03.026
830054新疆乌鲁木齐市,新疆医科大学第一附属医院介入放射科
任伟新,830054新疆乌鲁木齐市,新疆医科大学第一附属医院介入放射科;E-mail:rwx1031@163.com
2014-06-05;
2014-12-02)

