虫草素抗肿瘤作用分子机制研究进展
王多,鲍荣,王芳,胡永红,柴惠霞
(河北师范大学生命科学学院,河北石家庄050024)
虫草素抗肿瘤作用分子机制研究进展
王多,鲍荣,王芳,胡永红,柴惠霞
(河北师范大学生命科学学院,河北石家庄050024)
虫草素是一种脱氧核苷类似物,具有抗肿瘤、抗白血病、抗菌、抗衰老、免疫调节和清除体内自由基等多种药理作用,对多种疾病尤其是恶性肿瘤具有明确的治疗作用。虫草素抗肿瘤作用机制及其靶点目前已成为分子生物学研究的新热点。虫草素在细胞内有多个作用靶标,可调控肿瘤细胞生长、增殖和转移等过程。虫草素发挥抗肿瘤的作用机制包括抑制嘌呤、DNA和RNA合成及蛋白质翻译,诱导肿瘤细胞凋亡和调控细胞周期,抗肿瘤细胞侵袭,抑制血小板凝集和抗炎5个途径及其相应的信号通路。虫草素在体内迅速脱氨从而丧失生物活性。笔者提出了合成虫草素衍生物、虫草素与腺苷脱氨酶抑制剂联合用药及与纳米新材料等形成新的复合物等3个解决方案,旨在为虫草素的研发提供新的思路,为其临床应用奠定理论基础。
虫草素;抗肿瘤药;丝裂原活化蛋白激酶;细胞凋亡;细胞周期
虫草素(cordycepin)又名3′-脱氧腺苷(3′-deoxyadenosine),是由腺苷和具有碳支链的脱氧戊糖组成的一种核苷酸[1],1951年由Cunninghan等[2]首先从冬虫夏草(Cordyceps sinensis)中分离得到。虫草素参与人体细胞代谢的多个过程,具有抗肿瘤、抗白血病、抗菌、抗衰老、免疫调节、清除体内自由基及抗缺血再灌注损伤等多方面药理作用,同时参与腺苷的生物合成、DNA和RNA合成、调控哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号转导通路[3]、肿瘤细胞凋亡[4-5]、增殖[6]和侵袭[7]以及血小板聚集[8]和炎症反应[9-10]等多个分子生化过程。近年来,对虫草素药理作用机制尤其是抗肿瘤作用机制的研究已成为极其活跃的研究领域之一。已从菌株筛选、产量提高、人工合成修饰、作用机制及产品开发等多方面对虫草素进行了研究。本文从分子生物学角度对虫草素分子结构和抗肿瘤作用机制进行综述,并对虫草素研究和应用中存在的问题及解决对策提出自己的见解,以期为其药物开发提供新的思路,为临床应用奠定理论基础。
1 虫草素的理化特性及合成
虫草素,分子式为C10H13N5O3,分子质量为251 u,碱性,呈针状或片状结晶,熔点228~231°C,紫外最大吸收波长为259.0 nm。虫草素溶于生理盐水、热乙醇和甲醇,不溶于苯、乙醚和氯仿[11]。虫草素可通过提取纯化和人工合成两种途径获得。目前天然虫草素主要从蛹虫草中获取,但生产成本极高。以3′-O-对硝基苯磺酰基腺苷为原料合成虫草素的方法相对较多,主要有氯硫代甲酸苯酯法、2-乙酰氧基异丁酰溴法和原乙酸三甲酯法3种[12]。此外,Hansske等[13]利用叔戊酸2-羟基-4-戊炔酯为原料成功合成虫草素;Mc Donald等[14]利用6-N-苯甲酰基-5′-叔丁基二甲基硅氧基-2′,3′-环氧腺苷为原料成功合成虫草素。但虫草素化学合成技术尚未完全成熟,产率较低,且常伴反应副产物的污染。
2 虫草素抗肿瘤作用机制
2.1 抑制嘌呤、DNA和RNA生物合成及蛋白质翻译
人体细胞内,虫草素通过嘌呤核苷代谢途径转化为三磷酸虫草素——虫草素的生物活性形式。三磷酸虫草素抑制核糖磷酸焦磷酸激酶和5′-磷酸核糖焦磷酸转移酶活性,从而抑制嘌呤的合成,进而抑制肿瘤细胞DNA合成[15-17]。三磷酸虫草素结构和三磷酸腺苷相似,在多聚腺苷酸聚合酶或末端核糖腺苷酸转移酶作用下,参与RNA多聚腺苷酸链合成,参与其中的三磷酸虫草素作为链终止子,终止细胞RNA的合成[18-19]。虫草素还可通过激活腺苷酸活化蛋白激酶(adenosine monophosphateactivated protein kinase,AMPK)关闭mTOR信号转导通路,从而终止蛋白翻译,抑制细胞增殖和生长。同时,虫草素通过缩短mRNA的多聚腺苷酸长度抑制细胞间黏附[20]。
2.2 诱导肿瘤细胞凋亡和调控细胞周期
研究表明,与虫草素诱导肿瘤细胞凋亡相关的信号通路有NF-κB信号通路和丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路等。丁向萍等[21]报道,虫草素显著下调人肝癌细胞HepG-2中NF-κB P65蛋白的表达,降低细胞内端粒酶活性,最终促使HepG-2细胞凋亡。He等[22]报道,虫草素通过增强c-Jun氨基端激酶(c-Jun N-terminal kinase,JNK)蛋白激酶和P38蛋白激酶活性,从而上调Bcl-2家族中的促凋亡蛋白表达,诱导人结肠癌细胞SW480和SW620发生凋亡。Choi等[23]发现,将虫草素作用于人乳腺癌细胞MDAMB-231也表现出相似的细胞凋亡过程。胱天蛋白酶家族引发的级联反应也是细胞凋亡过程中的重要环节。虫草素可使X连锁凋亡抑制蛋白(X-linked inhibitor of apoptosis protein,XIAP)和细胞凋亡抑制蛋白1(cellular inhibitor of apoptosis protein-1,cIAP-1)水平降低,同时使促凋亡蛋白Bax和Bas水平升高,Bax蛋白从胞浆易位到线粒体引起细胞色素c释放,进而激活胱天蛋白酶9和胱天蛋白酶3,致使淋巴瘤细胞U937和THP-1发生凋亡[24]。Baik等[25]报道,虫草素使胱天蛋白酶3表达上调,激活多聚二磷酸腺苷核糖聚合酶(poly ADP-ribose polymerase,PARP),启动PARP蛋白促凋亡作用,致使人神经母细胞瘤细胞CRL-2268及人黑色素瘤细胞SK-Mel-2发生凋亡。Lee等[26]报道,在乳腺癌细胞中,虫草素通过激活DNA损伤反应,包括PARP蛋白裂解及共济失调毛细血管扩张突变基因蛋白(ataxia telangiectasia mutated,ATM)、共济失调毛细血管扩张突变基因Rad3相关蛋白(ataxia telangiectasia mutated and Rad3 related protein,ATR)及组蛋白2A变异体磷酸化(γ histone family 2A variant,γH2AX),从而使细胞凋亡。虫草素诱导肿瘤细胞凋亡的信号转导通路见图2。
虫草素不仅能诱导细胞凋亡,还可调控细胞周期。在人口腔鳞癌细胞中,虫草素使细胞周期中的G1期缩短,G2期和M期延长,且呈现浓度相关性[27]。在人膀胱癌细胞5637和T-24中,虫草素既上调JNK蛋白表达诱导肿瘤细胞凋亡,同时上调P21WAF1基因表达,从而抑制细胞周期蛋白B1/ CDC2表达,使细胞周期阻滞于G2/M期[28]。虫草素作用于大肠癌细胞HCT116也可观测到同样的结果[29]。虫草素通过上调P27表达和下调细胞周期蛋白D1/细胞周期蛋白依赖性激酶4(cyclin-dependent kinase 4,CDK4)表达,从而阻滞细胞周期[30]。Jung等[31]报道,虫草素通过Ras/细胞外调节蛋白激酶1(extracellular regulated protein kinases 1,ERK1)信号通路上调p27KIP1基因表达,抑制细胞周期蛋白E/CDK2表达,使细胞周期阻滞于G1/S期,进而抑制细胞增殖。虫草素阻滞肿瘤细胞周期的信号转导通路见图3。
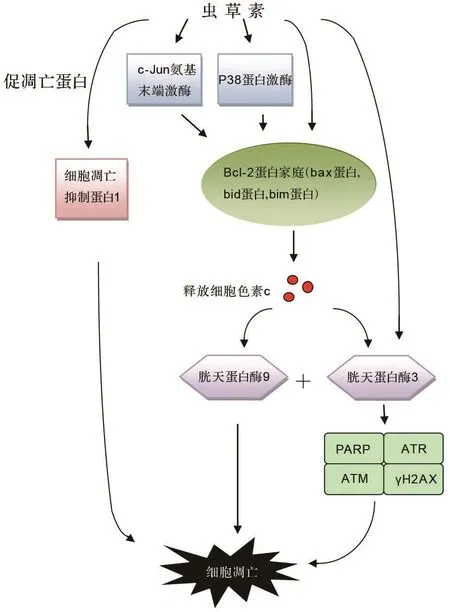
图2 虫草素诱导肿瘤细胞凋亡的信号通路.PARP:多聚二磷酸腺苷核糖聚合酶;γH2AX:组蛋白2A变异体磷酸化;ATM:共济失调毛细血管扩张突变基因蛋白;ATR:共济失调毛细血管扩张突变基因Rad3相关蛋白.
2.3 抗肿瘤细胞侵袭
癌症转移是多步骤且极其复杂的过程,而肿瘤细胞对细胞外基质(extracellular matrix,ECM)的侵袭是这一过程中极为重要的一步[32]。基质金属蛋白酶(metalloproteinases,MMP)是一个内肽酶家族,MMP蛋白表达上调使多种癌症(如脑肿瘤、膀胱癌、乳腺癌和前列腺癌)的侵袭性增强[33-34]。在MMP蛋白家族中,MMP-2和MMP-9由于能将基底膜中主要成分——胶原蛋白IV酶解而备受关注[35-36]。Ren等[37]报道,虫草素可以抑制NF-κB抑制蛋白α(inhibitor of nuclear factor kappa-B kinase α,IκBα)激酶γ亚基(inhibitor nuclear factor kappa-B kinase γ,IKKγ)泛素化,使IKKα和IKKβ活性降低,从而抑制IκBα的磷酸化及降解,抑制NF-κB信号通路,最终抑制MMP-9的表达。此外,IKKα和IKKβ还能调控干扰素调控因子信号通路和MAPK信号通路。在人乳腺癌细胞MCF-7中,虫草素通过抑制MAPK/转录因子激活蛋白1途径下调MMP-9表达,从而降低其侵袭性[38]。Lee等[39]研究发现,在表皮成纤维细胞中,虫草素可通过抑制NF-κB信号通路而完全抑制MMP-1和MMP-3的表达。Jeong等[40]研究发现,虫草素能减少紧密连接和下调MMP活性,进而抑制人前列腺癌细胞的转移和侵袭,而这一过程极有可能同阻滞磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/v-akt蛋白激酶(v-akt kinase,Akt)信号通路相关。虫草素作用于胃癌细胞MKN-28可阻止表皮生长因子(epidermal growth factor,EGF)信号通路活化,下调埃滋蛋白(ezrin protein)的表达,减少MMP-9的分泌,降低其破坏基底膜向周围组织侵袭和转移的能力[41]。虫草素通过MAPK、NF-κB和PI3K/Akt等信号转导通路干预MMP表达,从而抑制肿瘤细胞侵袭和转移(图4)。

图3 虫草素阻滞肿瘤细胞周期的信号通路.ERK1:细胞外调节蛋白激酶1;cdc2:裂殖酵母cdc2基因.

图4 虫草素抑制肿瘤细胞侵袭和转移的信号通路.IKKγ:IκB激酶γ亚基;IKKα:IκB激酶α亚基;IKKβ:IκB激酶β亚基;IκBα:NF-κB抑制蛋白α.
2.4 抑制血小板凝集作用
肿瘤细胞诱导血小板聚集,继而形成癌栓,降低癌细胞在宿主体内的免疫原性,阻断自然杀伤细胞与癌细胞直接接触,协助癌细胞在血流中安全转移[42]。Cho等[43]报道,虫草素通过降低胞质内Ca2+浓度和提高胞质内cAMP/cGMP水平,达到抑制血小板凝集的目的,且呈浓度相关性;虫草素500 μmol·L-1可使胞质内的Ca2+浓度和血栓素A2浓度分别下降74%和46%;虫草素可抑制Ca2+泵和血栓素类似物U46619从而使细胞内的Ca2+浓度上升,抑制血小板凝集,阻止癌栓形成。除此之外,虫草素还能抑制肿瘤细胞释放腺苷二磷酸所引起的血小板聚集[44]。
2.5 抗炎作用
Balkwill等[45]撰文中提到,德国病理学家Virchow于1863年观察到肿瘤组织中有一些白细胞存在,第一次提出炎症与癌症可能存在着某些联系。目前,大量流行病学、基因组学及分子生物学的研究表明,炎症在肿瘤发生发展过程中具有决定性作用[46-47]。研究发现,过量表达淋巴因子和趋化因子可诱导癌症发生;炎症和癌症过程中有相似的分子通路调控;非甾体类抗炎药如阿司匹林,能显著降低胃肠道癌症和乳腺癌等癌症的发病率。因而,控制炎症反应对预防和治疗肿瘤至关重要[48]。Kim等[49]报道,虫草素可抑制Akt活化及P38磷酸化,抑制肿瘤坏死因子α(tumor necrosis factor-α,TNF-α),从而抑制NF-κB的激活,最终下调诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)表达,减少NO产生,进而起到抗炎作用。Jeong等[50]研究报道,虫草素抑制IκBα降解,从而抑制NF-κB活化,最终显著减少炎症因子的释放。虫草素还能增加人外周血单核细胞中白细胞介素10的表达,显著减少炎症因子如白细胞介素2和干扰素γ等的产生[51]。Rao等[52]研究报道,虫草素能减少活性氮中间体、转录因子如TNF-α及炎症因子如白细胞介素12的产生。该研究提示,虫草素的抗炎作用有助于其预防癌症的发生。虫草素抑制炎症反应的信号通路见图5。

图5 虫草素抑制炎症反应的信号通路.Akt:安基因蛋白激酶.
3 虫草素研究和应用中存在的问题与对策
虫草素既诱导肿瘤细胞凋亡又阻滞肿瘤细胞周期,从而抑制肿瘤细胞生长;虫草素既调控肿瘤细胞侵袭性又抑制肿瘤细胞诱导的血小板聚集,使免疫系统识别并攻击肿瘤细胞,从而抑制肿瘤细胞血行转移,故虫草素抗肿瘤作用是从多个方面和多条路径协同发挥的。而在虫草素介导的信号通路中,一些信号通路又参与多个调控过程。MAPK信号通路涉及细胞凋亡的调控、细胞周期阻滞、肿瘤细胞侵袭性的调控、炎性因子释放的调控;NF-κB不仅调控肿瘤细胞侵袭性,还调控炎症因子的释放。因此,明确虫草素抗肿瘤作用所涉及的信号通路及信号通路之间的交互关系,既为药物开发提供了新的思路,又为虫草素临床应用奠定了理论基础。但虫草素介导的抗肿瘤相关的信号通路之间的交互关系及MAPK通路级联反应的一些上游调节激酶和下游作用底物等尚未完全清楚,仍需在今后研究中不断探索。
在人体细胞内,虫草素通过嘌呤核苷代谢途径,在腺苷脱氨酶(adenosine deaminase,ADA)作用下,快速脱氨基形成无生物活性的代谢产物——3′-脱氧次黄嘌呤核苷,只有极小部分磷酸化后形成具有生物活性的三磷酸虫草素[15]。虫草素被ADA迅速脱氨丧失生物活性,阻碍了其生物作用的发挥,这极大地限制了虫草素的开发和应用。目前,可从以下3个途径解决这一问题。①稳定虫草素的结构,添加具有防脱氨作用的侧键、基团或离子,构建结构合理的虫草素衍生物。Wei等[53]合成的N-酰基虫草素衍生物可保护其氨基,提高其生物活性;Shimada等[54]研究报道,以氟取代4′位上的氢,可延缓其代谢并减低其细胞毒性。目前已得到的虫草素衍生物种类有限,并且在虫草素上加某些侧键基团虽可以延缓脱氨作用,但仍然不能完全阻断ADA脱氨作用,在今后研究中仍需对虫草素衍生物侧键、基团的种类及位置进行完善。②将虫草素与ADA抑制剂联合应用。喷司他丁——ADA抑制剂,联合虫草素治疗急性淋巴细胞性白血病或慢性粒细胞性白血病在美国已完成Ⅰ期临床试验,Ⅱ期临床试验正在进行中[55]。脱氧柯福霉素联合虫草素能有效清除小鼠体内布氏锥虫[56]。虫草素与ADA抑制剂联合应用具有一定副作用,该副作用具有可逆性和剂量依赖性,这为虫草素临床应用提供了理论依据[57]。③将虫草素与纳米新材料组合形成新的复合物。陈望化等[58]将虫草素插入到层状双氢氧化物层之间,得到稳定虫草素/双氢氧化物纳米复合物,可有效防止其被ADA脱氨。目前,关于虫草素衍生物与ADA抑制剂联合应用以及虫草素衍生物与纳米材料组合形成新复合物药效评价的研究皆未见报道,其结果值得期待。
4 结语
虫草素抑制DNA和RNA合成,终止蛋白质翻译,诱导肿瘤细胞凋亡,阻滞其细胞周期,从而抑制肿瘤细胞的生长和增殖;降低肿瘤细胞侵袭性,减少转移;能抑制血小板聚集,使肿瘤细胞暴露于免疫系统监控之下;抑制炎症反应,避免过度免疫引起的损伤,有效预防和治疗癌症。虫草素在肿瘤的发生、发展及转移等多个过程中都可进行调控,故其在抗肿瘤临床应用中潜力巨大。而完善虫草素抗肿瘤相关信号通路及各通路之间的交互关系、降低虫草素在体内脱氨速率是虫草素研究与应用中亟待解决的问题。虽然北虫草和冬虫夏草是传统的中医药材,但是我国对冬虫夏草的研究较晚,故而对虫草素的研究也相对落后。国内研究仍偏重于虫草素的提取、检测和人工培养技术改进等方面,对虫草素作用机制、调控途径和衍生物合成等方面的研究尚处于起步阶段。因此,加强虫草素作用机制、调控途径及衍生物合成等方面的研究,对虫草素的开发及临床应用意义深远。
[1]Wu WD,Hu ZM,Shang MJ,Zhao DJ,Zhang CW, Hong DF,et al.Cordycepin down-regulates multiple drug resistant(MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells[J].Int J Mol Sci,2014,15(7):12778-12790.
[2]Cunningham KG,Hutchinson SA,Manson W,Spring FS.Cordycepin,a metabolic product from cultures ofCordyceps militaris(Linn.)link.Part I. Isolation and characterisation[J].J Chem Soc,1951,2229-2300.
[3]Wu WD,Hu ZM,Shang MJ,Zhao DJ,Zhang CW,Hong DF,et al.Cordycepin down-regulates multiple drug resistant(MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells[J].Int J Mol Sci,2014,15:12778-12790.
[4]Lee HH,Kim SO,Kim GY,Moon SK,Kim WJ, Jeong YK,et al.Involvement of autophagy in cordycepin-induced apoptosis in human prostate carcinoma LNCaP cells[J].Environ Toxicol Phar-macol,2014,38(1):239-250.
[5]Wang XA,Xiang SS,Li HF,Wu XS,Li ML,Shu YJ,et al.Cordycepin induces S phase arrest and apoptosis in human gallbladder cancer cells[J].Molecules,2014,19(8):11350-11365.
[6]Jeong MH,Lee CM,Lee SW,Seo SY,Seo MJ, Kang BW,et al.Cordycepin-enrichedCordyceps militarisinduces immunomodulation and tumor growth delay in mouse-derived breast cancer[J].Oncol Rep,2013,30(4):1996-2002.
[7]Lu H,Li X,Zhang J,Shi H,Zhu X,He X.Effects of cordycepin on HepG2 and EA.hy926 cells: Potential antiproliferative,antimetastatic and antiangiogenic effects on hepatocellular carcinoma[J].Oncol Lett,2014,7(5):1556-1562.
[8]Lee DH,Kim HH,Cho HJ,Yu YB,Kang HC,Kim JL,et al.Cordycepin-enriched WIB801C fromcordyceps militarisinhibits collagen-induced[Ca2+]imobilization via cAMP-dependent phosphorylation of inositol 1,4,5-trisphosphate receptor in human platelets[J].Biomol Ther(Seoul),2014,22(3):223-231.
[9]Choi YH,Kim GY,Lee HH.Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways[J].Drug Des Dev Ther,2014,8:1941-1953.
[10]Park ES,Kang DH,Yang MK,Kang JC,Jang YC, Park JS,et al.Cordycepin,3'-deoxyadenosine, prevents rat hearts from ischemia/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression[J].Cardiovasc Toxicol,2014,14(1):1-9.
[11]Yang J,Chen SZ.Progress in the study on cordycepin[J].Chin J Biochem Pharm(中国生化药物杂志),2008,29(6):414-417.
[12]Tu HY,Li XF,Lu XY.Review on chemosynthesis of 3'-deoxyadenosine[J].Chem Ind Times(化工时刊),2006,20(2):66-69.
[13]Hansske F,Cramer F.Reaction of the D-ribose moiety of adenosine and AMP with periodate and 5,5-dimethylcyclohexane-1,3-dione(dimedone)[J].Carbohydr Res,1975,41:366-369.
[14]McDonald FE,Gleason MM.Asymmetric synthesis of nucleosides via molybdenum-catalyzed alkynol cycloisomerization coupled with stereoselective glycosylations of deoxyfuranose glycalsand 3-amidofuranose glycals[J].J Am Chem Soc,1996,118(28):6648-6659.
[15]el-Khadem HS,el-Ashry SH.Synthesis of cordycepin-C(8-(3'-deoxy-beta-D-erythro-pentofuranosyl)adenine)[J].Carbohydr Res,1973,29(2):525-527.
[16]Overgaard-Hansen K.The inhibition of 5-phosphoribosyl-1-pyrophosphateformationby cordycepin triphosphate in extracts of ehrlich ascites tumor cells[J].Biochim Biophys Acta,1964,80:504-507.
[17]Kato K,Hayakawa H,Tanaka H,Kumamoto H, Shindoh S,Satoshi S,et al.A new entry to 2-substituted purine nucleosides based on lithiation-mediated stannyl transfer of 6-chloropurine nucleosides[J].J Org Chem,1997,60(20):6833-6841.
[18]Chen LS,Stellrecht CM,Gandhi V.RNA-directed agent,cordycepin,induces cell death in multiple myeloma cells[J].Br J Haematol,2008,140(6):682-687.
[19]Holbein S,Wengi A,Decourty L,Freimoser FM, Jacquier A,Dichtl B.Cordycepin interferes with 3′end formation in yeast independently of its potential to terminate RNA chain elongation[J].RNA,2009,15(5):837-849.
[20]Wong YY,Moon A,Duffin R,Barthet-Barateig A, Meijer HA,Clemens MJ,et al.Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction[J].J Biol Chem,2010,285(4):2610-2621.
[21]Ding XP,Ma L.Research progress of antitumour mechanisms of cordycepin[J].J Fourth Mil Med Univ(第四军医大学学报),2009,30(8):764-766.
[22]He W,Zhang MF,Ye J,Jiang TT,Fang X,Song Y. Cordycepin induces apoptosis by enhancing JNK and p38 kinase activity and increasing the protein expression of Bcl-2 pro-apoptotic molecules[J].J Zhejiang Univ Sci B,2010,11(9):654-660.
[23]Choi S,Lim MH,Kim KM,Jeon BH,Song WO, KimTW.Cordycepin-inducedapoptosisand autophagy in breast cancer cells are independent of the estrogen receptor[J].Toxicol Appl Pharmacol,2011,257(2):165-173.
[24]Jeong JW,Jin CY,Park C,Hong SH,Kim GY, Jeong YK,et al.Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells[J].Toxicol In Vitro,2011,25(4):817-824.
[25]Baik JS,Kim KS,Moon HI,An HK,Park SJ, Kim CH,et al.Cordycepin-mediated transcriptional regulation of human GD3 synthase(hST8Sia I)in human neuroblastoma SK-N-BE(2)-C cells[J].Acta Biochim Biophys Sin(Shanghai),2014,46(1):65-71.
[26]Lee HJ,Burger P,Vogel M,Friese K,Brüning A. Thenucleosideantagonistcordycepincauses DNA double strand breaks in breast cancer cells[J].Invest New Drugs,2012,30(5):1917-1925.
[27]Wu WC,Hsiao JR,Lian YY,Lin CY,Huang BM. The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line[J].Cancer Chemother Pharmacol,2007,60(1):103-111.
[28]Lee SJ,Moon GS,Jung KH,Kim WJ,Moon SK. c-Jun N-terminal kinase 1 is required for cordycepin-mediated induction of G2/M cell-cycle arrest via p21WAF1 expression in human colon cancer cells[J].Food Chem Toxicol,2010,48(1):277-283.
[29]Imesch P,Goerens A,Fink D,Fedier A.MLH1-deficient HCT116 colon tumor cells exhibit resistance to the cytostatic and cytotoxic effect of the poly(A)polymeraseinhibitorcordycepin(3′-deoxyadenosine)in vitro[J].Oncol Lett,2012,3(2):441-444.
[30]Yoshikawa N,Yamada S,Takeuchi C,Kagota S, Shinozuka K,Kunitomo M,et al.Cordycepin(3′-deoxyadenosine)inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosineA3receptorfollowedbyglycogen synthase kinase-3beta activation and cyclin D1 suppression[J].Naunyn Schmiedebergs Arch Pharmacol,2008,377(4-6):591-595.
[31]Jung SM,Park SS,Kim WJ,Moon SK.Ras/ERK1 pathwayregulationofp27KIP1-mediatedG1-phase cell-cycle arrest in cordycepin-induced inhibition of the proliferation of vascular smooth muscle cells[J].Eur J Pharmacol,2012,681(1-3):15-22.
[32]Keskinov AA,Shurin MR.Myeloid regulatory cells in tumor spreading and metastasis[J].Immunobiology,2014,220(2):236-242.
[33]FiorentiniC,BodeiS,BedussiF,FragniM,BoniniSA, Simeone C.GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity[J].Exp Cell Res,2014,323(1):100-111.
[34]Dodd T,Jadhav R,Wiggins L,Stewart J,Smith E,Russell JC,et al.MMPs 2 and 9 are essential for coronary collateral growth and are prominently regulated by p38 MAPK[J].J Mol Cell Cardiol,2011,5(6):1015-1025.
[35]Chung TW,Moon SK,Chang YC,Ko JH,Lee YC, Cho G,et al.Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells:complete regression of hepatoma growth and metastasis by dual mechanism[J].FASEB J,2004,18(14):1670-1681.
[36]Hong S,Park KK,Magae J,Ando K,Lee TS, Kwon TK,et al.Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the ERK1/2 signaling pathway:inhibitory effects of ascochlorin on the invasion of renal carcinoma cells[J].J Biol Chem,2005,280(26):25202-25207.
[37]Ren Z,Cui J,Huo Z,Xue J,Cui H,Luo B,et al. Cordycepin suppresses TNF-α-induced NF-κB activation by reducing p65 transcriptional activity, inhibitingIκBαphosphorylation,andblocking IKKγ ubiquitination[J].Int Immunopharmacol,2012,14(4):698-703.
[38]Noh EM,Youn HJ,Jung SH,Han JH,Jeong YJ, Chung EY,et al.Cordycepin inhibits TPA-induced matrix metalloproteinase-9 expression by suppressingtheMAPK/AP-1pathwayinMCF-7human breast cancer cells[J].Int J Mol Med,2010,25(2):255-260.
[39]Lee YR,Noh EM,Jeong EY,Yun SK,Jeong YJ, Kim JH,et al.Cordycepin inhibits UVB-induced matrix metalloproteinase expression by suppressing the NF-kappaB pathway in human dermal fibroblasts[J].ExpMolMed,2009,41(8):548-554.
[40]Jeong JW,Jin CY,Park C,Han MH,Kim GY, Moon SK,et al.Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt[J].Int J Oncol,2012,40(5):1697-1704.
[41]Sun GB,Lan L,Tang HM,Lin N,Wang.Effects ofCordyceps sinensison the migration and invasion capability of human gastric adenocarcinoma cell line MKN28[J].J Tianjin Univ Tradit Chin Med(天津中医药大学学报),2014,33(3):152-156.
[42]Jia J,Chen Y.Role of platelets in tumor metastasis[J].Chin Clin Oncol(临床肿瘤学杂志),2013,18(11):1033-1036.
[43]Cho HJ,Cho JY,Rhee MH,Park HJ.Cordycepin(3'-deoxyadenosine)inhibitshumanplatelet aggregation in a cyclic AMP-and cyclic GMP-dependent manner[J].Eur J Pharmacol,2007,558(1-3):43-51.
[44]Lee DH,Kwon HW,Kim HH,Lim DH,Nam GS, Shin JH,et al.Cordycepin-enriched WIB801C fromCordyceps militarisinhibits ADP-induced [Ca2+]imobilization andfibrinogen binding via phosphorylation of IP3R and VASP[J].Arch Pharm Res,2015,38(1):81-97.
[45]Balkwill F,Mantovani A.Cancer and inflammation:implications for pharmacology and therapeutics[J].ClinPharmacolTher,2010,87(4):401-406.
[46]Diakos CI,Charles KA,McMillan DC,Clarke SJ. Cancer-related inflammation and treatment effectiveness[J].Lancet Oncol,2014,15(11):e493-e503.
[47]WaldnerMJ,NeurathMF.Colitis-associated cancer:the role of T cells in tumor development[J].Semin Immunopathol,2009,31(2):249-256.
[48]Bower JE,Lamkin DM.Inflammation and cancerrelated fatigue:mechanisms,contributing factors, and treatment implications[J].Brain Behav Immun,2013,30 Suppl:S48-S57.
[49]Kim HG,Shrestha B,Lim SY,Yoon DH,Chang WC, Shin DJ,et al.Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells[J].Eur J Pharmacol,2006,545(2-3):192-199.
[50]Jeong JW,Jin CY,Kim GY,Lee JD,Park C, Kim GD,et al.Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells[J].Int Immunopharmacol,2010,10(12):1580-1586.
[51]Zhou X,Meyer CU,Schmidtke P,Zepp F.Effect ofcordycepinoninterleukin-10productionof human peripheral blood mononuclear cells[J].Eur J Pharmacol,2002,453(2-3):309-317.
[52]Rao YK,Fang SH,Wu WS,Tzeng YM.Constituents isolated fromCordyceps militarissuppress enhancedinflammatorymediator'sproduction and human cancer cell proliferation[J].J Ethnopharmacol,2010,131(2):363-367.
[53]Wei HP,Ye XL,Chen Z,Zhong YJ,Li PM,Pu SC,et al.Synthesis and pharmacokinetic evaluation of novel N-acyl-cordycepin derivatives with a normal alkyl chain[J].Eur J Med Chem,2009,44(2):665-669.
[54]Shimada H,Haraguchi K,Hotta K,Miyaike T, Kitagawa Y,Tanaka H,et al.Synthesis of 3',4'-difluoro-3'-deoxyribonucleosides and its evaluation of the biological activities:discovery of a novel type of anti-HCV agent 3',4'-difluorocordycepin[J].Bioorg Med Chem,2014,22(21):6174-6182.
[55]Chou SM,Lai WJ,Hong TW,Lai JY,Tsai SH, Chen YH,et al.Synergistic property of cordycepinincultivatedCordycepsmilitaris-mediated apoptosis in human leukemia cells[J].Phytomedicine,2014,21(12):1516-1524.
[56]Rottenberg ME,Masocha W,Ferella M,Petitto-Assis F,Goto H,Kristensson K,et al.Treatment of African trypanosomiasis with cordycepin and adenosine deaminase inhibitors in a mouse model[J].J Infect Dis,2005,192(9):1658-1665.
[57]Jiang N,Liu HJ,Liu F,Zhu YZ,Wang DY,Xu WM. Current situation and prospects of cordycepin research and exploitation[J].Acta Agric Jiangxi(江西农业学报),2011,23(1):121-123.
[58]Chen WH,Yang QZ,Sun YJ,Zhang CK.Cordycepin/LDHnanometercompoundinhabitsthe growth of U937 tumour cell[J].Chin J Biochem Pharm(中国生化药物杂志),2006,27(3):156-159.
Progress in molecular mechanisms of anticaner action of cordycepin
WANG Duo,BAO Rong,WANG Fang,HU Yong-hong,CHAI Hui-xia
(College of Life Sciences,Hebei Normal University,Shijiazhuang 050024,China)
Cordycepin(3'-deoxyadenosine),a nucleoside analog,is reported to have many pharmacological activities and reliable therapeutic effects on many diseases,especially on cancers.Molecular mechanisms of cordycepin against cancers and its targets have attracted extensive attention in this field.Cordycepin has many intracellular targets,through which it regulates cell growth,proliferation and metastasis in cancer cells.The anticancer mechanisms of cordycepin include inhibiting purine,DNA and RNA synthesis and protein translation,inducing cell apoptosis and regulating cell cycle,inhibiting invasion,platelet aggregation and inflammation.This paper offers some solutions to the synthesis of cordycepin derivatives,combination with adenosine deaminase,and creation of new compounds with nano-materials in order to provide useful information on further research and clinical application of cordycepin。
cordycepin;antineoplastic agents;mitogen-activated protein kinases;apoptosis;cell cycle
The project supported by National Natural Science Foundation of China(C040501);and Natural Science Foundation of Hebei Province(C190402)
CHAI Hui-xia,E-mail:wdcxycy@163.com,Tel:(0311)80787552
R285
A
1000-3002-(2015)04-0643-08
10.3867/j.issn.1000-3002.2015.04.018
2015-02-05接受日期:2015-07-22)
(本文编辑:齐春会)
国家自然科学基金项目(C040501);河北省自然科学基金项目(C190402)
王多,女,硕士,实验师,主要从事中药药理学研究。
柴惠霞,E-mail:wdcxycy@163.com,Tel:(0311)80787552

