西北干旱区典型固沙植物夜间耗水及其影响因素
徐世琴,吉喜斌,金博文
(1中国科学院寒区旱区环境与工程研究所,中国生态系统研究网络临泽内陆河流域研究站,兰州730000;2中国科学院大学,北京100049)
西北干旱区典型固沙植物夜间耗水及其影响因素
徐世琴1,2,吉喜斌1*,金博文1
(1中国科学院寒区旱区环境与工程研究所,中国生态系统研究网络临泽内陆河流域研究站,兰州730000;2中国科学院大学,北京100049)
利用热平衡包裹式Flow32茎干液流仪和环境要素监测系统研究了河西走廊中段典型固沙植物梭梭(Haloxylon ammodendron)、白刺(Nitraria tangutorum)、沙拐枣(Calligonum mongolicum)夜间液流活动特征,分析影响3种植物夜间耗水的主要环境因素及其利用途径。结果表明:(1)梭梭、白刺、沙拐枣茎干夜间液流密度在前半夜(20:00~0:00)较大且迅速降低,在后半夜(0:00~6:00)仍有微弱液流密度且波动较大;梭梭、白刺、沙拐枣的夜间液流密度差异极显著,平均夜间液流密度依次为3.73、1.12、6.07g·cm-2·h-1,且在典型降雨天气条件下3种植物夜间液流活动明显减弱。(2)在观测期间,总夜间耗水量与基茎显著相关,但3种植物夜间耗水分配在不同月份间差异不显著;梭梭、白刺、沙拐枣夜间耗水对日总耗水贡献率变化范围分别为1%~30%、0.1%~16%和1.5%~20%。(3)饱和水汽压差和风速仅能解释梭梭、沙拐枣、白刺夜间液流密度24%、25%、27%的变化,3种植物夜间液流主要用以茎干补水。
茎干液流;单因素方差分析;荒漠植物;夜间耗水
蒸腾是陆地表层物质与能量交换过程的关键环节,也是植物生命活动的重要过程。借助茎干液流监测系统,越来越多研究发现各种生境中的植物存在夜间液流,该过程对于调控植物冠层与大气水分交换通量起着重要作用[1-2],也是植物保持和恢复水分平衡的生理策略[3-5]。白天强烈的蒸腾会导致植物体内严重水分亏缺,而夜间液流能够缓冲夜间植物体内较低的水势从而有效平衡植物体内水分,另外夜间液流也是一些植物适应极端干旱环境的重要方式[6-8]。Phillips等[9]发现高大植物通过夜间液流能够潜在补偿水分传输限制(hydraulic limitation),另外夜间液流还能维持因暗呼吸导致的碳水化合物持续向外传输,这一功能对于速生型耐阴植物十分重要[10],同时也能防止植物茎干输水组织产生栓塞和空穴[11]。
研究表明,饱和水汽压差(saturate vapor pressure difference,VPD)和风速是影响植物夜间液流的主要环境因子,二者与液流的关系通常被用来判断夜间液流的利用途径[12-13]。当液流同VPD和风速高度相关时,夜间液流主要用以蒸腾,其主要原因在于尽管入夜后光合有效辐射为零,但当VPD很高时其仍然能够成为气孔张开的重要驱动因子从而发生冠层水分交换,较高风速则能够维持更低冠层边界层阻力从而加强植物叶片的水汽扩散速率[7]。当夜间液流与VPD和风速关系较弱时,夜间液流主要用以植物茎干水分补充[14]。
梭梭、白刺、沙拐枣是西北干旱区典型的固沙植物[15],它们在长期适应干旱环境的过程中形成了独特的生理生态性能[16-18]。目前,植物夜间液流的研究对象主要为生长于热带、亚热带、温带等环境条件相对湿润的高大乔木[1-2,10,19-21],对极端干旱环境条件下灌木的夜间液流开展的相关研究还比较少见。本实验基于2014年对西北荒漠地区梭梭群落3种主要植物茎干液流以及环境要素长期野外观测数据,旨在研究3种植物夜间液流活动,分析影响3种植物夜间耗水的主要环境因素及其利用途径。
1 材料和方法
1.1 研究区概况
野外观测实验样地位于中国西北河西走廊中段临泽绿洲-荒漠过渡带(39°22′07″N,100°08′48″E,海拔1 386m),为典型大陆性干旱气候,干旱高温和多风是其气候的主要特点。其中,年均日照时数和辐射总量分别为3 018h和6 254MJ·m-2;年平均气温8.9℃,年平均降水量为123mm,近80%降水集中于6~9月;本区主风向为西北风,年均风速2.7 m·s-1;土壤为壤质砂土。除了梭梭、白刺和沙拐枣3种建群植物外,还零星分布有柽柳(Tamarix chinensis)、花棒(Hedysarum scoparum)和芦苇(Phragmites communis)等,植被盖度15%左右。
1.2 茎干液流测定
2014年6月1日~9月30日期间,利用热平衡包裹式Flow32(Dynamax,Inc.,Houston,USA)茎干液流仪对生长状况良好的梭梭、白刺、沙拐枣茎干液流进行测定,探头的具体型号和相应基茎见表1。测定前,用砂纸轻轻将茎杆打磨光滑,然后用游标卡尺测量茎干直径,在打磨好的位置涂抹G4保护油脂后仔细将加热片安装于被测区,用铝箔包裹,最后用胶带密封,防止雨水进入。通过计算机分别将被测样枝的茎干类型、横截面积、探头电压、起始时间、数据记录间隔等参数输入到数据采集器CR1000中并定期采集数据,测定期间每两周更换一次探头。本研究的数据采集间隔为60s,每30min平均一次并储存,将光合有效辐射(PAR)为零时的液流值作为夜间液流[7,22]。
1.3 立地环境要素测定
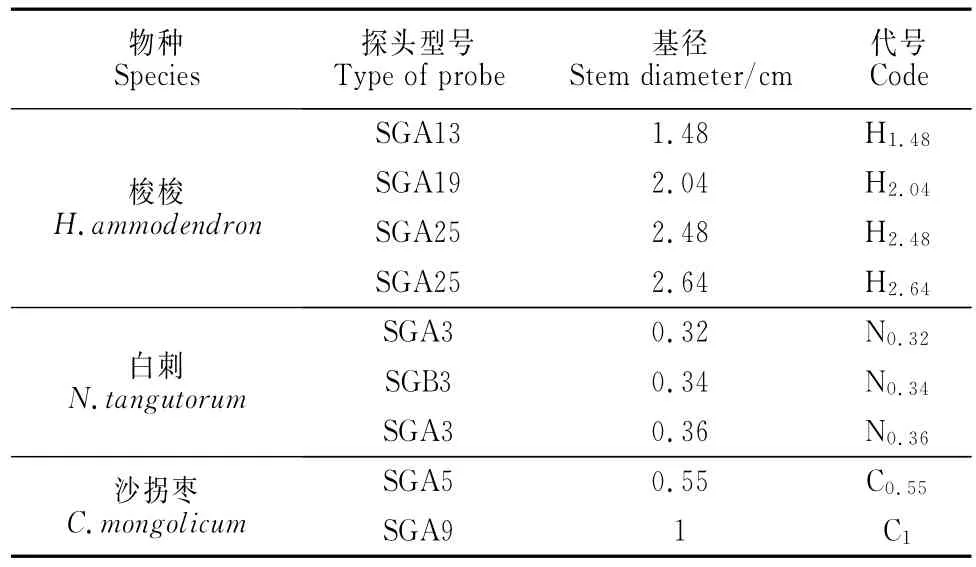
表1 被测茎干基本参数和探头型号Table 1 Basic parameters of measured stems and types of probe
试验地建有8m微气象观测塔,于冠层顶部2 m高度处安装有净辐射传感器(CNR4,Kipp &Zonen,Delft,Netherlands)、光合有效辐射传感器(LI-190SB,LI-COR Inc.,Lincoln,NE,USA)、大气温度和湿度传感器(HMP155A,Vaisala,Helsinki,Finlnd),和风速风向传感器(1405-PK-052Wind-Sonic anemometer,Gill Instruments,Ltd.,Lymington,UK)。植被冠层顶部安装有降雨量传感器(TE525MM tipping bucket rain gauges,Texas E-lectronics,Inc.,Texas,USA)。土壤温度探头(109-L,Campbell Scientific,Inc.,UT,USA)和水分探头(CS616,Campbell Scientific,Inc.,UT,USA),设置深度均为10、20、40、60、80、100cm(文中采用10 cm深度处土壤温度、水分数据)。所有数据通过数据采集器(CR1000-XT,Campbell Scientific Inc.,USA)自动记录,每30min计算平均值并进行存储。
1.4 数据处理
液流数据分析采用统计软件SPASS 16.0。单因素方差分析(One-way ANOVA)判断不同植物茎干夜间液流密度种间差异及不同月份夜间耗水、夜间耗水对日总耗水贡献率差异。夜间液流密度与环境要素的关系采用相关分析和回归分析,夜间耗水与基茎的关系采用回归分析。
2 结果与分析
2.1 植物茎干液流密度日变化特征
观测期间,梭梭茎干液流密度日变化过程在白天波动较大,呈双峰或多峰变化,不同基茎茎干液流密度在午间(13:00左右)有所降低(图1,a),白刺和沙拐枣不同茎径液流密度日变化均为宽幅单峰形,峰值出现在13:00左右(图1,b、c)。梭梭、白刺、沙拐枣3种植物茎干液流密度平均值变化范围分别为(10.7±9.3)~(17.1±15.9)、(23.6±23.1)~(25.6±29.6)、(19.9±14.8)~(29.0±25.2)g· cm-2·s-1,它们均存在微弱夜间液流(图1)。
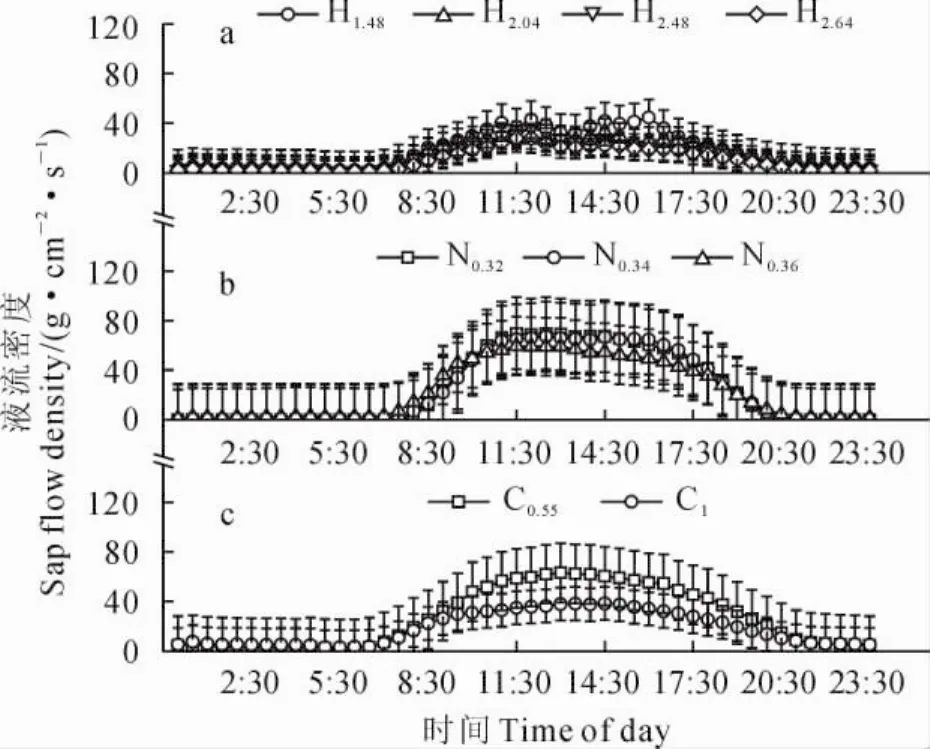
图1 梭梭(a)、白刺(b)和沙拐枣(c)茎干液流密度日变化Fig.1 Diurnal dynamics of sap flow density for H.ammodendron(a),N.tangutorum(b)and C.mongolicum(c)
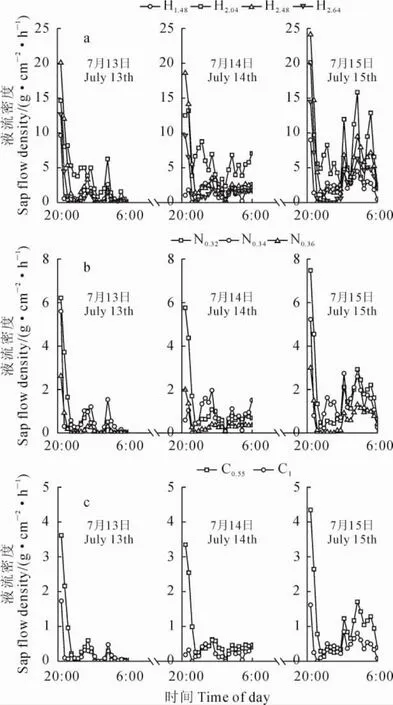
图2 梭梭(a)、白刺(b)和沙拐枣(c)在典型晴天的茎干夜间液流密度变化Fig.2 Variation in nighttime sap flow density for H.ammodendron(a),N.tangutorum(b)and C.mongolicum(c)in typical sunny days
2.2 植物夜间茎干液流密度变化特征
入夜之后,典型晴天条件下,3种植物茎干液流密度在前半夜(20:00~0:00)较大并迅速降低,在后半夜(0:00~6:00)有较大波动(图2)。典型降雨天气条件下,降雨发生后3种植物茎干液流密度均明显减弱甚至为零,且降雨对白刺夜间液流影响最大,仅在8月11日0.36cm基径的白刺枝条观测到微弱夜间液流;降雨量最大的6月11日,降雨结束后仅有基茎为2.04cm和2.64cm的梭梭观测到夜间液流密度(图3)。进一步用单因素方差分析结果表明,3种固沙植物夜间液流密度存在显著差异(F=15.7,P<0.01),梭梭、白刺、沙拐枣夜间茎干液流密度分别为3.73、1.12、6.07g·cm-2·s-1。
2.3 植物茎干夜间液流密度与环境因子的关系
观测期间,相关分析结果表明,3种植物夜间液流密度与主要环境要素的相关关系均达到显著水平(P<0.05),气温、VPD、土壤温度和风速是影响3种植物夜间液流密度的主要因素(表2)。由于VPD和土壤温度均受气温影响,且VPD更能表征空气的蒸腾需求,故选取VPD和风速作为分析影响3种植物夜间液流密度的环境要素。3种固沙植物夜间液流密度大体随VPD和风速的增加而增加。
其中,VPD的影响仅能解释梭梭、白刺、沙拐枣夜间液流密度17%、14%、25%的变化,风速影响仅能解释梭梭、白刺、沙拐枣夜间液流密度10%、10%、6%的变化(图4);多元线性回归分析的结果表明VPD和风速综合影响仅能分别解释梭梭、白刺、沙拐枣平均夜间液流密度24%,25%及27%的变化(表3)。
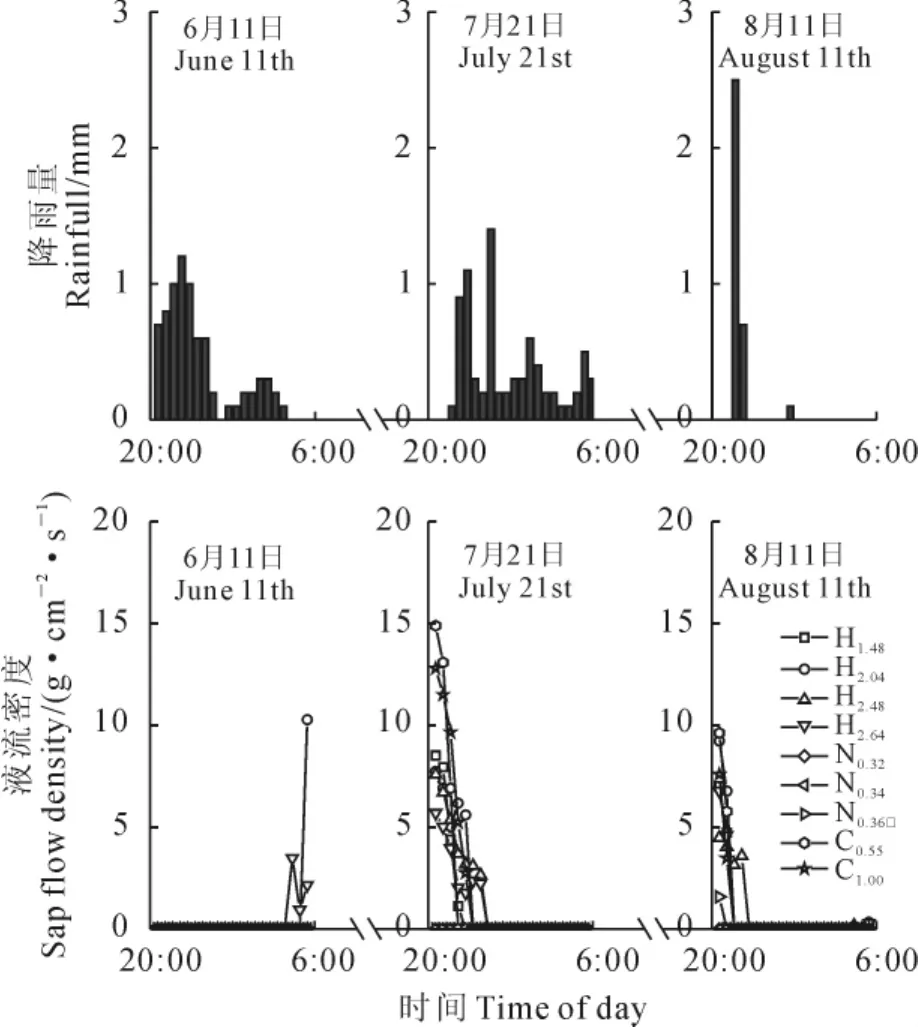
图3 典型降雨天气下3种植物夜间茎干液流密度变化Fig.3 Variation in nighttime sap flow density for the 3species in typical rainy days
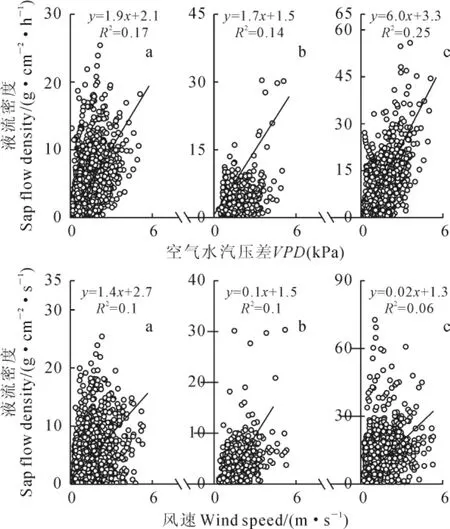
图4 梭梭(a)、白刺(b)和沙拐枣(c)茎干夜间液流密度与饱和空气水汽压差、风速的关系Fig.4 The relationship between nighttime sap flow density and VPD,wind speed for H.ammodendron(a),N.tangutorum(b)and C.mongolicum(c)
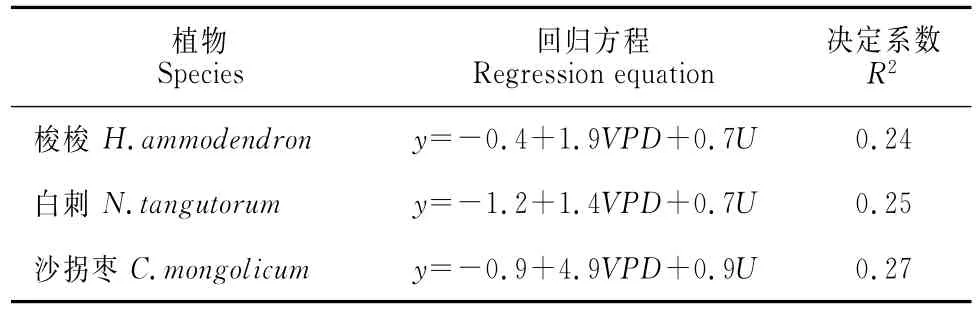
表3 植物夜间液流密度与气象因子VPD、风速(U)多元线性回归方程Table 3 Regression equation for nighttime sap flow density and environmental variables(VPDand wind speed)
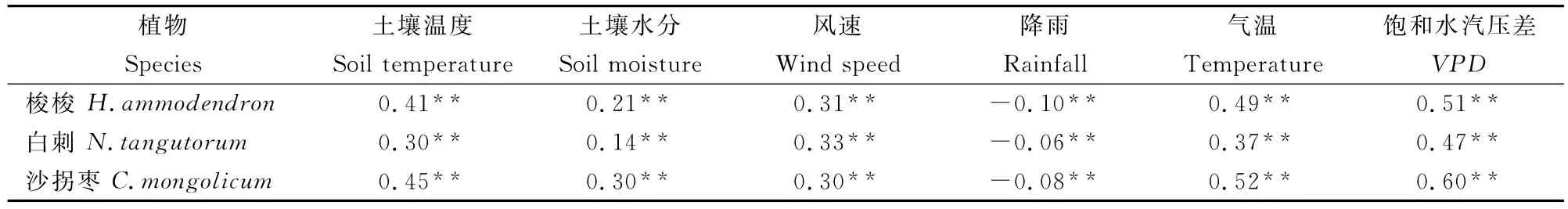
表2 夜间液流密度与主要环境要素的相关关系Table 2 The correlations between nighttime sap flow density and environmental variables during the study period
2.4 典型固沙植物耗水日变化特征
研究表明,植物茎干液流的90%通过叶片蒸腾散失到大气之中,因此能够将植物的茎干液流量作为植物耗水量[22]。观测期间,3种植物白天耗水量和夜间耗水量显著相关,梭梭、沙拐枣白天耗水和夜间耗水在最干旱的7月份均有所增加,而白刺白天耗水量总体呈上升趋势,夜间耗水则没有显著的变化;降雨天,3种植物白天和夜间的耗水显著降低(图5)。各基茎的梭梭总夜间耗水量变化范围为11.4~18.6kg,白刺为0.02~0.3kg,沙拐枣为1.8~6.4kg,而夜间耗水量与基茎呈正相关关系(图6)。
进一步用单因素方差分析方法来检测3种植物夜间耗水各月分配情况发现,3种植物在不同月份夜间耗水分配差异并不显著(梭梭F=1.3,P>0.05;白刺F=0.3,P>0.05;沙拐枣F=0.2,P>0.05),表明固沙植物夜间耗水比较稳定;另外,基茎为0.32cm、0.36cm的白刺仅在7月和8月有少量的夜间耗水(图7)。
2.5 固沙植物夜间耗水贡献率变化特征
观测期间,3种固沙植物夜间耗水对日总耗水量的贡献率变化范围较大,最大贡献率均出现在降雨之后;这一方面是由于降雨过后夜间耗水升高幅度较日间耗水升高幅度大,另一方面也存在降雨天气条件下仪器观测不稳定导致的测量误差。其中,梭梭夜间耗水对日总耗水的平均贡献率最大,而白刺最小,这与白刺微弱的夜间液流密度有关(表4)。
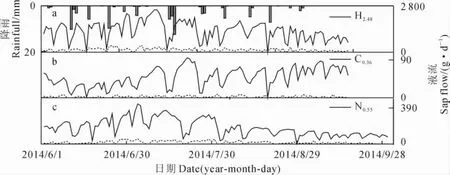
图5 不同时期梭梭(a)、白刺(b)和沙拐枣(c)耗水日变化Fig.5 Daytime and nighttime water use for H.ammodendron(a),N.tangutorum(b)and C.mongolicum(c)
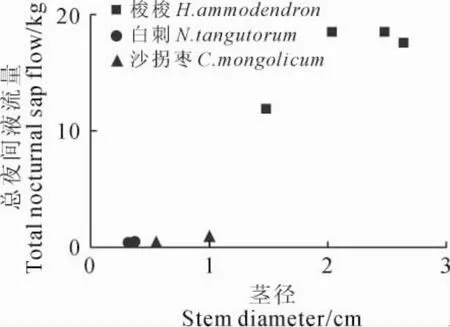
图6 观测期总夜间液流量与茎径关系Fig.6 Relationship between total nocturnal sap flow and stem diameter during study period
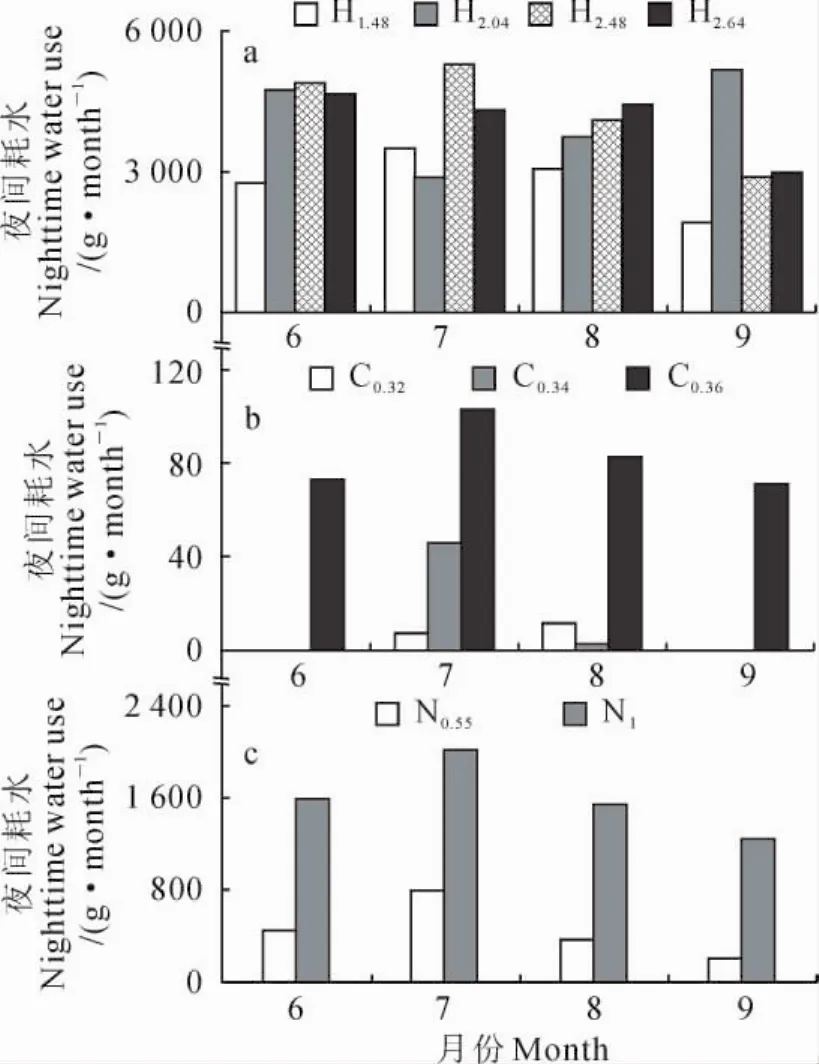
图7 梭梭(a)、白刺(b)和沙拐枣(c)不同月份夜间耗水分配Fig.7 Total nighttime water use for H.ammodendron(a),N.tangutorum(b)and C.mongolicum(c)in different months
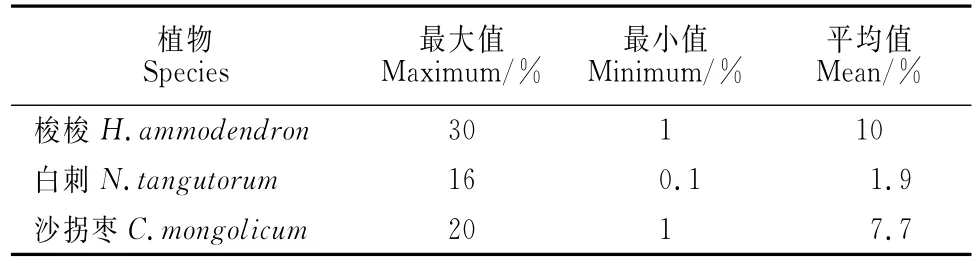
表4 茎干夜间液流量对日总液流量的贡献率Table 4 Parameters of ratio of nighttime sap flow to daily total water flow
同时,单因素方差分析结果表明,梭梭、白刺、沙拐枣夜间耗水对日总耗水贡献率在季节间差异并不显著(梭梭F=1.8,P>0.05;白刺F=0.8,P>0.05;沙拐枣F=1.7,P>0.05),又以白刺变动幅度略大(图8)。
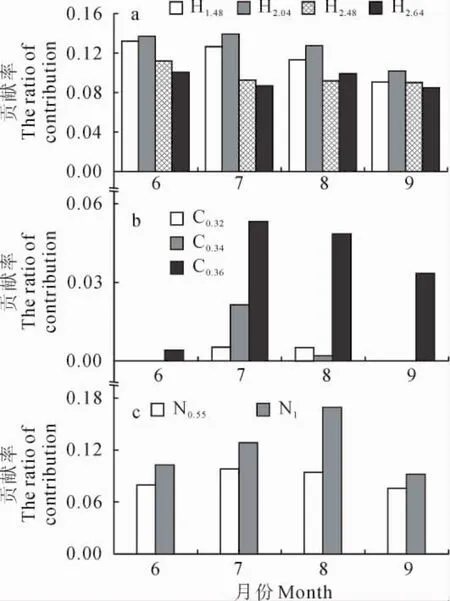
图8 梭梭(a)、白刺(b)和沙拐枣(c)不同月份夜间耗水贡献率变化Fig.8 The ration of nighttime water use to total daily water use for H.ammodendron(a),N.tangutorum(b)and C.mongolicum(c)in different months
3 讨 论
研究发现,VPD和风速是影响植物夜间茎干液流的主要环境因子[7,9,21]。本研究中,VPD和风速对3种植物夜间液流密度较低的解释率表明,这3种植物茎干夜间液流主要用以茎干补水,是否发生蒸腾以及蒸腾与茎干补水占夜间耗水的比例还需要辅以其他的研究手段进行深入分析。不同植物以及相同植物在不同季节夜间液流利用方式存在差异,如荷木的夜间液流主要用于茎干补水[2],雪松、大叶榉、丝棉木和水杉夜间液流在前半夜主要用以蒸腾,而后半夜则主要用以茎干补水[24],桉树夜间液流主要用以蒸腾[13]。除了VPD和风速以外,植物夜间液流也受其他环境因子的调节,如美国海岸松(Pinus pinaster)夜间耗水随土壤干旱程度的加重而升高[23],华北落叶松夜间耗水量与土壤含水量显著相关[25],Phillips等[21]发现当叶龄越小时,植物夜间耗水会增加。
最优化理论认为,由于光合有效辐射在夜间为零,C3、C4植物在夜间并不进行光合作用,气孔会在夜间关闭以防止水分损失,因此在估算植物蒸腾耗水时假设夜间蒸腾为零[7,26-27]。已有研究发现,不同植物类型和不同生境条件中叶片气孔在夜间仍然开放[28-31],因此会发生夜间水分交换。夜间蒸腾对日蒸腾总量的贡献率不同植物类型存在差异,桉树群落约为5%,花旗松可达7%,腰果树和伞树达9%~15%[13-14]。因此,当某些生境中植物群落夜间耗水比例较大时,忽略夜间蒸腾无论是在时间尺度还是空间尺度均会影响植物耗水估算的准确性,同时也会影响一些植物灌溉需水模型的计算精度[29]。
目前国内利用热平衡技术开展植物茎干液流研究已经比较广泛[32-36],但是该技术依然存在多个误差来源,如重要参数鞘传导率Ksh确定方法的选择、温差变化的有效范围、茎干热存储等均会降低测量结果的可靠性[37-39]。Coners等[40]结合热平衡茎流仪、分析天平称重法和容器测量分析法研究欧洲山毛榉根系液流变化时发现,当液流速率大于2g· h-1时,茎干液流仪测定结果与重量测定方法的结果十分一致,但是当液流速率低于上述值时,茎干液流仪测定结果不可靠。梭梭、白刺、沙拐枣生长于极端干旱环境之中,与生长于水分充足环境中的植物相比具有低液流密度的特点[41],因此在未来的工作中还需要考虑仪器的误差限。
综上所述,结合热平衡茎干液流仪和微气象监测系统研究3种典型固沙植物夜间耗水,得出如下几点结论:(1)梭梭液流密度日变化过程为双峰或者多峰型,白刺和沙拐枣均为宽幅单峰型;3种植物夜间液流密度存在显著差异,整个观测期,梭梭、白刺和沙拐枣平均夜间液流密度依次为3.73、6.07、1.12g·cm-2·h-1;典型降雨天气条件下3种植物夜间液流密度明显减弱,且对白刺的影响程度更大。(2)梭梭、沙拐枣夜间和白天耗水在最干旱的7月份均有所增加,而白刺则无明显变化。(3)多元线性回归结果表明VPD和风速综合影响仅能分别解释梭梭、白刺、沙拐枣夜间液流密度24%,25%,27%的变化,据此推测3种植物夜间液流主要用以茎干补水。(4)3种植物夜间耗水和夜间耗水占日总耗水量的贡献率在不同月份间差异并不显著;梭梭夜间液流对日总液流量贡献率变化范围为1%~30%,平均值为10%,白刺为0.1%~16%,平均值为1.9%,沙拐枣为1%~20%,平均值为7%。研究固沙植物夜间耗水对于估算群落耗水、固沙物种选育具有重要意义,由于其受环境、木质部导水性能、植物抗旱性能等多个方面的综合影响,要深入研究夜间液流问题除了考虑仪器测定误差外,还需要开展夜间气孔导度、气体交换参数、基茎收缩特征等相关观测工作。
[1] ZHAO X W(赵晓伟),ZHAO P(赵 平),et al.Seasonal dynamics of night-time stem water recharge of Schima superbaand its relation to tree architecture and leaf biomass[J].Chinese Journal of Plant Ecology(植物生态学报),2013,37(3):239-247(in Chinese).
[2] ZHOU M C(周翠鸣),ZHAO P(赵 平),NI G Y(倪广艳),et al.Water recharge through nighttime stem sap flow of Schima superba in Guangzhou region of Guangdong Province,South China:affecting factors and contribution to transpiration[J].Chinese Journal of Applied Ecology(用生态学报),2012,23(7):1 751-1 757(in Chinese).
[3] SCHÂFER K V R,ORNE R,TENHUNEN J D.The effect of tree height on crown level stomatal conductance[J].Plant,Cell and Environment,2000,23:365-375.
[4] MOTZER T,MUNZ N,KÜPPERS M,et al.Stomatal conductance,transpiration and sap flow of tropical montane rain forest in the southern Ecuadorian Andes[J].Tree Physiology,2005,25:1 283-1 293.
[5] ORTUÑO M F,YELITZA G O,CONEJERO W.Stem and leaf water potentials,gas exchange,sap flow,and trunk diameter fluctions for detecting water stress in lemon trees[J].Trees,2006,20:1-8.
[6] HOLBROOK N M.Stem water storage.Physiology and Functional Morphology[M].Academic Press,San Diego,1995:151-174.
[7] DALEY M J,PHILLIPS N G.Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest[J].Tree Physiology,2006,26(4):411-419.
[8] VERBEECK H,STEPPE K,NADEZHDINA N,et al.Stored water use and transpiration in Scots pine:a modeling analysis with ANAFORE[J].Tree Physiology,2007,27(12):1 671-1 685.
[9] PHILLIPS N G,RYAN M G,BOND B J,et al.Reliance on stored water increases with tree size in three species in the Pacific Northwest[J].Tree Physiology,2003,23(4):237-245.
[10] MARKS C O,LECHOWICZ M J.The ecological and functional correlates of nocturnal transpiration[J].Tree Physiology,2007,27(4):577-584.
[11] BUSH S E,PATAKI D E,HULTINE K R,et al.Wood anatomy constrains stomatal responses to atmospheric vapor pressure deficit in irrigated,urban trees[J].Oecologia,2008,156(1):13-20.
[12] HOGG E H,HURDLE P A.Sap flow in trembling aspen:implications for stomatal responses to vapor pressure deficit[J].Tree Physiology,1997,17(89):501-509.
[13] BENYON.Nighttime water use in an irrigated Eucalyptus grandis plantation[J].Tree Physiology,1999,19(13):853-859.
[14] GOLDSTEIN G,ANDRADE J L,MEINZER F C,et al.Stem water storage and diurnal patterns of water use in tropical forest canopy trees[J].Plant,Cell and Environment,1998,21(4):397-406.
[15] DAI Y(戴 岳),ZHEN X J(郑新军),TAN L S(唐立松),et al.Dynamics of water usage in Haloxylon ammodendronin the southern edge of the Gurbantonggut Desert[J].Chinese Journal of Plant Ecology(植物生态学报),2004,38(11):1 214-1 225(in Chinese).
[16] SU P X(苏培玺),AN L ZH(安黎哲),MA R J(马瑞君),et al.Kranz anatomy and C4photosynthetic character plants,Haloxylon ammodenron and Calligonum mongolicum[J].Acta Physiological Sinica(植物生态学报),2005,29(1):1-7(in Chinese).
[17] SU P X(苏培玺),YAN Q D(严巧娣).Photosynthetic characteristics of C4desert species Haloxylon ammodendron and Calligonum mongolicumunder different moisture conditions[J].Acta Ecologica Sinica(生态学报),2006,26(1):75-82(in Chinese).
[18] BAI X F(柏新富),ZHU J J(朱建军),ZHAO A F(赵爱芬).Comparison of physiological adapt abilities of several desert plants to drying stress[J].China Journal Applied Environmental Biology(应用与环境生物学报),2008,14(6):763-768(in Chinese).
[19] WANG H(王 华),ZHAO P(赵 平),CAI X A(蔡锡安),et al.Partitioning of night sap flow of Acaclia mangiumand its implication for estimating whole-tree transpiration[J].Chinese Journal of Ecology(植物生态学报),2007,31(5):777-786(in Chinese).
[20] CHEN L X(陈立欣),ZHANG ZH Q(张志强),LI ZH D(李湛东),et al.Nocturnal sap flow of four urban greening tree species in Dalian,Liaoning Province[J].Chinese Journal of Ecology(植物生态学报),2010,34(5):535-546(in Chinese).
[21] PHILLIPS N G,LEWIS J D,LOGAN B A,et al.Intera-and intra-specific variation in nocturnal water transport in Eucalyptus[J].Tree Physiology,2010,30:586-596.
[22] JIANG G M(蒋高明).Plant exophysiology 3rd[M].Beijing:China Higher Education Press.
[23] OLIVEIRA R S,DAWSON T E,et al.Hydraulic redistribution in three Amazonian trees[J].Oecologia,2005,145(3):354-363.
[24] LOUSTAU D,BERBIGIER P,ARRUDA P R C,et al.Transpiration of a 64-year-old maritime pine stand in Portugal.1.Seasonal course of water flux through maritime pine[J].Oecologia,1996,107:33-42.
[25] WANG Y B(王艳兵),DE Y J(德永军),et al.The characteristics of nocturnal sap flow and stem water recharge pattern in growing sea-son for a Larix principis-rupprechtii plantation[J].Acta Ecology Sinica(生态学报),2013,33(5):1 375-1 385(in Chinese).
[26] VERTESSY R A,HATTON T J,REECE P,et al.Estimating stand water use of large mountain ash trees and validation of the sap flow measurement technique[J].Tree Physiology,1997,17(12):747-756.
[27] OREN R,PHILLIPS N,EWERS B E,et al.Sap-flux-scaled transpiration responses to light,vapor pressure deficit,and leaf area reduction in a flooded Taxodium distichumforest[J].Tree Physiology,1999,19(6):337-347.
[28] MUSSELMAN R C,MINNICK T J.Nocturnal stomatal conductance and ambient air quality standards for ozone[J].Atmospheric Environment,2000,34:719-733.
[29] SNYDER K A,RICHARDS J H,DONOVAN L A.Night-time conductance in C3and C4species:do plants lose water at night?[J].Journal of Experimental Botany,2003,54(383):861-865.
[30] KAVANAGH L,PANGLE,SCHOTZKO D.Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho[J].Tree Physiology,2007,27(4):621-629.
[31] DAWSON T D,BURGESS S S O,TU K P,et al.Nighttime transpiration in woody plants from contrasting ecosystems[J].Tree Physiology,2007,27(4):561-575.
[32] XU X Y(徐先英),SONG B G(松保国),DING G D(丁国栋),et al.Sap flow patterns of three main sand-fixing shrubs and their response to environmental factors in desert areas[J].Acta Ecology Sinica(生态学报),2008,28(3):895-905(in Chinese).
[33] XU H(许 浩),ZHANG X M(张希明),YAN H L(闫海龙),et al.Water consumption and transpiration of Haloxylon ammodendronin interland of Taklinakan Desert[J].Acta Ecoligy Sinica(生态学报),2008,28(8):3 713-3 720(in Chinese).
[34] YUE G Y(岳广阳),ZHANG T H(张铜会),et al.Characteristics of sap flow and transpiration of Salix gordejevii and Caragana microphyllain Horqin Sandy Land,Northeast China[J].Acta Ecologica Sinica(生态学报),2006,26(10):3 205-3 213(in Chinese).
[35] ZHAO W Z,LIU B.The response of sap flow in shrubs to rainfull pulses in the desert region on China[J].Agriculrural and Forest Meterology,2010,150(9):1 297-1 306.
[36] LIU B,ZHAO W Z,JIN B W.The response of sap flow in desert shrubs to environmental variables in an arid region of China[J].Ecology,2011,4:448-457.
[37] KENNETH A S,JOHNSONS R S,CHARLES K M.Substantial errors in estimates of sap flow using the heat balance technique on woody stems under field conditions[J].Journal of the American Society for Horticultural Science,1992,117(2):351-356.
[38] GORDON R,DIXON M A,BROWN D M.Verification of sap flow by heat balance method on three potato cultivars[J].Potato Research,1997,40(3):267-276.
[39] MATTHIAS L,KUPISCH M,GRAF A,et al.Improving the stem heat balance method for determining sap-flow in wheat[J].Agricultural and Forest Meteorology,2014,186:34-42.
[40] CONERS H,LEUSCHNER C.In situ water absorption by tree fine roots measured in real measured in real time using miniature sap-flow gauges[J].Functional Ecology,2002,16(5):696-703.
[41] LI SH(李 双),XIAO H L(肖洪浪),WANG F(王 芳),et al.Accuracy and error sources of stem heat balance method in sap flow measurements[J].Journal of Desert Research(中国沙漠),2014,34(6):1 544-1 551(in Chinese).
(编辑:裴阿卫)
Nighttime Water Use and Its Influencing Factors for Typical Sand Binding Plants in the Arid Region of Northwest China
XU Shiqin1,2,JI Xibin1*,JIN Bowen1
(1Linze Inland River Basin Research Station,Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou 730000,China;2University of Chinese Academy of Sciences,Beijing 100049,China)
Independent measurements of sap flow in stems of Haloxylon ammodendron,Nitraria tangutorumand Calligonum mongolicumand environmental variables using a commercial sap-flow gauges and micrometeorological monitoring system,respectively,were made to study nighttime sap flow activities and its influencing factors,and to estimate the nighttime water consumption for an H.ammodendronstand in an oasis-desert ecotone,located in the middle range of Hexi Corridor,Northwest China.(1)Nighttime sap flow density for these species was high before 0o'clock and decreased quickly,it was weak and fluctuated dramatically between 0o'clock and 6o'clock.We found nighttime sap flow densities between these species were very significant(P<0.01),and the mean value for H.ammodendron,N.tangutorum,and C.mongolicum was 3.73,1.12,and 6.07g·cm-2·h-1,respectively.The results showed that sap flow density of three species all decreased in typical rainy days.(2)The total nighttime water use for these species was close related to the stem diameter during the study period(R2=0.92),and it was not significant difference between different months(H.ammodendron,P>0.05;N.tangutorum,P>0.05;C.mongolicum,P>0.05).We found that H.ammodendroncontributed 1%to 30%nighttime water use to daily total water,N.tangutorumcontribute 0.1%to 16%,and C.mongolicumconribute 1.5%to 20%,respectively.(3)We presumed that nighttime sap flow for these species were mainly used to refilling because VPD and wind speedonly explained 24%,25%and 27%of their variation,respectively.
sap flow;One-way ANOVA;desert plants;nighttime water use
Q945.79
A
10.7606/j.issn.1000-4025.2015.07.1443
1000-4025(2015)07-1443-08
2015-04-03;修改稿收到日期:2015-05-04
国家重点基础研究发展计划(973)课题(2013CB429902);国家自然科学基金项目(41271036)
徐世琴(1989-),女,硕士,主要从事干旱区生态水文研究。E-mail:xushiqin@lzb.ac.cn
*通信作者:吉喜斌,副研究员,硕士生导师,主要从事生态水文和气象研究。E-mail:xuanzhij@ns.lzb.ac.cn

