示踪法测定九龙江河口沉积中硫酸盐还原速率
尹希杰,孙治雷,徐勇航,李云海,邵长伟
(1. 国家海洋局 第三海洋研究所 海洋与海岸地质环境开放实验室,福建 厦门361005;2.青岛海洋地质研究所 国土资源部海洋油气资源和环境地质重点实验室,山东 青岛266071;3. 山东省物化探勘查院,山东 济南250013)
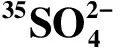
尹希杰1,孙治雷2,徐勇航1,李云海1,邵长伟3
(1. 国家海洋局 第三海洋研究所 海洋与海岸地质环境开放实验室,福建 厦门361005;2.青岛海洋地质研究所 国土资源部海洋油气资源和环境地质重点实验室,山东 青岛266071;3. 山东省物化探勘查院,山东 济南250013)

1 引言

河口海岸地区作为海陆的交汇地带,有大量陆源有机物输入,也具有高的初级生产力和沉积速率,因此其沉积物中有机质含量较高。这些有机质可以为不同的生物地球化学过程提供能量和电子供体,硫酸盐还原过程就是其中之一[3,6—7,11]。据估计,河口海岸沉积物中约有一半的有机质是通过硫酸盐还原反应的方式被矿化[6—7]。因此硫酸盐还原是河口海岸带沉积物中生物地球化学循环的主要组成部分,也是沉积物中硫元素生物地球化学循环的基础[10,12]。

2 研究方法
2.1 样品采集


图1 采样站位分布Fig.1 The locations of sampling sites in the Jiulong River Estuary
表1 两个站位沉积环境参数
Tab.1 Characteristics of sampling localities

站位经纬度柱样长/cm沉积物组成水深/m盐度底层水温度/℃溶解氧/mg·L-1A24°25′50 22″N,117°51′34 05″E50黏土、粉砂约2约4 0923 55 6B24°25′22 57″N,117°58′51 57″E88黏土、粉砂约4约23 322 86 9
2.2 孔隙水采集
2.3 沉积物中硫酸盐还原速率(SRR)的测定
还原态无机硫的分离:沉积物中被还原的无机硫采用冷铬还原-被动吸收法进行分离[4,7]。将离心后的沉积物样品与20 mL N,N-二甲基甲酰胺(DMF)混合后,转移到反应瓶中,将浸润醋酸锌溶液的玻璃纤维膜悬挂在反应瓶上部,用高纯氮气吹尽反应瓶中的氧气,10 min之后加入20 mL 6 mol/dm3的 HCl和16 mL 1 mol/dm3的CrCl2溶液,总还原无机硫(TRIS,包括挥发性硫、黄铁矿和元素S)以H2S的形式释放出来,被吸附到玻璃纤维膜上。
取离心后的清液5 mL和吸附还原硫的玻璃纤维膜,分别加入5 mL闪烁液(Triton X-100),用液相闪烁计数仪(LS-6500)测定其活度值。沉积物中各层位硫酸盐还原速率(SRR)用下面公式计算[4—5,19]:
24/t×1.06,
(1)
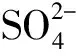
孔隙水甲烷浓度测定:在20 mL顶空瓶中预先加入3 mL 1 mol/dm3氢氧化钠溶液,用来抑制沉积物产甲烷菌的活动,然后放入3 mL沉积物,丁基橡胶塞密封,压盖旋紧,摇匀后低温保存。用气密针抽取2 mL顶空的气体,将针筒拔出后缓慢推出1 mL的气体样品,之后迅速将剩余1 mL气体注入色谱进样阀中,并按下start键开始测定。色谱条件:检测器,FID,温度300℃,进样口温度120℃,柱温箱60℃,色谱柱为Porpark Q填充柱(2 m×3 mm,80/100目);载气,99.999%氮气,流速30 mL/min。每个样品重复测2次,测定误差±3.0%。沉积物中甲烷浓度根据孔隙度换算为单位体积孔隙水中含甲烷摩尔数(μmol/dm3)。
2.5 沉积物孔隙度、总有机碳(TOC)和氧化还原电位测定
沉积物孔隙度测定:将3 mL原始沉积物样品放置于称量瓶内称重,于105℃放置24 h,恒重后称量,计算样品前后质量差。孔隙度以单位体积沉积物所含孔隙水的体积表示。
沉积物TOC测定:取一定量经冷冻干燥后的沉积物样品,加入过量4 mol/dm3HCl,反应24 h。用去离子水洗酸3次,将样品置于烘箱内60℃烘干,恒重后称量样品质量。称取一定量磨匀的样品,用元素分析仪(Vario EL III,德国制造)测定有机碳含量。每个样品平行测定2次,测量误差为±0.2%(n=5),TOC含量以有机碳占样品总干重百分数表示。
沉积物氧化还原电位测定:在分样过程中用EXTECH RE300氧化还原电位计探头直接插入沉积物中测其氧化电位值。
3 结果
3.1 沉积物中硫酸盐还原速率分布
A站位位于河口中段红树林潮滩附近,硫酸盐还原速率从表层随深度增加先增大后减小(见图2),其值由表层的54 nmol/(cm3·d)逐渐增大到19 cm深度的2 345 nmol/(cm3·d);随后硫酸盐还原速率逐渐降低,55 cm深度降为121 nmol/(cm3·d)。B站位于河口下端海相区,其沉积物中硫酸盐还原速率的最大值比A站位明显偏低,在垂直剖面上的分布也有显著的差异(见图2)。B站位硫酸盐还原速率在10 cm和78 cm深度附近出现两个峰值,其值分别为843 nmol/(cm3·d)和987 nmol/(cm3·d)。对两个站位测得的各层位沉积物中硫酸盐还原速率进行积分,估算得A和B站位沉积物中硫酸盐还原通量(以硫计)分别为527.9 mmol/(m2·d)和 357.1 mmol/(m2·d)。

图2 A站位(•)和B站位(○)沉积物中硫酸盐还原速率垂直分布Fig.2 Vertical profiles of sulfate reduction rates in sediments at A(•) and B(○) cores
3.2 孔隙水中硫酸盐和甲烷浓度分布
3.3 沉积物氧化还原电位和总有机碳含量
硫酸盐还原过程是在硫酸盐还原菌为媒介的作用下进行的,而硫酸盐还原菌属于严格的厌氧细菌,因此沉积物中氧化还原电位变化对硫酸盐还原菌活性有重要的影响,从而间接影响沉积物中硫酸盐还原速率。图4显示,A站位表层沉积物(0~3 cm深度)的氧化还原电位为-87 mV,随深度增加快速降低,在10 cm深度减小到-289 mV,之后随深度增加没有明显的变化趋势。B站位表层沉积物的氧化还原电位值为-12 mV,随深度增加急剧减小,在28 cm深度附近减小到-245 mV,之后随深度增加缓慢减小,至沉积物底部减小至-296 mV。两个站位沉积物的氧化还原电位表明沉积物为厌氧的还原环境。图4显示A站位TOC含量的变化范围1.51%~1.98%,平均值为1.75%;B站位TOC含量的变化范围1.19%~1.61%,平均值为1.36%。
4 讨论
4.1 九龙江河口硫酸盐还原带空间分布及环境控制因素
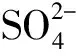

图3 A站位(•)和B站位(○)孔隙水中甲烷和浓度垂直分布Fig.3 Vertical profiles of sulfate and methane concentration in pore water at A(•)and B(○) cores
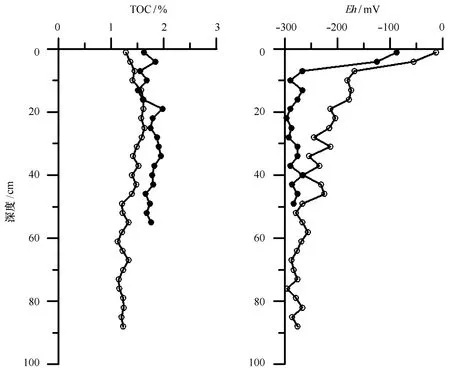
图4 A站位(•)和B站位(○)沉积物中TOC和氧化还原电位垂直分布Fig.4 Vertical profiles of TOC and Eh in sediments of A (•)and B(○) cores
4.1 九龙江河口硫酸盐还原速率及环境控制因素
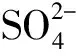

(2)

(3)
因此在A站位从沉积物表层至20 cm深度存在高的硫酸盐还原速率,20 cm深度以下,随着孔隙水中硫酸盐浓度快速的减小,硫酸盐还原速率随着深度的增加也呈现减小的趋势。
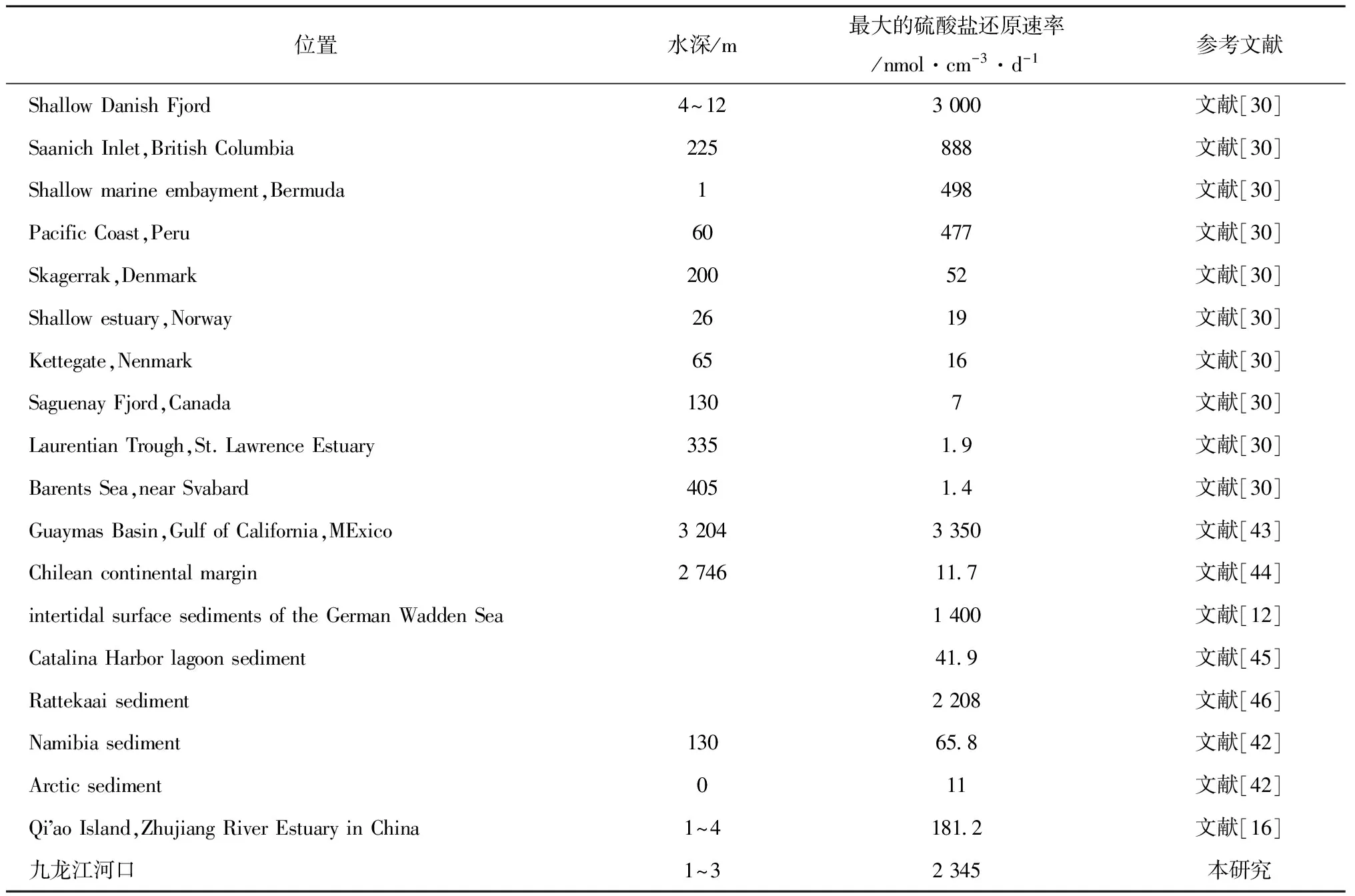
表2 世界不同地区沉积物硫酸盐还原速率最大值
B站位沉积物的上部(约20 mm)和下部(约78 mm)分别存在较高的硫酸盐还原速率,但其峰值均低于A站位的最大值。这两个高的硫酸盐还原速率是由不同的硫酸盐还原路径所导致[33—35],上部硫酸盐还原作用主要由氧化降解沉积物活性有机质而产生;随着深度增加,硫酸盐浓度逐渐降低,沉积物中剩余的部分难降解的有机质经发酵产生甲烷[36],B站位在60 cm深度以下孔隙水中甲烷浓度表现出随着深度而逐渐增加的趋势,生成的甲烷在向上层扩散的过程中,在78cm深度附近发生硫酸盐还原与甲烷厌氧氧化的耦合作用,化学计量式可以表示如下[35—36]:
(4)
在该层位硫酸盐还原和甲烷厌氧氧化同时进行,导致硫酸盐还原速率的第二个极大值[987 nmol/(cm3·d)]的出现。对B站位沉积物中活性古菌的群落组成进行研究,发现该层位以甲烷厌氧氧化菌 ANME-2a 为主,进一步验证了该层位甲烷厌氧氧化和硫酸盐还原耦合的存在[37]。沉积物中的有机质都是经由水体沉降矿化之后而逐渐被埋藏,B站位水深明显大于A站位,水体中活性有机质被埋藏之前在水柱沉降过程中被大量氧化而消耗[38],最后进入沉积物厌氧带中的有机质主要以难降解长链化合物为主[39],因此B站位沉积物中有机质埋藏的通量和有机质活性都比A站位降低[24],因此沉积物中没有足够活性有机质为硫酸盐还原提供的电子供体,硫酸盐还原菌的活性受到抑制,导致该站位沉积物上部硫酸盐还原速率相对A站位偏低,对一些海洋和湖沉积物研究结果也表明硫酸盐还原速率主要受到新沉降的有机质通量及活性所控制[10—12,28,33,40—41]。其次A站位表层沉积物温度(23.5℃)高于B站位(22.8℃),已有的研究显示在温度低于36℃时,沉积物中硫酸盐还原速率与温度存在正相关性[12,16,28,42]。因此A站位和B站位沉积物中硫酸盐还原反应速率的差异,反映了该地区沉积物中硫酸盐还原的速率受到有机质埋藏的通量和活性以及沉积物温度的综合影响。
4.3 九龙江河口硫酸盐还原对有机质矿化通量的估算
国内外对河口海岸沉积物有机质矿化路径进行了大量的研究[47—49],其中对硫酸盐还原研究最为广泛和深入,其原因是硫酸盐还原一直被认为是河口海岸地区有机质厌氧矿化最主要的方式[3,6—7,10,39]。大量研究发现河口海岸地区通过硫酸盐还原矿化的有机质量占到有机质矿化总量的(62±17)%[3,6,50]。如在缺氧的黑海、智利陆架和纳米比亚近海上升流区,沉积物乃至深部水柱中的有机质几乎都是由硫酸盐还原的方式矿化[51—53]。本研究分别对A和B两个站位各层位硫酸盐还原速率进行积分计算,得到两个站位硫酸盐还原通量(以硫计)分别为 527.9 mmol/(m2·d)和 357.1 mmol/(m2·d)。沉积物中硫酸盐还原主要通过有机质矿化和甲烷厌氧氧化两种方式进行,反应关系式如下:

(2)
(4)
CH3COOH→CH4+CO2.
(5)
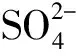

表3 世界不同地区沉积物中硫酸盐还原通量

续表3
5 结论
(1)九龙江河口沉积物中硫酸盐还原带深度,随着上覆水盐度的增加而逐渐增大,表明该地区硫酸盐还原深度分布主要受到上覆水体硫酸盐浓度控制。近岸红树林地区沉积物中硫酸盐还原速率最大值明显高于河口下端海相区,表明硫酸盐还原速率主要受到沉积物中有机质浓度和活性以及温度等环境因素的影响。
(2)通过对两个站位硫酸盐还原带中不同层位硫酸盐还原速率积分计算,表明九龙江河口沉积物中存在较高的硫酸盐还原通量,硫酸盐还原作用在九龙江河口沉积物有机质矿化中具有重要的作用。
[1] Vairavamurthy M A,Orr W L,Manowitz B. Geochemical transformation of sedimentary sulfur: an introduction[M]// Vairavamurthy M A,Schoonen M A A. Geochemical Tranformation of Sedimentary Sulfur. Washington,DC: ACS Symposium,1995: 1-17.
[2] Bottrell S H,Newton R J. Reconstruction of changes in global sulfur cycling from marine sulfate isotopes[J]. Earth-Science Reviews,2006,75(1/4): 59-83.
[3] Jørgensen B B. The sulfur cycle of coastal marine sediment (Limfjorden,Denmark)[J]. Limnology and Oceanography,1977,22(5): 814-832.
[4] Kallmeyer J,Ferdelman T G,Weber A,et al. A cold chromium distillation procedure for radio labeled sulfide applied to sulfate reduction measurements[J]. Limnology and Oceanography Methods,2004,2: 171-180.
[5] Fossing H,Jørgensen B B. Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method[J]. Biogeochemistry,1989,8(3): 205-222.
[6] Jørgensen B B,Fenchel T. The sulfur cycle of a marine sediment model system[J]. Marine Biology,1974,24(3): 189-201.
[7] Sweeney R E,Kaplan I R. Diagenetic sulfate reduction in marine sediments[J]. Marine Chemistry,1980,9(3): 165-174.
[8] Berner R A. Sulfate reduction and the rate of deposition of marine sediments[J]. Earth and Planetary Science Letters,1978,37(3): 492-498.
[9] Bowles M W,Samarkin V A,Bowles K M,et al. Weak coupling between sulfate reduction and the anaerobic oxidation of methane in methane-rich seafloor sediments during ex situ incubation[J]. Geochimica et Cosmochimica Acta,2011,75(2): 500-519.
[10] Lee T,Hyun J H,Mok J S,et al. Organic carbon accumulation and sulfate reduction rates in slope and basin sediments of the Ulleung Basin,East/Japan Sea[J]. Geo-Marine Letters,2008,28(3): 153-159.
[11] Meister P,Liu B,Ferdelman T G,et al. Control of sulphate and methane distributions in marine sediments by organic matter reactivity[J]. Geochimica et Cosmochimica Acta,2013,104: 183-193.
[12] Al-Raei A M,Bosselmann K,Böttcher M E,et al. Seasonal dynamics of microbial sulfate reduction in temperate intertidal surface sediments: controls by temperature and organic matter[J]. Ocean Dynamics,2009,59(2): 351-370.
[13] Gribsholt B,Kristensen E. Benthic metabolism and sulfur cycling along an inundation gradient in a tidalSpartinaanglicasalt marsh[J]. Limnology and Oceanography,2003,48(6): 2151-2162.
[14] Thang N M,Brüchert V,Formolo M,et al. The impact of sediment and carbon fluxes on the biogeochemistry of methane and sulfur in Littoral Baltic Sea Sediments (Himmerfjärden,Sweden)[J]. Estuaries and Coasts,2013,36(1): 98-115.
[15] 孙炳寅,经美德. 废黄河口盐沼土硫酸盐还原速率的研究[J]. 应用生态学报,1990,1(3): 248-253.
Sun Bingyin, Jing Meide. A study on sulfate reduction in salt marsh near the estuary of obsolete Huanghe River[J]. Chinese Journal of Applied Ecology, 1990, 1(3):248-253.
[16] Wu Z J,Zhou H Y,Peng X T,et al. Rates of bacterial sulfate reduction and their response to experimental temperature changes in coastal sediments of Qi’ao Island,Zhujiang River Estuary in China[J]. Acta Oceanologica Sinica,2014,33(8): 10-17.
[17] 程思海,陆红锋. 海洋沉积物孔隙水的制备方法[J]. 岩矿测试,2005,24(2): 102-104.
Cheng Sihai, LU Hongfeng. Techniques for marine sediment pore-water sampling[J]. Rock and Mineral Analysis, 2005, 24(2):102-104.
[18] 吴自军,周怀阳,彭晓彤,等. 甲烷厌氧氧化作用: 来自珠江口淇澳岛海岸带沉积物间隙水的地球化学证据[J]. 科学通报,2006,51(17): 2052-2059.
Wu Zijun,Zhou Huaiyang,Peng Xiaotong,et al.Anaerobic oxidation of methane: Geochemical evidence from pore-water in coastal sediments of Qi’ao Island(Pearl River Estuary), southern China[J]. Chinese Science Bulletin, 2006, 51(17): 2052-2059.
[19] Schulz H D,Zabel M. Marine Geochemistry[M]. Berlin: Springer,2006: 198-199.
[20] 张胜,张翠云,张云,等. 地质微生物地球化学作用的意义与展望[J]. 地质通报,2005,24(10/11): 1027-1031.
Zhang Sheng, Zhang Cuiyun, Zhang Yun, et al. Geomicrobial geochemical processes: Significance and prospects[J]. Regional Geology of China, 2005, 24(10/11):1027-1031.
[21] Froelich P N,Klinkhammer G P,BenderM L,et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis[J]. Geochimica et Cosmochimica Acta,1979,43(7): 1075-1090.
[22] Canfield D E. Organic matter oxidation in marine sediments[C]∥Wollast R,Mackenzie F T,Chou L,et al. Interactions of C,N,P and S Biogeochemical Cycles and Global Change. Berlin Heidelberg: Springer-Verlag,1993: 333-363.
[23] Burdige D J. Geochemistry of Marine Sediments[M]. USA: Princeton University Press,2006.
[24] 尹希杰,陈坚,郭莹莹,等. 九龙江河口沉积物中硫酸盐还原与甲烷厌氧氧化:同位素地球化学证据[J]. 海洋学报,2011,33(4): 121-128.
Yin Xijie, Chen Jian, Guo Yingying, et al. Sulfate reduction and methane anaerobic oxidation: isotope geochemical evidence from the pore water of coastal sediments in the Jiulong Estuary[J]. Haiyang Xuebao, 2011,33(4),121-128.
[25] Wijsman J W M,Middelburg J J,Herman P M J,et al. Sulfur and iron speciation in surface sediments along the northwestern margin of the Black Sea[J]. Marine Chemistry,2001,74(4): 261-278.
[26] 尹希杰,周怀阳,杨群慧,等. 珠江口淇澳岛海岸带沉积物中硫酸盐还原和不同形态硫的分布[J]. 海洋学报,2010,32(3): 31-39.
Yin Xijie, Zhou Huaiyang, Yang Qunhui, et al. Sulfate reduction and reduced sulfur speciation in the coastal sediments of Qi’ao Island in the Zhujiang Estuary in China[J]. Haiyang Xuebao, 2010, 32(3):31-39.
[27] Marvin-DiPasquale M C,Boynton W R,Capone D G. Benthic sulfate reduction along the Chesapeake Bay central channel. Ⅱ. Temporal controls[J]. Marine Ecology Progress Series,2003,260: 55-70.
[28] Beck M,Dellwig O,Liebezeit G,et al. Spatial and seasonal variations of sulphate,dissolved organic carbon,and nutrients in deep pore waters of intertidal flat sediments[J]. Estuarine,Coast Shelf Science,2008,79(2): 307-316.
[29] Manous J J,Gantzer C J,Stefan H G. Spatial Variation of Sediment Sulfate Reduction Rates in a Saline Lake [J]. Journal of Environmental Engineering,2007,133(12): 1106-1116.
[30] Edenborn H M,Silverberg N,Mucci A,et al. Sulfate reduction in deep coastal marine sediments[J]. Marine Chemistry,1987,21(4): 329-345.
[31] Coleman M L,Raiswell R. Source of carbonate and origin of zonation in pyritiferous carbonate concretions: evaluation of a dynamic model[J]. American Journal of Science,1995,295(3): 282-308.
[32] Pallud C,Cappellen P V. Kinetics of microbial sulfate reduction in estuarine sediments[J]. Geochimica et Cosmochimica Acta,2006,70(5): 1148-1162.
[33] Schubert C S,Ferdelman T G,Strotmann B. Organic matter composition and sulfate reduction rates in sediments off Chile[J]. Organic Geochemistry,2000,31(5): 351-361.
[34] Canfield D E. Sulfate reduction in deep sea sediments[J]. American Journal of Science,1991,291(2): 177-188.
[35] Devol A H,Ahmend S I. Are high rates of sulphate reduction associated with anaerobic oxidation of methane? [J]. Nature,1981,291(5814): 407-408.
[36] Pohlman J W,Ruppel C,Hutchinson D R,et al. Assessing sulfate reduction and methane cycling in a high salinity pore water system in the northern Gulf of Mexico[J]. Marine and Petroleum Geology,2008,25(9): 942-951.
[37] Li Q Q,Wang F P,Chen Z W,et al. Stratified active archaeal communities in the sediments of Jiulong river estuary China[J]. Frontiers in Microbiology,2012,3: 311.
[38] Wenzhofer F,Glud R N. Benthic carbon mineralization in the Atlantic: a synthesis based on in situ data from the last decade[J]. Deep-Sea Research,2002,49(7): 1255-1279.
[39] Jahnke R A. The global ocean flux of particulate organic carbon: A real distribution and magnitude[J]. Global Biogeochemical Cycles,1996,10(1): 71-88.
[40] Hadas O. Sulfate reduction in Lake Agmon,Israel[J]. Science of the Total Environment,2001,266(1/3): 203-209.
[41] Julies E M,Fuchs B M,Arnosti C,et al. Organic carbon degradation in anoxic Organic-Rich shelf sediments: Biogeochemical rates and microbial abundance[J]. Geomicrobiology Journal,2010,27(4): 303-314.
[42] Sawicka1 J E,Jørgensen B B,Brüchert V. Temperature characteristics of bacterial sulfate reduction in continental shelf and slope sediments[J]. Biogeosciences,2012,9(8): 3425-3435.
[43] Weber A,Jørgensen B B. Bacterial sulfate reduction in hydrothermal sediments of the Guaymas Basin,Gulf of California,Mexico[J]. Deep-Sea Research I,2002,49(5): 827-841.
[44] Treude T,Niggemann J,Kallmeyer J,et al. Anaerobic oxidation of methane and sulfate reduction along the Chilean continental margin[J]. Geochimica et Cosmochimica Acta,2005,69(11): 2767-2779.
[45] Bertics V J,Ziebis W. Bioturbation and the role of microniches for sulfatereduction in coastal marine sediments[J]. Environmental Microbiology,2010,12(11): 3022-3034.
[46] Laverman A M,Pallud C,Abell J. et al. Comparative survey of potential nitrate and sulfate reduction rates in aquatic sediments[J]. Geochimica et Cosmochimica Acta,2012,77: 474-488.
[47] Hines M E,Knollmeyer S L,Tugel J B. Sulfate reduction and other sedimentary biogeochemistry in a northern New England salt marsh[J]. Limnology and Oceanography,1989,34(3): 578-590.
[48] Maltby E,Immirzi C P. Carbon dynamics in peatlands and other wetlands soils: regional and global perspectives[J]. Chemosphere,1993,27(6): 999-1023.
[49] Hyun J H,Smith A C,Kostka J E. Relative contributions of sulfate-and iron(III) reduction to organic matter minrtalization and process controls in contrasting habitats of the Georgia saltmarsh[J]. Applied Geochemistry,2007,22(12): 2637-2651.
[50] Thamdrup B. Bacterial manganese and iron reduction in aquatic sediments[J]. Advances in Microbiology and Ecology,2000,16: 41-84.
[51] Weber A,Riess W,Wenzhoefer F,et al. Sulfate reduction in Black Sea sediments:Insituand laboratory radiotracer measurements from the shelf to 2000 m depth[J]. Deep-Sea Research,2001,48(9): 2073-2096.
[52] Bruchert V,Gorgensen B B,Neumann K,et al. Regulation of bacterial sulfate reduction and hydrogen sulfide fluxes in the central Namibian coastal upwelling zone[J]. Geochimica et Cosmochimica Acta,2003,67(23): 4505-4518.
[53] Zopfi J,BöttcherM,Jørgensen B B. Biogeochemistry of sulfur and iron in Thioploca-colonized surface sediments in the upwelling area off central Chile[J]. Geochimica et Cosmochimica Acta,2008,72(3): 827-843.
[54] Devol A H,Anderson J J,Kuivila K,et al. A model for coupled sulfate reduction and methane oxidation in the sediments of Saanich Inlet[J]. Geochimica et Cosmochimica Acta,1984,48(5): 993-1004.
[55] Iversen N,Jørgensen B B. Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark)[J]. Limnology and Oceanography,1985,30(5): 944-955.
[56] Alperin M J,Reeburgh W S,Whiticar M J. Carbon and hydrogen isotope fractionation resulting from anaerobic methane oxidation[J]. Global Biogeochemical Cycles,1988,2(3): 279-288.
[57] Thode-Andersen S,Jørgensen B B. Sulfate reduction and the formation of 35S-labeled FeS,FeS2,and So in coastal marine sediments[J]. Limnology and Oceanography,1989,34(5): 793-806.
[58] Jørgensen B B,Bang M,Blackburn H T. Anaerobic mineralization in marine sediments from the Baltic Sea-North Sea transition[J]. Marine Ecology Progress Series,1990,59: 39-54.
[59] Oenema O. Sulfate reduction in fine-grained sediments in the Eastern Scheldt,southwest Netherlands[J]. Biogeochemistry,1990,9(1): 53-74.
[60] Roden E E,Tuttle J H. Inorganic sulfur cycling in mid and lower Chesapeake Bay sediments[J]. Marine Ecology Progress Series,1993,93: 101-118.
[61] Fossing H,Ferdelman T G,Berg P. Sulfate reduction and methane oxidation in continental margin sediments influenced by irrigation (South-East Atlantic off Namibia) [J]. Geochimica et Cosmochimica Acta,2000,64(5): 897-910.
[62] Jørgensen B B,Weber A,Zopfi J. Sulfate reduction and anaerobic methane oxidation in Black Sea sediments[J]. Deep-Sea Research,2001,48(9): 2097-2120.
[63] Mazumdar A,Paropkari A L,Borole D V,et al. Pore-water sulfate concentration profiles of sediment cores from Krishna-Godavari and Goa basins,India[J]. Geochemical Journal,2007,41: 259-269.
[64] Bowles M W,Samarkin V A,Bowles K M. Weak coupling between sulfate reduction and the anaerobic oxidation of methane in methane-rich seafloor sediments during ex situ incubation[J]. Geochimica et Cosmochimica Acta,2011,75(2): 500-519.
[65] Crill P M,Martens C S. Biogeochemical cycling in an organic-rich coastal marine basin. 6. Temporal and spatial variations in sulfate reduction rates[J]. Geochimica et Cosmochimica Acta,1987,51(5): 1175-1186.
[66] Takii S,Tanaka H,Kohata K,et al. Seasonal changes in sulfate reduction in sediments in the Inner Part of Tokyo Bay[J]. Microbes and Environments,2002,17(1): 10-17.
[67] Panutrakul S,Monteny F,Baeyens W,et al. Seasonal variations in sediment sulfur cycling in the Ballastplaat Mudflat,Belgium[J]. Estuaries,2001,24(2): 257-265.
Measurement of sulfate reduction rate in coastal sediments of Jiulong River Estuary with a radiotracer technique
Yin Xijie1,Sun Zhilei2,Xu Yonghang1,Li Yunhai1,Shao Changwei3
(1.OpenLaboratoryofOcean&CoastEnvironmenttalGeology,ThirdInstituteofOceanographyStateOceanicAdministration,Xiamen361005,China; 2.KeyLaboratoryofMinistryofLandandResourcesforMarineOilGasResourcesandEnvironmentalGeology,QingdaoInstituteofMarineGeology,Qingdao266071,China; 3.ShandongGeophysicalandGeochemicalExplorationInstitute,Jinan250013,China)
sulfate reduction rate; sulfate; anaerobic methane oxidation; Jiulong River Estuary
10.3969/j.issn.0253-4193.2015.04.008
2014-03-31;
2014-09-23。
国家青年基金(41006072,41276059);福建省青年基金项目(2010J05095)。
尹希杰(1977—),男,山东省潍坊市人,副研究员,主要研究方向为海洋生物地球化学。E-mail:yinxijie2003@163.com
P736.41
A
0253-4193(2015)04-0083-11
Yin Xijie,Sun Zhilei,Xu Yonghang,et al. Measurement of sulfate reduction rate in coastal sediments of Jiulong River Estuary with a radiotracer technique[J]. Haiyang Xuebao,2015,37(2):83—93,doi:10.3969/j.issn.0253-4193.2015.04.008

