青花菜转录因子基因BoWRKY2的克隆与表达分析
蒋明, 陈贝贝, 管铭, 李金枝, 黄笑梅, 顾云吉
(台州学院生命科学学院,植物进化生态学与保护省重点实验室/生态学省重点学科,浙江 椒江 318000)
青花菜转录因子基因BoWRKY2的克隆与表达分析
蒋明*, 陈贝贝, 管铭, 李金枝, 黄笑梅, 顾云吉
(台州学院生命科学学院,植物进化生态学与保护省重点实验室/生态学省重点学科,浙江 椒江 318000)
以青花菜为材料,克隆到1个WRKY转录因子基因BoWRKY2,在序列分析的基础上,利用反转录聚合酶链反应(reverse transcription-polymerase chain reaction, RT-PCR)检测其在核盘菌和霜霉菌侵染下的表达模式。结果表明:BoWRKY2的基因组全长为1 507 bp,具有2个内含子,编码区全长为987 bp;BoWRKY2编码328个氨基酸,具有1个WRKYGQK残基和C-X5-C-X23-H-X1-H锌指结构,WRKY结构域与甘蓝型油菜的最为相似,仅存在1个氨基酸残基的差异。进化分析结果表明,BoWRKY2与同为十字花科的甘蓝型油菜、拟南芥、深山南芥、荠菜和盐芥聚为一组,支持率达97%。RT-PCR结果表明,BoWRKY2的表达受霜霉菌和核盘菌的诱导,二者的表达模式相似,在6 h和12 h时表达量增加,但在24 h后下降,暗示BoWRKY2与2种病菌的早期抗性反应相关.
青花菜; WRKY转录因子; 克隆; 基因表达; 抗病
Summary Broccoli (Brassicaoleraceavar.italica), which belongs to Cruciferae family, is a cash crop widely cultivated in China, and it is regarded as one of the most consumed vegetables in the world. The flower head of broccoli is rich in minerals, vitamins, fibers as well as anti-oxidants, so it is recognized as a healthy vegetable with anti-cancer properties. As a major broccoli production center in China, the average plantation areas reach to 8 000 hm2in Taizhou of Zhejiang Province. However, broccoli cultivation suffered from plant diseases of downy mildew and stalk break which were caused byHyaloperonosporaparasiticaandSclerotiniasclerotiorum, respectively, resulting in yield and quality loss.
WRKY transcription factors played important roles in plant stress responses, and WRKY domains were defined as an approximately 60-amino acid motif named WRKYGQK as well as a zinc finger structure of C-X4-5-C-X22-23-H-X1-H at their C-terminus. The WRKY domain binds specifically to DNA sequence of (T)(T)TGAC(C/T) known as the W-box which exists in defense-related promoters. Enhanced disease resistance by overexpression of WRKY genes in different crop plants has been reported in recent years.
In this study, a WRKY gene designated asBoWRKY2, was isolated fromB.oleraceavar.italica. Based on sequence analysis, expression patterns ofBoWRKY2 were detected using reverse transcription-polymerase chain reaction (RT-PCR) method while challenged byH.parasiticaandS.sclerotiorum, respectively.
The results showed that the genome DNA sequence was 1 507 bp in length with two introns and a complete coding sequence of 987 bp, and the length of two introns were 425 and 95 bp, respectively;BoWRKY2 encoded 328 amino acids with a WRKYGQK residue and a zinc finger structure of C-X5-C-X23-H-X1-H. The WRKY domain located between 247 and 313 residues, and several DNA binding sites were found at sites of 66, 67, 69, 71, 74, 281, 282, 283 and 317. The WRKY domain was similar to that of oilseed rape with only one amino acid residue difference between them. Phylogenetic analysis indicated the BoWRKY2 was grouped with other Cruciferae plants such asB.napus,Arabidopsisthaliana,A.lyrata,CapsellarubellaandEutremasalsugineum, with 97% confidence. RT-PCR results revealed that theBoWRKY2 was induced by bothH.parasiticaandS.sclerotiorumwith similar expression patterns. The expression level both increased at 6 h and 12 h, and decreased after 24 h, indicating the resistance responses ofBoWRKY2 against two plant fungi.
In a word, the cloning and expression analysis ofBoWRKY2 gene lay the foundation for further studies in gene function identification and molecular breeding of broccoli.
青花菜(Brassicaoleraceavar.italica)为十字花科(Cruciferae)芸薹属蔬菜,别名西兰花、绿花椰菜和绿菜花等,是一种在我国栽培面积较大的蔬菜作物。因花茎和花球中富含矿物质、维生素、纤维素和抗氧化物质,青花菜已成为一种深受人们喜爱的保健蔬菜[1-2]。浙江省台州地区是我国青花菜主产区,建有国内规模最大的生产和出口基地,常年种植面积8 000 hm2左右[3]。霜霉病和菌核病是青花菜生产过程中的2大病害,它们分别由寄生霜霉菌(Hyaloperonosporaparasitica)和核盘菌(Sclerotiniasclerotiorum)引起,危害幼苗、成株和花球,造成产量和品质下降,给菜农带来一定的损失[4-5]。分子育种是培育抗病品种的重要途径之一,利用现代分子生物学技术,挖掘与青花菜抗病相关的功能基因,将为分子育种提供重要的目标基因。
WRKY转录因子在高等植物中以家族形式存在,因蛋白质序列中含保守的WRKY结构域而得名[6]。WRKY结构域由WRKYGQK残基和锌指结构组成,C-X4-5-C-X22-23-H-X1-H为最常见的锌指结构模式[7]。WRKY结构域特异结合(T)(T)TGAC(C/T)序列(W盒),W盒存在于跟防卫反应相关的启动子中,因此WRKY能调节逆境胁迫相关基因的表达[8]。近年来,WRKY在抗病中的作用已有一些报道,如拟南芥(Arabidopsisthaliana)AtWRKY28和AtWRKY75的过量表达可增强对核盘菌的抗性[9];在拟南芥中超量表达水稻(Oryzasativa)的OsWRKY77可抑制丁香假单孢菌(Pseudomonassyringaepv.tomatoDC3000)的生长,同时能激发抗病基因PR1、PR2和PR5的表达[10]。本研究以青花菜为材料,克隆到1个WRKY转录因子基因BoWRKY2,在序列分析的基础上,研究了BoWRKY2在寄生霜霉菌及核盘菌侵染下的表达模式,以期为该基因功能鉴定和抗病分子育种奠定基础。
1 材料与方法
1.1 材料
青花菜材料选用“绿雄”品种,由实验室栽植;将其播种于无菌基质中,待长出2片真叶时接种霜霉菌和核盘菌。带霜霉菌的病叶采自浙江省临海市上盘镇青花菜种植基地,在实验室用无菌ddH2O小心冲洗白色霉层以收集孢子,稀释后备用;核盘菌菌核采自浙江省临海市江南青花菜试验田,在超净工作台上用无菌ddH2O清洗3次,再用1%氯化汞表面消毒7 min,切成小块后接种到马铃薯葡萄糖琼脂(potato dextrose agar, PDA)培养基上,用于培养菌丝。霜霉菌接种采用喷雾法[11];核盘菌接种采用菌丝块法[12];采集接种0、6、12、24、36和72 h的叶片,置于-80 ℃备用。
1.2 方法
1.2.1 基因克隆和转化 基因组DNA的提取采用十二烷基硫酸钠(sodium dodecyl sulfate, SDS)法,RNA提取用TRIzol法,cDNA的合成采用SMARTTMcDNA合成试剂盒。基因克隆所用引物为BoWYup:5′-ATGACCGTCGACATCATGC-3′和BoWYdn:5′-TCAAGCAGAGCCAAACAC-3′,委托生工生物工程(上海)股份有限公司合成。分别以叶片DNA和混合cDNA为模板进行聚合酶链反应(polymerase chain reaction, PCR)扩增,在0.2 mL薄壁管中分别加入2 μL 10×PCR缓冲液(含20 mmol/L Mg2+),1.0 UTaqDNA聚合酶(北京鼎国生物技术有限责任公司),0.4 μL 10 mmol/L dNTPs,20 μmol/L引物各0.5 μL,30 ng模板,加ddH2O至20 μL。PCR扩增程序:94 ℃预变性5 min;94 ℃变性35 s,54.5 ℃退火 45 s,72 ℃延伸100 s,共33个循环。
1.2.2 PCR产物回收与转化 PCR产物经电泳分离后,用DNA凝胶回收试剂盒(上海碧云天生物技术研究所)回收。取2 μL回收产物与p-GEM-T-easy载体(Promega公司,美国)连接,室温放置3 h后转入DH-5α感受态细胞中,经LB(Luria-Bertani)固体培养基涂布培养,挑取白色单菌落于37 ℃振荡培养12 h。菌液PCR体系和程序同1.2.1节,模板改为0.5 μL菌液,经验证后各取3个阳性克隆用于测序。
1.2.3 生物信息学分析 用分子软件DNAMAN 5.2.2推导BoWRKY2的蛋白质序列;用Scanprosite在线工具http://prosite.expasy.org/scanprosite/[13]检索编码蛋白的保守结构域;用Clustal X 1.81软件[14]比对WRKY序列;用Mega 3.1软件[15]构建进化树,采用邻接法,自举检测1 000次。
1.2.4 表达分析 根据测序结果设计反转录聚合酶链反应(reverse transcription-polymerase chain reaction, RT-PCR)引物,分别为Bo2up:5′-GACCACAACGCTGACTG-3′和Bo2dn:5′-TTTCCTCGCTGGACACC-3′,以等量cDNA为模板进行PCR扩增。在20 μL反应体系中加入1.2 UTaqDNA聚合酶,20 μmol/L引物各0.6 μL,其余PCR试剂及用量同1.2.1节。PCR扩增程序:94 ℃预变性5 min;94 ℃变性30 s,57.8 ℃退火45 s,72 ℃延伸60 s,共35个循环。PCR产物于1%琼脂糖凝胶上电泳并拍照,RT-PCR重复3次。以肌动蛋白基因为内标,上、下游引物分别为5′-TCTCGATG GAAGAGCTGGTT-3′和5′-GATCCTTACCGAG GGAGGTT-3′,扩增程序为94 ℃预变性5 min;94 ℃变性30 s,55.6 ℃退火45 s,72 ℃延伸90 s,共32个循环。
2 结果与分析
2.1 BoWRKY2的序列特征
分别以叶片基因组DNA和cDNA为模板,用BoWYup/BoWYdn引物对扩增出目的条带。测序结果表明,BoWRKY2基因组全长为1 507 bp,编码区全长为987 bp。BoWRKY2具有2个内含子,长度分别为425和95 bp,外显子长度分别为689、129和169 bp(图1)。
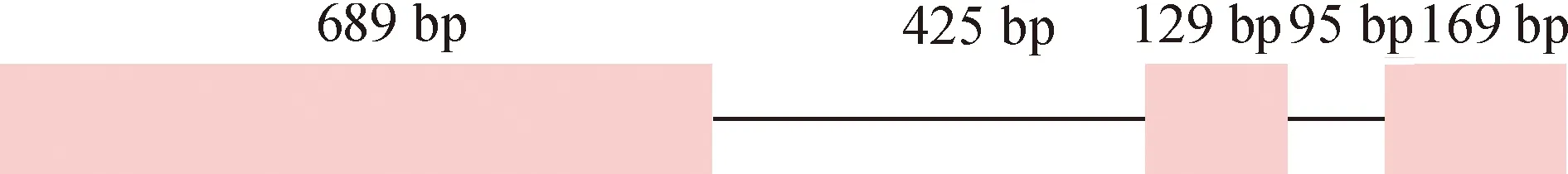
实心方块及上面的数字表示外显子及其长度;直线及上面的数字表示内含子及其长度。Solid box and number above indicate exon and its length; Line and number above indicate intron and its length.
2.2 BoWRKY2编码蛋白的特征
推导的BoWRKY2编码328个氨基酸,相对分子质量为3.557 42×104,等电点为9.77,具有1个WRKYGQK残基(图2)。该残基的C端有1个锌指结构,类型为C2H2,根据半胱氨酸(cysteine, C)和组氨酸(histidine, H)之间的残基数,该锌指结构可用C-X5-C-X23-H-X1-H表示。WRKY结构域位于247~313残基处,在66、67、69、71、74、281、282、283和317位置处各有1个DNA结合位点(图3)。
2.3 BoWRKY2与同源序列的比对与进化分析
从NCBI下载到29个WRKY基因的编码蛋白序列,分别为森林草莓(Fragariavesca)(登录号:XP_004299798.1)、苹果(Malusdomestica)(ADL36859.1)、烟草(Nicotianatabacum)(BAA77358.1)、马铃薯(Solanumtuberosum)(XP_006342466.1)、番茄(Solanumlycopersicum)(XP_004244484.1)、甘蓝型油菜(B.napus)(ACI14386.1)、拟南芥(A.thaliana)(AEC07593.1)、深山南芥(A.lyrata) (EFH56854.1)、盐 芥 (Eutremasalsugineum) (ESQ46443.1)、 荠 菜 (Capsella(rubella)(AAS66778.1)、蒺藜苜蓿(Medicagotruncatula)(AES58882.1)、鹰嘴豆(Cicerarietinum)(XP_004498566.1)、大豆(Glycinemax)(ABS18446.1)、陆地棉(Gossypiumhirsutum)(AET36544.1)、黄瓜(Cucumissativus)(ADU52516.1)、蓖麻(Ricinuscommunis)(EEF46802.1)、苹果(M.domestica)(ADL36858.1)、橙(Citrussinensis)(XP_006483548.1)、石斛(Dendrobiumnobile)(AHH81834.1)、铁皮石斛(D.officinale)(AHG94294.1)、玉米(Zeamays)(ACG39023.1)、小米(Setariaitalica)(XP_004975218.1)、小麦(Triticumaestivum)(AFR66647.1)、大麦(Hordeumvulgare)(ABI13376.1)、燕麦(Avenasativa)(AAD32676.1)、水稻(O.sativa)(EEC76600.1)、玉米(Z.mays)(ACG45417.1)、高粱(Sorghumbicolor)(EES11384.1)和大麦(H.vulgare)(BAK03156.1)。

阴影处为WRKY结构域;斜体和下划线处为保守的氨基酸残基。WRKY domain was highlighted under the shade. Italic and underlined sequences indicate conserved amino acid residues.

(247~313):WRKY结构域位置;(66,67,69,71,74,281,282,283,317):DNA结合位点的位置。(247-313): Location of WRKY domain; (66, 67, 69, 71, 74, 281, 282, 283 and 317): DNA binding sites.
结果(图4)表明:BoWRKY2与从NCBI上下载的29条蛋白质序列长度范围为280~350,其中,黄瓜和棉花的WRKY序列最长,鹰嘴豆次之(346),苹果(ADL36859.1)最短;它们均带有WRKYGQK保守残基,锌指结构模式都为C-X4-5-C-X22-23-H-X1-H;BoWRKY2与甘蓝型油菜的WRKY结构域最为相似,仅有1个氨基酸残基的差异,与其余3种十字花科植物也只有2个残基的差异,与番茄的差异最大,残基数差异为8。
以BoWRKY2和从NCBI上下载的29条蛋白质序列为材料,用Mega 3.1软件构建系统进化树。结果(图5)表明,这30条序列可分为7组,BoWRKY2与同为十字花科的甘蓝型油菜、拟南芥、深山南芥、荠菜和盐芥聚为一组,支持率为97%;豆科的蒺藜苜蓿、鹰嘴豆和大豆聚为一组,支持率为99%;茄科的番茄、烟草和马铃薯聚为一组,支持率也为99%;草莓和苹果(ADL36859.1)、石斛与铁皮石斛两两聚为一组,支持率均为100%;黄瓜、苹果(ADL36858.1)、蓖麻、橙和棉花聚为一组,支持率仅为55%;9种禾本科植物聚为一大组,支持率为91%,该组可分为2个亚组,其中玉米的 ACG45417.1 和 ACG39023.1、 大麦的BAK03156.1和ABI13376.1分别位于不同的亚组。

图4 BoWRKY2与同源序列WRKY结构域的比对
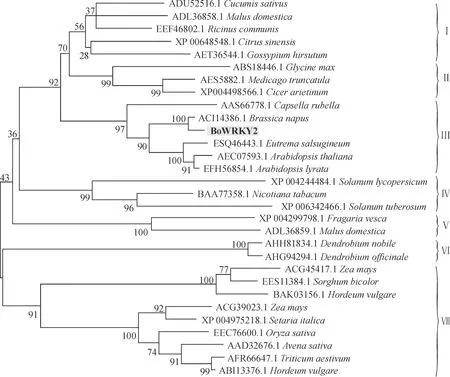
图5 BoWRKY2及其同源序列的系统进化树
2.4 BoWRKY2表达分析
分别以等量经霜霉菌和核盘菌处理的叶片cDNA为模板,用引物对Bo2up和Bo2dn进行PCR扩增。RT-PCR结果表明:在霜霉菌处理6 h和12 h时,BoWRKY2表达量增加,但24 h时的表达量下降(图6A);核盘菌处理后的表达模式与霜霉菌类似,在6 h和12 h时的表达量增加,但在24 h后下降(图6C)。
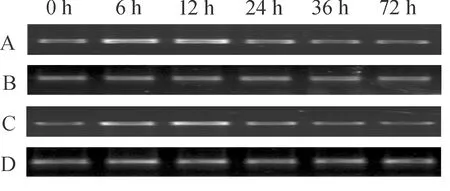
(A,C):分别为霜霉菌和核盘菌浸染下的BoWRKY2表达模式;(B,D):肌动蛋白内标。A and C: Expression patterns of BoWRKY2 incubated with Hyaloperonospora parasitica and Sclerotinia sclerotiorum, respectively; B and D: Internal control of actin.
3 讨论
WRKY以基因家族的形式存在于高等植物中,数量从几十到上百个不等。黄瓜(C.sativus)有55个WRKY成员,其中23个与非生物胁迫相关[16];桐油树(Jatrophacurcas)有58个成员,其中47个与非生物胁迫有关[17];粳稻与籼稻分别有98和102个WRKY基因,它们参与抗病反应、种子发育和非生物胁迫[18];欧洲大叶杨(Populustrichocarpa)有104个成员,而拟南芥相对较少,仅72个[19-20]。近年来,在白菜(B.campestris)、青花菜和甘蓝型油菜等十字花科植物中克隆到了WRKY基因[21-24]。本研究以青花菜为材料,克隆到1个WRKY基因BoWRKY2,该基因组全长为1 507 bp,具有2个内含子,编码区全长为987 bp,编码328个氨基酸,与前期克隆到的BoWRKY3[21]相比,BoWRKY2的基因组DNA和编码区较长,但内含子少1个。
在WRKY结构域中,以WRKYGQK最为常见,另有WKKYGQK、WRKYGKK、WRKYGEK、WRKYSEK和WRKYEQK等变异类型[24]。锌指结构除C2H2外,还有C2HC和C2XX等类型,其中,C2XX在水稻中已有报道[18]。WRKY转录因子通常分为3类:第1类有2个WRKY结构域,锌指结构为C2H2;第2类具有1个WRKY结构域,锌指结构为C2H2型;第3类也只有1个WRKY结构域,但锌指结构为C2HC[25]。BoWRKY2具常见的WRKYGQK残基,锌指结构为C2H2,属第2类WRKY转录因子。本研究表明,30个WRKY转录因子在进化树上分为7组,除黄瓜、苹果(ADL36858.1)、蓖麻、橙和棉花外,其他物种WRKY的聚类方式与分类学一致;ADL36859.1和ADL36858.1为苹果WRKY转录因子家族的不同成员,它们在进化树上分属V和I组。
WRKY转录因子广泛参与植物代谢、生长发育和逆境响应,在抗病反应中也有广泛研究[26]。陆地棉GhWRKY15表达受棉刺盘孢菌(Colletotrichumgossypii)、枯萎病菌(Fusariumoxysporumf. sp.vasinfectum)和立枯病菌(Rhizoctoniasolani)诱导,将该基因导入烟草,转基因植株过氧化物酶和抗坏血酸过氧化物酶等防御酶的活性增强[27];拟南芥经水杨酸处理后,AtWRKY33的表达量增加,同时,它的表达受霜霉菌的诱导[28]。在本研究中,青花菜BoWRKY2受霜霉菌和核盘菌的诱导,表达模式十分相似,即在6 h和12 h时的表达量增加,24 h后下降:暗示BoWRKY2与2种病菌的抗性反应有关。前期克隆的BoWRKY3同样受霜霉菌的诱导,但表达模式存在一定的差异,BoWRKY3在6~36 h时的表达量最高,高表达持续的时间较长。这说明同一基因家族的2个成员对霜霉菌有着不同的响应[21]。
总之,对BoWRKY2基因的克隆和表达分析,为进一步开展对该基因的功能鉴定和抗病分子育种奠定了基础。本实验室下一步将开展BoWRKY2的遗传转化研究,以明确该基因在抗病反应中的功能。
[1] Gliszczyńska-Swigo A, Ciska E, Pawlak-Lemańska K,etal. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing.FoodAdditives&Contaminants, 2006,23(11):1088-1098.
[2] Finley J W, Davis C D, Feng Y. Selenium from high selenium broccoli protects rats from colon cancer.JournalofNutrition, 2000,130(9):2384-2389.
[3] 王会福,钟列权,余山红,等.青花菜茎瘤病、根肿病和根结线虫病的识别与防治.江苏农业科学,2013,41(9):127-129.
Wang H F, Zhong L Q, Yu S H,etal. Recognition and control of broccoli stem gall, clubroot and root knot nematode disease.JiangsuAgriculturalSciences, 2013,41(9):127-129. (in Chinese)
[4] 陈海平,董荷玲,冯春梅.青花菜霜霉病的发生和流行规律.浙江农业科学,2013(1):56-58.
Chen H P, Dong H L, Feng C M. Occurrence and epidemic law of broccoli downy mildew.ZhejiangAgriculturalSciences, 2013(1):56-58. (in Chinese)
[5] 任典东,汪恩国,王永才.台州西兰花主要病虫发生为害规律研究.蔬菜,2013(2):65-67.
Ren D D, Wang E G, Wang Y C. Occurrence and injury regularity of major broccoli diseases and insects in Taizhou.Vegetables, 2013(2):65-67. (in Chinese)
[6] Eulgem T, Rushton P J, Robatzek S,etal. The WRKY superfamily of plant transcription factors.TrendsinPlantScience, 2000,5(5):199-206.
[7] Rushton P J, Macdonald H, Huttly A K,etal. Members of a new family of DNA-binding proteins bind to a conservedcis-element in the promoters ofα-Amy2 genes.PlantMolecularBiology, 1995,29(4):691-702.
[8] Eulgem T, Rushton P J, Schmelzer E,etal. Early nuclear events in plant defence signalling: Rapid gene activation by WRKY transcription factors.TheEMBOJournal, 1999,18(17):4689-4699.
[9] Chen X T, Liu J, Lin G F,etal. Overexpression ofAtWRKY28 andAtWRKY75 inArabidopsisenhances resistance to oxalic acid andSclerotiniasclerotiorum.PlantCellReports, 2013,32(10):1589-1599.
[10] Lan A, Huang J, Zhao W,etal. A salicylic acid-induced rice (OryzasativaL.) transcription factor OsWRKY77 is involved in disease resistance ofArabidopsisthaliana.PlantBiology, 2013,15(3):452-461.
[11] Peart J R, Lu R, Sadanandom A,etal. Ubiquitin ligase-associated protein SGT1 is required for host and nonhost disease resistance in plants.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStates, 2002,99(16):10865-10869.
[12] Silué D, Nashaat N I, Tirilly Y. Differential responses ofBrassicaoleraceaandB.rapaaccessions to seven isolates ofPeronosporaparasiticaat the cotyledon stage.PlantDisease, 1996,80(2):142-144.
[13] Sigrist C J A, de Castro E, Cerutti L,etal. New and continuing developments at PROSITE.NucleicAcidsResearch, 2013,41:344-347.
[14] Thompson J D, Gibson T J, Plewniak F,etal. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools.NucleicAcidsResearch, 1997,25(24):4876-4882.
[15] Kumar S, Tamura K, Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment.BriefingsinBioinformatics, 2004,5(2):150-163.
[16] Ling J, Jiang W J, Zhang Y,etal. Genome-wide analysis ofWRKYgene family inCucumissativus.BMCGenomics, 2011,12:471.
[17] Xiong W D, Xu X Q, Zhang L,etal. Genome-wide analysis of theWRKYgene family in physic nut (JatrophacurcasL.).Gene, 2013,524(2):124-132.
[18] Ross C A, Liu Y, Shen Q J. TheWRKYgene family in rice (Oryzasativa).JournalofIntegrativePlantBiology, 2007,49(6):827-842.
[19] Wu K L, Guo Z J, Wang H H,etal. TheWRKYfamily of transcription factors in rice andArabidopsisand their origins.DNAResearch, 2005,12(1):9-26.
[20] 宋琴,赵福宽,孙清鹏,等.白菜型油菜WRKY基因片段的克隆与表达分析.中国农学通报,2011,27(2):99-103.
Song Q, Zhao F K, Sun Q P,etal. Cloning & expression analysis of WRKY gene segment inBrassicacampestris.ChineseAgriculturalScienceBulletin, 2011,27(2):99-103. (in Chinese with English abstract)
[21] 陈贝贝,蒋明,苗立祥,等.青花菜转录因子基因BoWRKY3的克隆与表达分析.浙江大学学报:农业与生命科学版,2012,38(3):243-249.
Chen B B, Jiang M, Miao L X,etal. Cloning and expression analysis of a transcription factor geneBoWRKY3 fromBrassicaoleraceavar.italica.JournalofZhejiangUniversity:AgricultureandLifeSciences, 2012,38(3):243-249. (in Chinese with English abstract)
[22] Liu X, Wang X, Pang Y,etal. Molecular cloning and characterization of a novelWRKYgene fromBrassicachinensis.MolecularBiology, 2006,40(5):732-740.
[23] Yang B, Jiang Y, Rahman M H,etal. Identification and expression analysis of WRKY transcription factor genes in canola (BrassicanapusL.) in response to fungal pathogens and hormone treatments.BMCPlantBiology, 2009,9:68.
[24] Zhang Y J, Wang L J. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants.BMCEvolutionaryBiology, 2005,5:1.
[25] Eulgem T, Rushton P J, Robatzek S,etal. The WRKY superfamily of plant transcription factors.TrendsinPlantScience, 2000,5(5):199-206.
[26] Pandey S P, Somssich I E. The role of WRKY transcription factors in plant immunity.PlantPhysiology, 2009,150(4):1648-1655.
[27] Yu F F, Huaxia Y F, Lu W J,etal.GhWRKY15, a member of the WRKY transcription factor family identified from cotton (GossypiumhirsutumL.), is involved in disease resistance and plant development.BMCPlantBiology, 2012,12:144.
[28] Lippok B, Birkenbihl R P, Rivory G,etal. Expression ofAtWRKY33 encoding a pathogen- or PAMP-responsive WRKY transcription factor is regulated by a composite DNA motif containing W box elements.MolecularPlant-MicrobeInteractions, 2007,20(4):420-429.
Cloning and expression analysis of a transcription factor geneBoWRKY2 from broccoli. Journal of Zhejiang University (Agric. & Life Sci.), 2015,41(2):153-159
Jiang Ming*, Chen Beibei, Guan Ming, Li Jinzhi, Huang Xiaomei, Gu Yunji
(ZhejiangProvincialKeyLaboratoryofPlantEvolutionaryEcologyandConservation/EcologyKeyDisciplineofZhejiangProvince,CollegeofLifeSciences,TaizhouUniversity,Jiaojiang318000,Zhejiang,China)
Brassicaoleraceavar.italica; WRKY transcription factor; cloning; gene expression; disease resistance
浙江省自然科学基金(LY13C150003);浙江省公益性技术应用研究计划项目(2012C32011);浙江省重点学科(浙江省台州学院生态学)开放课题(EKD2013-03);浙江省植物进化生态学与保护重点实验室植物进化生态学人才培育项目.
2014-05-20;接受日期(Accepted):2014-12-09;网络出版日期(Published online):2015-03-20
Q 78
A
*通信作者(Corresponding author):蒋明,E-mail:jiangming1973@139.com
URL:http://www.cnki.net/kcms/detail/33.1247.S.20150320.2118.013.html

