脊髓脂质运载蛋白-2对大鼠吗啡耐受形成的影响
王 芬,刘清珍,刘 健,江姿潞,谢 滔,李伟彦
(1.徐州医学院江苏省麻醉学重点实验室,2.徐州医学院江苏省麻醉与镇痛应用技术重点实验室,江苏 徐州 221004;3.南京军区南京总医院麻醉科,江苏 南京 210002;4.第二军医大学研究生院,上海 200433)
脊髓脂质运载蛋白-2对大鼠吗啡耐受形成的影响
王 芬1,2,刘清珍3,刘 健3,江姿潞1,2,谢 滔4,李伟彦3
(1.徐州医学院江苏省麻醉学重点实验室,2.徐州医学院江苏省麻醉与镇痛应用技术重点实验室,江苏 徐州 221004;3.南京军区南京总医院麻醉科,江苏 南京 210002;4.第二军医大学研究生院,上海 200433)
目的 探索用RNA干扰(RNAi)法沉默脊髓脂质运载蛋白-2(lipocalin-2, LCN2)的表达及其对正常大鼠吗啡耐受形成的影响。方法 鞘内置管成功的♂SD大鼠48只,体质量180~220 g,随机均分为4组,每组12只:Ⅰ组对照组、Ⅱ组吗啡耐受组、Ⅲ组错义小干扰RNA(mismatch siRNA, MM siRNA)组、Ⅳ组LCN2小干扰RNA(LCN2 siRNA)组。所有大鼠鞘内置管术后d 6确定导管位置,记为d 0。d 2~8连续7 d,每天2次进行皮下注射,每次注射剂量10 μg·g-1,Ⅰ组大鼠皮下注射生理盐水(normal saline, NS),Ⅱ-Ⅳ组大鼠皮下注射吗啡建立吗啡耐受模型。各组大鼠每天皮下给药前分别鞘内注射10 μL DEPC溶液、10 μL DEPC溶液、10 μL含4 μg MM siRNA的DEPC溶液和10 μL含4 μg LCN2 siRNA的DEPC溶液。d 1、d 9,测定所有大鼠的基础热缩足反应潜伏期(paw withdrawal thermal latency, PWTL)及皮下注射吗啡后45 min的PWTL,并计算吗啡的最大可能镇痛效应百分比值(% maximal possible effect, %MPE)。d 9行为学测试结束后,取脊髓腰膨大,用Western blot法检测磷酸化p38 丝裂原活化蛋白激酶(p-p38 MAPK)及LCN2蛋白表达水平,用免疫组织荧光染色法检测小胶质细胞标记物Iba1的表达。结果 d 9,与Ⅰ组比,Ⅱ、Ⅲ组大鼠%MPE值明显下降(P<0.05),腰段脊髓LCN2、p-p38 MAPK、Iba1表达明显增加(P<0.05)。与Ⅱ组比,Ⅳ组大鼠的%MPE值明显增加(P<0.05),脊髓LCN2、p-p38MAPK、Iba1表达明显下降(P<0.05)。结论 鞘内注射LCN2 siRNA沉默脊髓LCN2的表达能够部分抑制吗啡耐受的形成,该现象可能与其抑制脊髓背角小胶质细胞的活化及脊髓p-p38 MAPK的表达有关。
吗啡耐受;脂质运载蛋白-2;小干扰RNA;小胶质细胞;磷酸化p38 丝裂原活化蛋白激酶;脊髓
吗啡(morphine)耐受是指长期反复使用吗啡后,其镇痛作用逐渐减退甚至消失,需加大剂量才能产生既定药理效应的现象。大量研究显示,胶质细胞活化是吗啡耐受的重要机制,其中小胶质细胞活化主要参与了吗啡耐受的形成,星型胶质细胞活化则参与了吗啡耐受的维持[1-2]。脂质运载蛋白-2(lipocalin-2, LCN2),也被称作24p3或中性粒细胞明胶酶相关脂质运载蛋白(neutrophil gelatinase-associated lipocalin, NGAL),是一个分子质量为25 ku的糖蛋白,最初在中性粒细胞颗粒中发现。研究发现,LCN2能够诱导小胶质细胞和p38 MAPK活化,进而诱导炎症因子释放而导致神经病理性疼痛[3],而小胶质细胞、p38 MPAK通路均参与了吗啡耐受的形成,其中p-p38MAPK主要表达于小胶质细胞[4,5],因此,我们推测LCN2亦可能参与了吗啡耐受的发生。本实验拟用鞘内注射LCN2 siRNA选择性抑制LCN2表达的方法,探讨LCN2对大鼠吗啡耐受形成的影响,并探索相关机制,为防治吗啡耐受提供新的依据。
1 材料与方法
1.1 实验动物与分组清洁级♂SD大鼠,体质量180~220 g(南京军区南京总医院比较医学科提供)。盐酸吗啡注射液(130704-2,东北制药集团沈阳第一制药有限公司,中国),DEPC溶液,LCN2 siRNA序列:sense: 5′-GCCUCAAGGAUAACAACAUTT-3′; antisense: 5′-AUGUUGUUAUCCUUGAGGCTT-3′; 错义siRNA序列:sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′- ACGUGACACGUUCGGAGAATT-3′(上海吉玛制药技术有限公司,中国)。所有大鼠鞘内置管成功后,采用随机数字表法将其随机分为4组(n=12):Ⅰ组,对照组(DEPC+NS组);Ⅱ组,吗啡耐受组(DEPC+morphine组);Ⅲ组,错义 siRNA组(MM siRNA+morphine组);Ⅳ组,LCN2 siRNA组(LCN2 siRNA+morphine组)。
1.2 鞘内置管参考Milligan等[6]的方法,大鼠腹腔注射质量分数为2 %的戊巴比妥钠50 μg·g-1麻醉,以L5-6为中心作纵切口,分离椎旁肌肉,暴露椎间隙,以稍钝针头穿破黄韧带及硬脊膜,可见大鼠出现甩尾反射及脑脊液外溢。将一段20 cm的PE-10导管(宁波市科技园区安来软件科技有限公司,中国)经椎间隙向头端插入2 cm。经皮下自颈部将其引出后缝合固定导管,热熔封口。术后分笼饲养,自由饮水和摄食,恢复5 d。置管d 6经导管注射质量分数为2%的利多卡因15 μL,如注射后30 s内大鼠出现双后肢麻痹现象说明导管位置正确,记为d 0。置管不成功大鼠予以麻醉处死。
1.3 给药方案参照Raghavendra等[4]方法,d 2~8,连续7 d,每天8 ∶00和16 ∶00,Ⅱ~Ⅳ组大鼠皮下注射10 μg·g-1吗啡建立吗啡耐受模型,Ⅰ组大鼠皮下注射10 μg·g-1生理盐水。各组大鼠每天皮下给药前分别鞘内注射10 μL DEPC溶剂(Ⅰ组、Ⅱ组)、4 μg MM siRNA(Ⅲ组)、4 μg LCN2 siRNA的DEPC溶液(Ⅳ组),siRNA均用10 μL DEPC溶剂稀释。
1.4 行为学测试d 1、d 9,参照Hargreaves等[7]方法,使用足底辐射热痛觉测试仪(Model 390, IITC Life ScienceInc, 美国),测定所有大鼠的基础热缩足反应潜伏期(paw withdrawal thermal latency, PWTL),皮下注射吗啡后45 min的PWTL,每只大鼠重复测量5次,单次光照时间不超过20 s(cut-off time),去掉最大及最小值,取平均值即为大鼠的PWTL,并计算吗啡的最大可能镇痛效应百分比值(% maximal possible effect, MPE/%),MPE/%=(给药后PWTL-基础PWTL) / (cut-off time-基础PWTL)×100%。
1.5 标本采集d 9行为学测试结束后,大鼠腹腔注射质量分数为2%的戊巴比妥钠80 μg·g-1麻醉。取8只大鼠快速截取脊髓腰膨大于液氮中保存,留待Western blot法检测蛋白表达。另取4只大鼠开胸灌注,留取脊髓腰膨大用于免疫荧光染色。
1.6 Western blot用含蛋白酶抑制剂、磷酸酶抑制剂和PMSF的RIPA裂解液将L4-6脊髓组织进行匀浆。蛋白定量煮样后,配置SDS-PAGE凝胶,每孔上样量50 μg(10 μL),电泳,转膜,质量分数为5 %的脱脂牛奶室温封闭1 h,加一抗:兔抗大鼠LCN2(1 ∶200,Millipore,美国)或兔抗大鼠p-p38 MAPK(1 ∶800, Cell Signaling Technology,美国),4 ℃孵育过夜。d 2,弃一抗,TBST洗5 min×6次,加辣根过氧化物酶标记的羊抗兔二抗(1 ∶1 000,Cell Signaling Technology,美国),室温孵育1 h,TBST洗5 min × 6次,增强型化学发光试剂显色曝光,显影,定影。 内参为GAPDH(1 ∶1 000,Cell Signaling Technology,美国)或β-actin(1 ∶1 000,Cell Signaling Technology,美国 )。扫描后蛋白条带用Image J软件分析,以目的条带灰度值与内参蛋白的比值反映目的蛋白表达的相对水平,进行半定量分析。
1.7 免疫组织荧光染色取L4-6脊髓,置入质量分数为4 %的多聚甲醛溶液,4 ℃固定4~6 h。然后转入质量分数为15 %的蔗糖溶液,待组织沉底后再转入质量分数为30 %的蔗糖溶液,4 ℃放置48 h。OCT包埋组织后,放入-22 ℃冰冻切片机(CM1900, Leica,美国),40 min后行冰冻切片,片厚35 μm。PBST洗5 min×3次,质量分数为5 %的驴血清封闭2 h,加山羊抗大鼠Iba1一抗(1 ∶100, Abcam,美国),4 ℃孵育过夜,PBST洗3次,加入荧光标记二抗(1 ∶1 000, Life Technologies,美国)室温避光孵育2 h,PBST洗5 min×3次。避光贴片、甘油封片。普通荧光显微镜下观察摄片。

2 结果
2.1 行为学测试d 1、d 9各组大鼠基础PWTL(Basic PWTL)差异均无显著性(Fig 1)。d 1,各组大鼠%MPE差异无显著性,d 9,与Ⅰ组比较,Ⅱ、Ⅲ、Ⅳ组大鼠%MPE明显降低(P<0.05)。与Ⅱ组比较,Ⅲ组大鼠的%MPE无明显变化,而Ⅳ组大鼠的%MPE明显升高(P<0.05)见Fig 2。
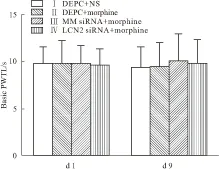
Fig 1 Basic PWTL of rats on d 1 and d 9(n=8)
On d 1 and d 9, there was no significant difference in the basic PWTL among four groups (P>0.05).
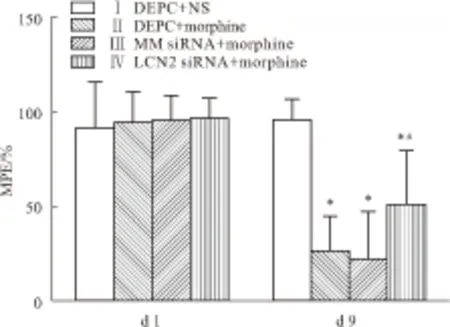
Fig 2 MPE of rats on d 1 and d 9(n=8)
On d 1, there was no significant difference in the %MPE among four groups (P>0.05), while on d 9, the MPE significantly declined in group Ⅱ-Ⅳ compared to group I (P<0.05) and the MPE significantly increased in group Ⅳ compared to group Ⅱ (P<0.05).*P<0.05vsgroup Ⅰ;#P<0.05vsgroup Ⅱ
2.2 脊髓腰膨大LCN2与p-p38 MAPK蛋白的表达变化与Ⅰ组相比,其余各组大鼠脊髓腰膨大的LCN2表达量均升高(P<0.05)。与Ⅱ组相比,Ⅲ组大鼠脊髓腰膨大的LCN2表达无明显变化,而Ⅳ组大鼠脊髓腰膨大的LCN2表达明显下降(P<0.05)(Fig 3)。与Ⅰ组相比,Ⅱ、Ⅲ组p-p38 MAPK表达量均升高(P<0.05)。与Ⅱ组相比,Ⅲ组p-p38 MAPK表达无明显变化,而Ⅳ组p-p38 MAPK表达明显下降(P<0.05),见Fig 4。
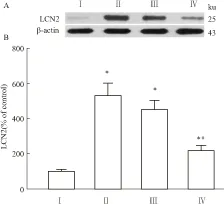
Fig 3 Expression of LCN2 in L5 spinal cord of rats on d 9(n=3)
On d 9, compared with group Ⅰ, the expression of LCN2 in the L5 spinal cord of rats was significantly increased in group Ⅱ-Ⅳ (P<0.05), while compared with group Ⅱ, the expression of LCN2 was significantly decreased in group Ⅳ (P<0.05).*P<0.05vsgroup Ⅰ;#P<0.05vsgroup Ⅱ.
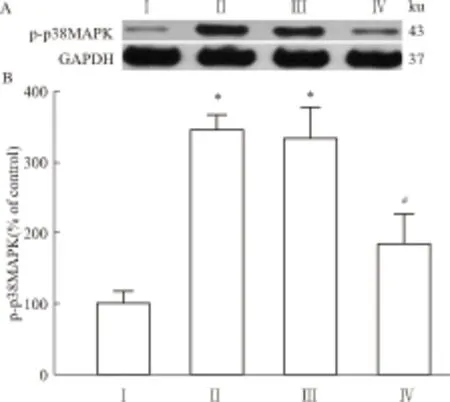
Fig 4 Expression of p-p38 MAPK in L5 spinal cord of rats on d 9(n=3)
On d 9, compared with group Ⅰ, the expression of p-p38 MAPK in the L5 spinal cord of rats was significantly increased in group Ⅱ and Ⅲ (P<0.05), while compared with group Ⅱ, the expression of p-p38 MAPK was significantly decreased in group Ⅳ (P<0.05).*P<0.05vsgroup Ⅰ;#P<0.05vsgroup Ⅱ.
2.3 腰段脊髓背角小胶质细胞标记物Iba1的表达变化免疫荧光染色结果显示,Ⅰ组大鼠腰段脊髓背角Iba1表达量较少。与Ⅰ组相比,Ⅱ组、Ⅲ组Iba1表达量明显增加,小胶质细胞胞体变大,分支增加。与Ⅱ组相比,Ⅲ组Iba1表达无明显变化,Ⅳ组Iba1表达量明显降低,小胶质细胞胞体较小,分支也减少(Fig 5)。
3 讨论
近年来研究发现,LCN2在胶质细胞活化及神经病理性痛中发挥重要作用。静止的小胶质细胞受炎症刺激后释放LCN2,而LCN2能进一步激活小胶质细胞,使小胶质细胞变为分枝状小胶质细胞,且导致小胶质细胞凋亡敏感性增加[8]。而LCN2主要通过激活小鼠或大鼠脊髓水平小胶质细胞及其p-p38 MAPK信号通路诱导痛觉过敏的产生,从而参与神经病理性疼痛及慢性炎症性痛[3,9]。已有研究显示,吗啡耐受能够促进大鼠腰段脊髓水平小胶质细胞活化及p-p38 MAPK的表达,且p-p38MAPK主要表达于活化的小胶质细胞中,这表明小胶质细胞及其内的p-p38 MAPK信号通路参与了吗啡耐受的形成过程,且多项研究表明,趋化因子也参与了吗啡耐受形成[10-11]。因此,我们推测LCN2可能通过激活脊髓小胶质细胞及其内p-p38 MAPK信号通路,进而诱导趋化因子及炎症因子的释放,从而参与了吗啡耐受的形成。
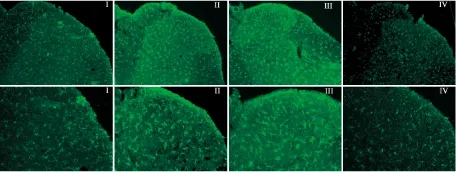
Fig 5 Expression of Iba1 in dorsal horn of L5 spinal cord of rats on d 9
On d 9, compared with group Ⅰ, the immunoreactivity of Iba1 in the dorsal horn of L5 spinal cord of rats was markedly increased in group Ⅱ and Ⅲ, while compared with group Ⅱ, the immunoreactivity was markedly decreased in group Ⅳ.
最近,小干扰RNA(siRNA)基因沉默技术在分子生物学领域迅速发展。siRNA是一段长度约21~22个碱基的双链RNA,可以特异性地诱导转录后基因沉默,即RNAi[12]。近年来,LCN2的研究主要采用基因敲除动物[3]、短发夹RNA(short hairpin)[13]或siRNA[14]等技术达到抑制LCN2表达的作用,本实验采用鞘内注射特异性LCN2 siRNA的方法进行研究,参照文献[15-17],在每天给予吗啡前鞘内注射LCN2 siRNA 4 μg,连续7 d,并检测脊髓内LCN2 mRNA及蛋白表达水平,结果显示此种给药方法及给药剂量能产生较好的基因沉默效果而无明显不良反应。本实验参照Raghavendra等[4]方法建立慢性吗啡耐受模型,d 9吗啡耐受组MPE值较对照组明显下降,表示吗啡镇痛效能下降,吗啡耐受已经形成。与吗啡耐受组比较,LCN2 siRNA处理组大鼠MPE值明显升高,即吗啡的镇痛效能得到改善,提示LCN2 siRNA能够缓解吗啡耐受的形成。进一步检测显示,与对照组相比,吗啡耐受组大鼠脊髓内Iba1及p-p38 MAPK的表达明显增加,而LCN2 siRNA处理后两者表达均明显降低。
本研究表明,吗啡耐受能够促进脊髓水平LCN2表达,进而促进小胶质细胞活化及其内p-p38 MAPK的表达。而通过向大鼠鞘内注射LCN2 siRNA后,脊髓水平的LCN2表达减少,进而小胶质细胞活化及其p-p38 MAPK表达水平亦下降,而相应地,大鼠的吗啡耐受部分缓解。因此,我们推测,脊髓水平LCN2可能通过激活小胶质细胞及其p-p38 MAPK信号通路,进一步诱导趋化因子及炎症因子的释放,从而参与吗啡耐受的形成,而鞘内注射LCN2 siRNA特异性抑制LCN2表达后,可能通过抑制小胶质细胞活化及其p-p38 MAPK信号通路,进而提高吗啡镇痛效能,达到缓解吗啡耐受形成的作用。
总之,鞘内注射LCN2 siRNA能特异性沉默脊髓LCN2的表达,进而部分抑制吗啡耐受的形成。该效应可能与其抑制吗啡诱导的腰段脊髓内小胶质细胞活化及其p-p38 MAPK信号通路有关。本研究提示,阻断脊髓LCN2,有望为吗啡耐受的机制研究提供新思路,并为吗啡耐受的临床防治提供新的途径。
[1] Song P, Zhao Z Q. The involvement of glial cells in the development of morphine tolerance[J].NeurosciRes, 2001, 39(3): 281-6.
[2] 舒银银,谢军明,李永乐,等.鞘内注射IL-18中和抗体对大鼠吗啡耐受形成的影响[J].中国药理学通报,2013,29(5):693-7.
[2] Shu Y Y, Xie J M, Li Y L, et al. Effect of intrathecal injection of anti-rat IL-18 antibody on the development of morphine tolerance in rats[J].ChinPharmacolBull, 2013, 29(5): 693-7.
[3] Jeon S, Jha M K, Ock J, et al. Role of lipocalin-2-chemokine axis in the development of neuropathic pain following peripheral nerve injury[J].JBiolChem, 2013, 288(33): 24116-27.
[4] Raghavendra V, Rutkowski M D, DeLeo J A. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats[J].JNeurosci, 2002, 22(22): 9980-9.
[5] Wen Y R, Tan P H, Cheng J K, et al. Microglia:a promising target for treating neuropathic and postoperative pain,and morphine tolerance[J].JFormosMedAssoc, 2011, 110(8): 487-94.
[6] Milligan E D, Hinde J L, Mehmert K K, et al. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats[J].JNeurosciMethods, 1999, 90(1): 81-6.
[7] Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia[J].Pain, 1988, 32(1): 77-88.
[8] Lee S, Lee J, Kim S, et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia[J].JImmunol, 2007, 179(5): 3231-41.
[9] Jha M K, Jeon S, Jin M, et al. The pivotal role played by lipocalin-2 in chronic inflammatory pain[J].Expneurol, 2014, 254: 41-53.
[10] Hutchinson M R, Coats B D, Lewis S S, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia[J].BrainBehavImmun, 2008, 22(8): 1178-89.
[11] Johnston I N, Milligan E D, Wieseler-Frank J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine[J].JNeurosci, 2004, 24(33): 7353-65.
[12] Dorn G, Patel S, Wotherspoon G, et al. siRNA relieves chronic neuropathic pain[J].NucleicAcidsRes, 2004, 32(5): e49.
[13] Zhang J, Wu Y J, Zhang Y, et al. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages[J].MolEndocrinol, 2008, 22(6): 1416-26.
[14] Yang J, Bielenberg D R, Rodig S J, et al. Lipocalin 2 promotes breast cancer progression[J].ProcNatlAcadSciUSA, 2009, 106(10): 3913-8.
[15] Tumati S, Roeske W R, Largent-Milnes T, et al. Sustained morphine-mediated pain sensitization and antinociceptive tolerance are blocked by intrathecal treatment with Raf-1-selective siRNA[J].BrJPharmacol, 2010, 161(1): 51-64.
[16] Yang C H, Huang H W, Chen K H, et al. Antinociceptive potentiation and attenuation of tolerance by intrathecal β-arrestin 2 small interfering RNA in rats[J].BrJAnaesth, 2011: 107(5): 774-81.
[17] Tumati S, Roeske W R, Largent-Milnes T M, et al. Intrathecal PKA-selective siRNA treatment blocks sustained morphine-mediated pain sensitization and antinociceptive tolerance in rats[J].JNeurosciMethods, 2011, 199(1): 62-8.
Effect of spinal cord lipocalin-2 on development of morphine tolerance in normal rats
WANG Fen1,2, LIU Qing-zhen3, LIU Jian3, JIANG Zi-lu1,2, XIE Tao4, LI Wei-yan3
(1.KeyLaboratoryofAnesthesiologyofJiangsuProvince; 2.KeyLaboratoryofAnesthesiaandAnalgesiaApplicationTechnologyofJiangsuProvince,XuzhouMedicalCollege,XuzhouJiangsu221004,China;3.DeptofAnesthesiology,NanjingGeneralHospitalofNanjingMilitaryCommand,Nanjing210002,China;4.GraduateSchoolofSecondMilitaryMedicalUniversity,Shanghai200433,China)
Aim To explore the effect of knockdown spinal cord LCN2 by RNAi on the development of morphine tolerance in normal rats. Methods After successful intrathecal implantation, fourty-eight male Sprague-Dawley rats weighing 180 - 220 grams were randomly divided into 4 groups (n=12): group I: control group, group II: morphine tolerance group, group Ⅲ: mismatch siRNA group, group IV: LCN2 siRNA group. The sixth day after intrathecal implantation, rats were tested to ensure the position of catheters, and it was recorded as d 0. On d 2 - 8, rats were subcutaneously (s.c) injected of normal saline (NS) (group I) or morphine (group Ⅱ,Ⅲ,Ⅳ) 10 μg·g-1twice a day at 8:00 and 16:00. Before everyday s.c injection, rats were intrathecally injected of 10 μL DEPC solution (group Ⅰ,Ⅱ), 10 μL DEPC solution containing 4 μg mismatch siRNA (group III) and 4 μg LCN2 siRNA solution (group IV). Paw withdrawal latencies to thermal stimuli (PWTL) were tested before morphine injection and 45 minutes after morphine injection on d 1 and d 9. The percentage of maximal possible effect (%MPE) was calculated later. Animals were sacrificed on d 9 after the behavioral test and the lumbar enlargement segments of the spinal cord were removed for detecting the expression of phosphorylated-p38 mitogen-activated protein kinase (p-p38 MAPK) and LCN2 by Western blot and microglia marker Iba1 by immunofluorecence. Results On d 1, there was no significant difference in %MPE among four groups. On d 9, compared to group Ⅰ, %MPE was significantly reduced (P<0.05) while p-p38MAPK, LCN2 and Iba1 were markedly up-regulated in group Ⅱ and Ⅲ (P<0.05). On d 9, compared to group Ⅱ, %MPE was significantly increased while p-p38MAPK, LCN2 and Iba1 were markedly reduced in group IV (P<0.05). Conclusion Using LCN2 siRNA to knockdown spinal LCN2 relieves the development of morphine tolerance in normal rats possibly through inhibiting the activation of microglia and p38 MAPK in the spinal cord.
morphine tolerance; LCN2; siRNA; microglia; p-p38 MAPK; spinal cord
时间:2015-3-16 15:41 网络出版地址:http://www.cnki.net/kcms/detail/34.1086.R.20150316.1541.011.html
2014-12-25,
2015-02-26
江苏省自然科学基金面上研究项目(No BK20131328)
王 芬(1989-)女,硕士,研究方向:麻醉与镇痛的基础与临床,E-mail:DocWangFen@126.com; 李伟彦(1965-),男,博士,主任医师,硕士生导师,研究方向:麻醉与镇痛的基础与临床,Tel:025-80860147,E-mail:weiyanlee@sina.com
10.3969/j.issn.1001-1978.2015.04.025
A
1001-1978(2015)04-0565-05
R-332;R322.81;R341;R342.2;R345.57; R971.2

