引线框架上的高光亮度银电镀
丁辉龙, 何莼, 叶家明, 陈喆垚*
With the increasing demand for greener and more energy efficient products, there is a rapid growth of the light-emitting diode (LED) market, especially the LED lighting market, due to LED’s superior features such as lower power consumption, ultra-long source life, low maintenance, digitally controllable, able to withstand strong vibrations,no ultra-violet (UV) or infrared (IR) radiation and no mercury content[1-2].According to the Climate Group’s report[3],lighting is responsible for 19% of global electricity use and around 6% of global greenhouse gas emissions.Saving 40%of lighting energy globally would have a climate impact equivalent to eliminating half the emissions of all electricity and heat production in the European Union (EU).In the United States alone, cutting the energy used by lighting by 40%would save US$53 billion in annual energy costs, and reduce energy demand equivalent to 198 mid-size power stations.U.S.Department of Energy (DoE) also estimates that in the USA annual energy savings from solid-state lighting (SSL)will be approximately 190 TW⋅h when switched to LED, which can replace 24 (1.000 MW) power plants and reduce greenhouse gas emissions by 31.4 million metric tons of carbon[2].Nowadays, nations across the world including Canada, China, India, Italy, Japan, Korea, Malaysia, the Netherlands, Spain and the United States are racing to develop leading-edge LED industries.It is said that LEDs are bringing a lighting revolution to our cities not seen since the days of Thomas Edison.
A leadframe is a core component of an LED, which supports the semiconductor chip and serves as connecting medium between chips and printed circuit boards (PCBs).Usually a layer of silver is electroplated on the leadframe as a reflective surface to improve the light extraction efficiency of an LED owing to its excellent electrical and thermal conductivity and intrinsic high reflectivity across the visible spectrum (390-700 nm)[4].As the life and power of an LED is greatly impacted by the reflectance of the reflective surface, particularly the high-output LED packages used for general lighting such as residential lighting, commercial lighting, street lighting, automobile headlights and the like, the reflectance of the reflective surface is a critical factor affecting product performance and the reflectance in particular should be as high as possible.
Recently, Dow Electronic Material developed a new high brightness silver electrolytic plating product for leadframes to meet the stringent requirement of the rapid growth and expected continuing rapid growth of the LED lighting market.The new silver plating electrolyte has low cyanide content, which significantly minimizes the potentially hazardous risk from conventional cyanide-based plating products that contain a large amount of free cyanide and lowers the cost for safety controls.Although a few cyanide-free silver plating products were developed in recent years, cyanide-based silver electrolytes are still predominantly used due to a number of drawbacks of the existing cyanide-free silver electrolytes such as unsatisfactory deposit appearance, electrolyte instability, difficulties in bath maintenance, limited plating rate and relatively high cost[5].In addition, this newly developed high brightness silver plating product has a wide operation window, applicable in high speed selective electroplating between 40 and 100 A/dm2(ASD),giving silver coatings with stable high brightness and excellent performance.The resulting silver deposits have high brightness (≥ 2.0 GAM) and high reflectance at 450 nm (≥ 94%) and exhibit good adhesion to the leadframe substrate with excellent wire bondability and solderability.In this paper, the characteristics of the new high brightness silver electrolyte and the performance of electroplated silver deposit are discussed in detail.
1 Characteristics of the high brightness silver electrolyte
Silver can be metallized on a substrate surface either by electrolytic or electroless plating processes.The electrolytic process is driven by an external electrical current with an applied voltage and silver would be deposited on electrically connected areas.The electroless silver is plating through the redox reaction without an external electrical current.It consists of technologies of immersion silver, for which the less noble ion (such as Cu and Ni) is oxidized while silver ions are reduced and deposited on the pads, and autocatalytic silver, for which oxidation and reduction of chemical species occur in the bath.For the high brightness silver plating in LED applications, electrolytic silver plating technology is preferred due to the following key advantages:
(1) Electrolytic silver plating has no thickness limitation.In general, thicker silver deposits can provide higher reflection density (brightness), better wire bonding performance and die attach performance.As shown in Figure 1Figure , silver deposits with thickness of 1.7 µm or above maintain high brightness.

Figure 1 Brightness of silver coatings with different thicknesses图1 不同厚度的银镀层的亮度
(2) The plating speed can be directly controlled by varying the applied current or current density.For the electrolytic silver, the required silver thickness can be achieved within only a few seconds and can therefore greatly enhance the production capability.
(3) Deposit performance generally depends on plating parameters, such as current density, bath temperature,metal ion concentration, plating time, solution circulation, and agitation.By adjusting these parameters, the required brightness, thickness, uniformity and roughness can be achieved.
(4) Bath life of electrolytic plating bath with good maintenance is normally longer than that of an electroless bath.For example, in some cases the bath life of an electrolytic silver plating bath can be more than 2 years.While the bath life of autocatalytic silver is only a few metal turnovers (MTO) and it also requires stringent process control.
In this paper, a new high brightness silver electrolytic plating product containing minimal amount of cyanide was developed.It is reported that with cyanide in the electrolyte, the silver plating electrolyte shows good stability and provides fine grain morphology and uniform deposits.For the bright silver product, cyanide may even have a synergistic effect with the brightener.The new silver electrolytic plating product offers a silver deposit with high reflection density(brightness) over 2.0 GAM.It comprises of several components to enable plating at high current densities from 40 to 100 A/dm2.The optimum plating parameters for high speed plating application, such as jet plating and spot plating, are as follows:
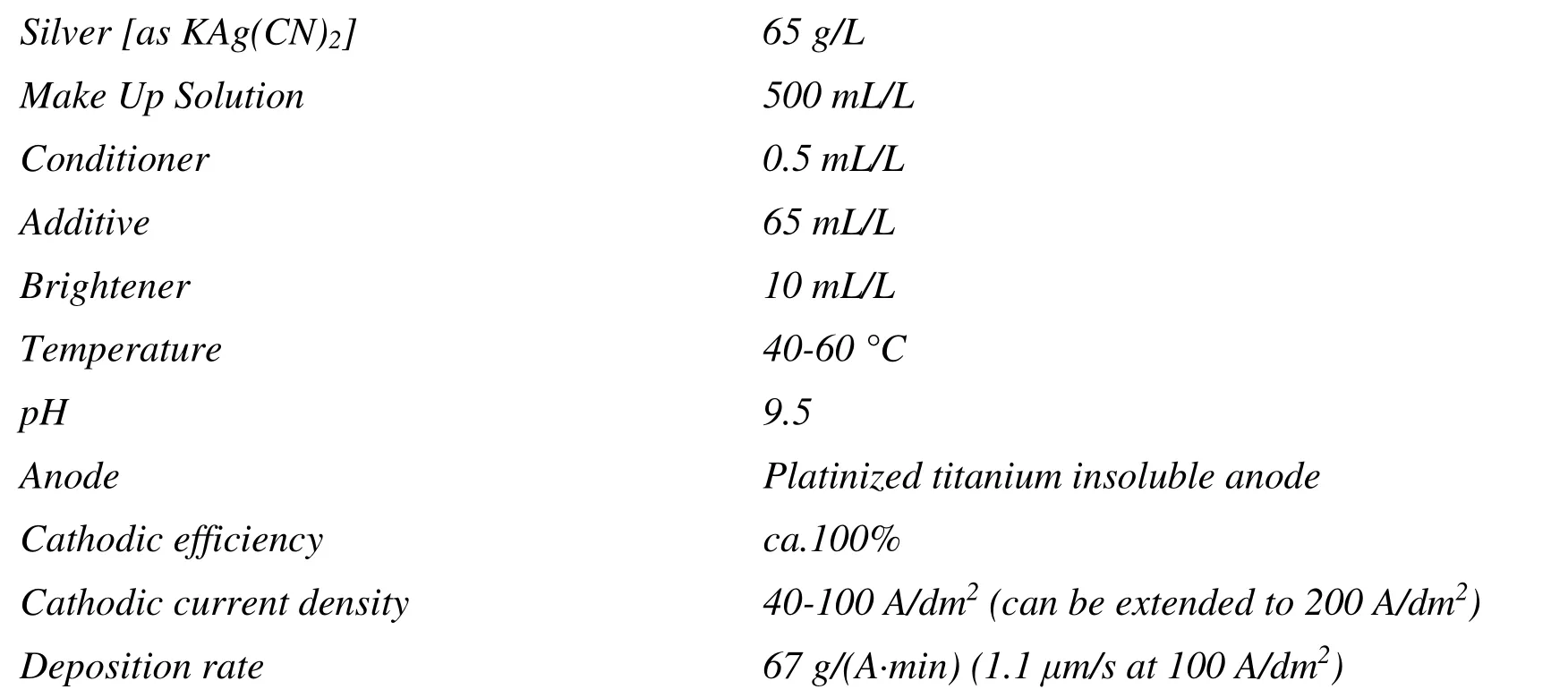
For this new high brightness silver plating product, potassium silver cyanide KAg(CN)2is preferred as the silver metal source.Normally, higher silver content favors high current density applications.A concentration of 65 g/L silver metal is recommended for applications at 40-200 A/dm2.The Make Up Solution component provides the required conductivity and buffering environment and should be in the range of 400-600 mL/L.The Conditioner is the component containing the surface active agent, which can enhance the uniformity of silver deposit and should be in range of 0.4-0.6 mL/L.The Additive contains active materials able to inhibit the immersion of silver onto copper or copper alloy substrates which results in rough or matte silver deposit and should be maintained between 50 mL/L and 70 mL/L.To improve the brightness performance of the new silver plating product, lots of effort was put on selecting a suitable brightener to produce a smooth silver deposit with a high reflection density and reflectance percentage as well as an excellent anti-tarnish performance.The Brightener is recommended to be controlled between 5 mL/L and 15 mL/L.As to the bath pH, a pH range of 8.0-9.5 is recommended for this new product.The bath may not be stable when the bath pH is lower than 8.0, especially for high silver content operation.Free cyanide may build up if the bath pH is higher than 9.5.
2 Characteristics of the high brightness silver deposits
2.1 Reflection density (GAM)
Gold plating and silver plating industries generally evaluate the gloss or brightness of metal coating using reflection density or optical density in units of GAM.A reflection densitometer is an instrument used for reflection density measurement which fundamentally measures the amount of light reflected from a surface.Currently in the silver plating industry, low speed cyanide-containing silver is mainly used for rack or barrel plating applications and can provide silver deposits with brightness over 2.0 GAM.However, for high speed processes such as spot or jet plating at current density between 40 A/dm2and 120 A/dm2, the brightness of the silver surface is normally 1.2-1.5 GAM.Some silver plating products can now achieve brightness up to 1.8 GAM.Brightness with 2.0 GAM may be achievable in some specific conditions but it is very difficult to be controlled in mass production.
The newly developed high speed silver electrolyte provides a silver deposit with a uniform mirror bright surface suitable for LED application.Figure 2 shows that the silver deposit plated by the new high brightness silver electrolyte has brightness over 2.0 GAM, which is much higher than that of the silver deposits plated by conventional high speed bright silver products and competitive silver products.Current density acts as a significant factor governing the brightness performance of the silver deposit.Under the optimized conditions, as shown in Figure 3, brightness of silver deposits can be maintained at 2.0 GAM or above within a wide current density range (40-100 A/dm2), indicating that this new silver plating product is suitable for the high speed plating application.
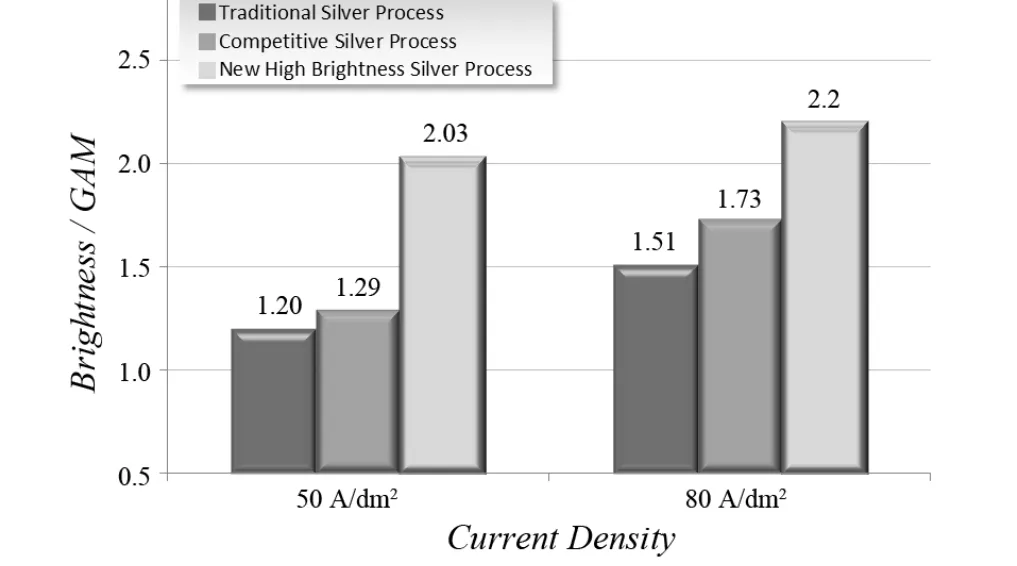
Figure 2 Silver coating brightness of several silver plating products图2 一些镀银产品的银层光亮度
2.2 Reflectance
Silver is one of very few metals that have high reflectivity at 400-700 nm, the wavelength of interest for LED applications.For high power-output LED packages for main illumination used in household, automobile headlight, or other applications, reflectance is required as high as possible in order to enhance the performance of the LED products.The reflectance percentage of silver in the wavelength range of 400-450 nm which is near an LED excitation wavelength is critical because the reflectance in this range is normally lower than 92%.Therefore, in order to achieve a high brightness silver deposit, it is necessary to ensure that the reflectance percentage in this wavelength range is as high as possible.Figure 4 shows the reflectance at 450 nm of the silver deposit plated by the new high brightness silver product.These silver deposits have a reflectance of 94%-96%, indicating that the silver deposits are applicable to LED devices with high performance requirements.

Figure 3 Brightness performance of the new high brightness silver electrolyte at 30-100 A/dm2图3 新型高光亮度镀银液在30 ~ 100 A/dm2 范围内所得镀层的光亮度
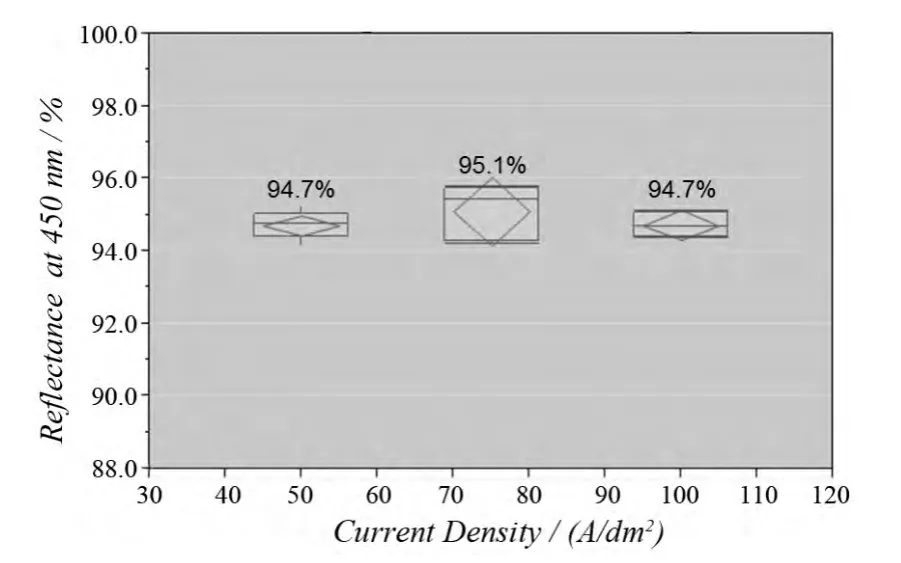
Figure 4 Reflectance performance at 450 nm of the new high brightness silver electrolyte at 50-100 A/dm2图4 50 ~ 100 A/dm2 范围内新型高光亮度镀银液所得镀层在450 nm 波长下的反射率
2.3 Color
For the expression of color of the material surface, several color systems are used to convert the subjective color display into a scientific value for objective comparison among samples, such as the Munsell Color System, CIE XYZ,and CIE LAB.For the silver deposit on the leadframe pad for LED applications, a light white color is required in order to ensure the light energy within the visible wavelength (400-700 nm) is reflected from the metal surface with a high reflectance (or with minimal or even without energy absorption).In this study, the L*a*b* color system was utilized.It is a rectangular coordinate system, which consists of a* and b* axes with the vertical L* axis indicating the brightness.Figure 5 shows the L*a*b* diagram and ideal L*a*b* values (gray spots which are in light red on the original colored diagram) of the high reflective silver deposit.As shown in Figure 5, for the high reflective silver deposit, the value of L*should be close to 100 (representing light color) and values of a* and b* should be close to 0 (representing white color).Figure 6 shows the L*a*b* values of the silver deposit plated by the new high brightness silver process.It indicates that the silver deposits have the required light and white color as the L*a*b* values of the silver deposits is over 98, 0.0-0.1 and 0.5-1.0, respectively.

Figure 5 Ideal color value of high reflective silver coating on L*a*b* diagram图5 L*a*b*图上高反射率银镀层的理想颜色值
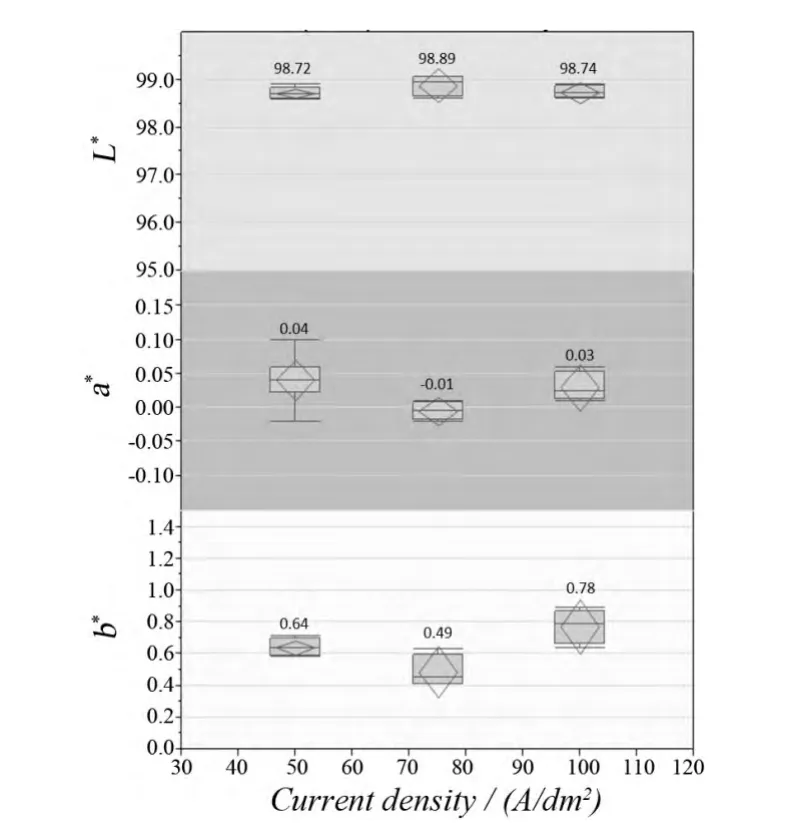
Figure 6 Color performance of the new high brightness silver electrolyte at 50-100 A/dm2图6 新型高光亮度镀银液在50 ~ 100 A/dm2 范围内所得镀层的色泽
2.4 Anti-tarnish performance
During the manufacturing of LED packages or devices, the leadframe components with the high brightness silver coating are generally subject to several processes, such as soldering and wire bonding, which are normally conducted at high temperature.These processes may impose a risk of tarnish on the silver surface.The tarnished silver surface would deteriorate its brightness performance, which consequently affects the LED product’s performance.Therefore, the anti-tarnish performance of the silver coating is also very important.In this paper, heat treatment was applied to the silver deposit samples plated at 50 A/dm2with a thickness of 2.5-3.0 μm.Four baking conditions commonly used in the LED industry were selected: 1) 180 °C, 3 hours; 2) 150 °C, 30 min; 3) 260 °C, 2 min; and 4) 300 °C, 1 min.Table 1 shows the brightness of samples after baking.Almost no or minor reduction in reflection density (GAM) and reflectance percentage at 450 nm was observed.In addition, the baking test did not cause any significant change in the color of the silver coating, especially the value of b*.The baked sample can still have a b* value less than 1.0.
3 Functional tests
3.1 Solderability
Solderability evaluation is usually made to verify the solderability of leads and terminations which are normallyjoined by a soldering operation.An accelerated precondition is generally used before solderability testing to simulate the condition of the components after long-term storage.In this paper, ‘dip and look’ solderability testing method was adopted to evaluate the solderability of the new high brightness silver deposit.Both as plated and baked samples(preconditioning: dry baked at 180 °C for 3 hours) with a rosin-based Type R flux were immersed in a Sn/Ag/Cu lead-free solder (245 °C) for 3 seconds and then inspected using an optical microscope.As shown in Table 2, the silver deposit can be 100% covered by the solder, indicating excellent solderability.Solderability testing of the baked samples confirmed that the aging condition has no significant impact on the solderability.

Table 1 Anti-tarnish performance of the new high brightness silver electrolyte表1 新型高光亮度镀银液所得镀层的抗变色性能

Table 2 Solderability test results of silver deposit plated at three current densities表2 3 个电流密度下所得银镀层的可焊性测试结果
3.2 Adhesion
The durability of coatings is of prime importance in many applications and one of the main factors that govern this durability is adhesion.For a coating to stay on the substrate and to perform its function, its adhesion to the substrate must tolerate mechanical stresses and elastoplastic distortions, thermal stress, and environment or process fluid displacement[6].Like other electrodeposited metal coatings, adhesion of the silver deposit to the leadframe substrate is critical to its function.A commonly used cross-hatch tape test was used to evaluate the adhesion of the new high brightness silver deposit, including as plated and aged samples which were baked at 500 °C for 1 min.A lattice pattern with eleven cuts with about 1 mm space in a perpendicular direction was made in the film to the substrate, then a pressure-sensitive tape (3M 610 Scotch) was applied over the lattice and removed.The deposits were then inspected using an optical microscope.The test results showed that the adhesive force between the silver coating and the substrate is strong enough to prevent peel off or blisters, even for the baked samples as shown in Table 3.

Table 3 Adhesion test results of ag deposit plated at three current densities表3 3 个电流密度下所得银镀层的附着力测试结果
3.3 Wire bonding capability
Wire bonding is the most common, cost-effective and flexible interconnect technology for making interconnections between an integrated circuit (IC) or other semiconductor device like an LED chip and its packaging during semiconductor device fabrication.The reliability of the devices is very dependent on the quality of the wire bond interconnection.Gold wire bondability analysis of the new bright silver deposit (as plated and baked at 170 °C for 3 hours)was conducted using a bonding machine and a pull tester.The wire bonding and pull test conditions are summarized in Table 4.The wire bonding test results are shown in Figure 7 and Figure 8.Figure 9 illustrates the position of five failure modes of wire pull test.

Table 4 Wire bonding & pull test conditions表4 引线键合及拉拔试验条件
As shown in Figure 7 and Figure 8, the pull strength of all samples (as-plated and baked) is higher than 60 mN(equivalent to 6.12 g) and no failure mode A and D is observed, indicating excellent wire bondability.
4 Summary
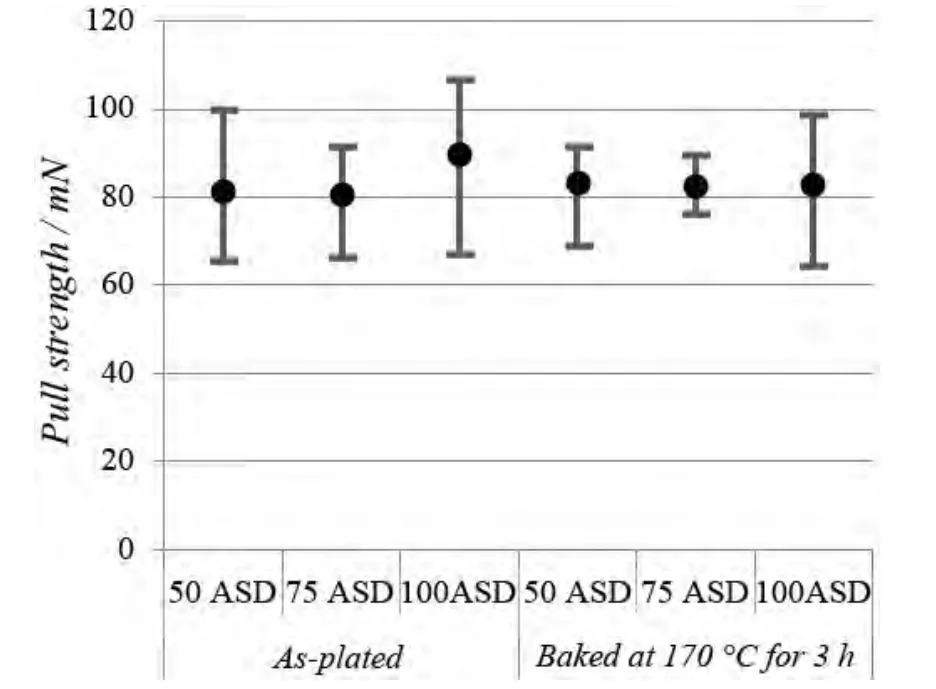
Figure 7 Pull strength of silver deposits (as-plated & baked)plated at three current densities图7 3 个电流密度下所得银镀层在镀态及烘烤后的抗拉强度
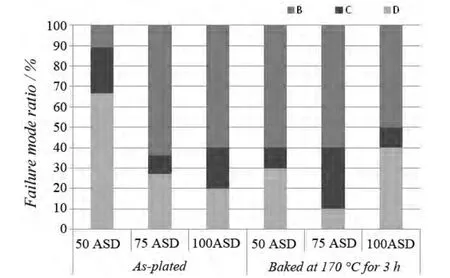
Figure 8 Failure mode ratio of silver deposits (as-plated &baked) plated at three current densities图8 3 个电流密度下所得银镀层在镀态及烘烤后键合失效形式的统计比例
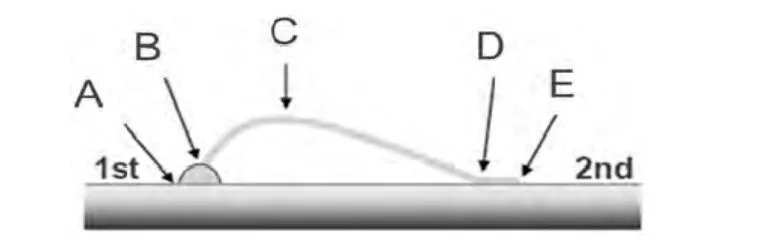
Figure 9 Illustration of failure modes (A––Lift at first bond foot; B––Broken heel of interconnect wire at first bond foot;C––Center span failure; D––Broken heel at second bond foot; E––Lift at the second bond foot)图9 键合失效形式图解
In summary, the newly developed high brightness silver electroplating product for leadframes can meet the stringent requirements for the LED lighting market.The resulting silver deposit has stable high brightness (≥ 2.0 GAM)and high reflectance at 450 nm (≥ 94%) and exhibits excellent performance in wire bonding and solderability.It has low cyanide content and is designed to be operated in high-speed application with jet-stream selective plating equipment to produce high purity, high brightness and high reflectance silver deposits, suitable for LED lighting applications.
5 Acknowledgements
The authors would like to thank Tony Ng in Dow Electronic Materials Hong Kong and Takashi Ohtsuka in Dow Electronic Materials Japan for their assistance in the brightness measurement and wire bondability test.
[1] U.S.Department of Energy.Solid-state lighting: brilliant solutions for America's energy future [R/OL].(2014–09–05) [2015–05–13].http://apps1.eere.energy.gov/buildings/publications/pdfs/ssl/ssl-overview_brochure_feb2013.pdf.
[2] DENNEMAN J.Global LED market overview and forecast [C/OL] // Taiwan International Lighting Show–—International Forum, Taiwan, 13th March 2012.(2012–03–16) [2015–05–13].http://www.globallightingassociation.org/documents/gla_papers/Global_LED_Market_Overview.pdf.
[3] The Climate Group.Lighting the clean revolution: the rise of LEDs and what it means for cities [R/OL].(2014–09–08) [2015–05–13].http://www.theclimategroup.org/what-we-do/publications/lighting-the-clean-revolution-the-rise-of-leds-and-what-it-means-for-cities/.
[4] JDS Uniphase Corporation.Mirrors-Coating Choice Makes a Difference [M/OL] // Photonics Handbook.[S.l.: s.n.], [2009].(2014–09–05) [2015–05–13].http://www.photonics.com/EDU/Handbook.aspx?AID=25501.
[5] CLAUSS M, FOYET A, ZHANG W.Development of an improved cyanide-free silver electrolyte [R/OL].(2012–06–20) [2015–05–13].http://www.metalfinishing.com/view/26438/development-of-an-improved-cyanide-free-silver-electrolyte/.
[6] DINI J W.Electrodeposition—The Materials Science of Coatings and Substrates [M].Westwood, NJ: Noyes Publications, 1993.

