反应物振动量子数对O(1D)+HBr→OH+Br立体动力学影响的研究
许慎德
(山东省莒县第一中学, 莒县276500)
反应物振动量子数对O(1D)+HBr→OH+Br立体动力学影响的研究
许慎德
(山东省莒县第一中学, 莒县276500)

立体动力学; 准经典轨线; 产物转动取向
1 引 言
本文所研究的O(1D)+HBr体系是星际中的一类重要的化学反应.随着立体化学动力学的发展, 此类反应引起了高度的关注[1-3]. 而且它作为一个典型的原子与分子的基元反应, 可用来检测新的理论与实验的标准. 该反应可以在单个势能面得以完成[4], 并且存在两种重要的竞争途径.
→Br+OHΔH0=-60.0±1.0kcal/mol
O(1D)+HBr→
→H+BrOΔH0=-13.9±1.4kcal/mol
在近三十年里, 从实验以及理论方面对该反应进行了大量的研究. 包括HOBr的振动转动光谱[5-6]以及分子偶极矩[7].Wineetal.采用时间分辨的共振荧光探测了反应的产物通道[8]. 利用交叉分子束实验技术, Balucanietal. 获得了产物的分支比以及相应的反应截面[4].由于该反应时间短, 产物BrO可以采用时间分辨的UV吸收光谱进行测量[9].
理论方面, 也对该反应进行了一系列的研究. 最早的理论研究可以追溯到McGrath和Rowland等人计算得到了HOBr和异构体HBrO的平衡结构以及谐振频率[10]. Petersonetal. 等人用从头算的方法获得了比较精确的势能面[11].然而, 以往的工作主要研究对象为反应的标量属性, 例如Tangetal. 采用含时波包理论计算了该反应的反应几率和速率常数, 结果表明反应几率与速率常数对温度影响不敏感[12]. 为了更充分地揭示出标题反应的动力学性质, 研究的重点不应该局限于反应的标量属性, 对反应矢量性质同样应该给予了足够地重视.
准经典轨线(QCT)是一种研究反应体系矢量性质的有效方法, 多年来得到人们的广泛应用[13-14]. 已经对许多重-轻-重反应体系, 如 O+HCl[15], Sr+HF[16], 以及 Ca+HCl[17]进行了研究. 迄今为止, 对于反应物的振动量子数对于具有深势阱影响的研究还没有报道. 采用量子的方法由于计算太大而非常困难, 准经典轨线方法可以解决上述的问题. 本文中我们采用准经典轨线方法研究了反应物v=0-7时该反应的立体动力学性质.
2 理论方法
本文计算是基于由Peterson等构造的精确基态势能面[18]. QCT详细描述参见文献[19-23]. 我们选用质心坐标系如图1, Z轴平行于反应物相对速度k, Y轴垂直于含有反应物相对速度k和产物相对速度k′的xz平面.

图 1 质心坐标系下描述 k, k′ 和 j′ 相关图Fig.1 The center-of-mass coordinate system used to describe the k, k′ and j′ correlations
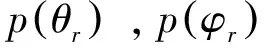
(1)

(2)
其中
an=2
bn=2
产物转动角动量j’ 的空间分布可用如下公式展开
(3)
参数
描述-k′、k′、j′三矢量的全三维角分布函数写为:
(4)

3 结果与讨论
我们给出了反应振动量子数分别为0-7时的反应物相对速度矢量k和产物的转动角动量矢量j’ 两矢量相关的函P(θr)分布,P(θr)的分布直接反映了k,j′的两矢量相关. 从图2可以很清晰地看出,P(θr)分布的峰值均位于θr=90°, 并且同时每一个反应都关于θr=90°对称. 随着振动量子数的增加, 峰值越来越大, 半高宽变窄表明反应的产物OH的转动角动量的取向性非常强. 具体地说, 产物的转动角动量矢量j′强烈地取向于垂直相对速度k的方向.

图 2 反应物在不同的振动态时k-j′相关的p(θr)分布图Fig.2 The distributions of p(θr) reflecting k-j′ correlations at different vibrational levels v=0-7 for the reagent vibrational excitation


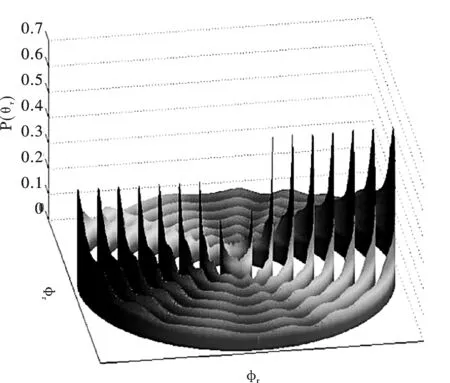
图 3 反应物在不同的振动态时相应于k-k′平面的p(θr)分布图,从里向外振动量子数分别为0到7Fig.3 The dihedral angle distribution of p(φr) with respect to the k-k′ plane, plotted at reagent vibrational quantum numbers from inside to outside are v=0 to v=7, respectively


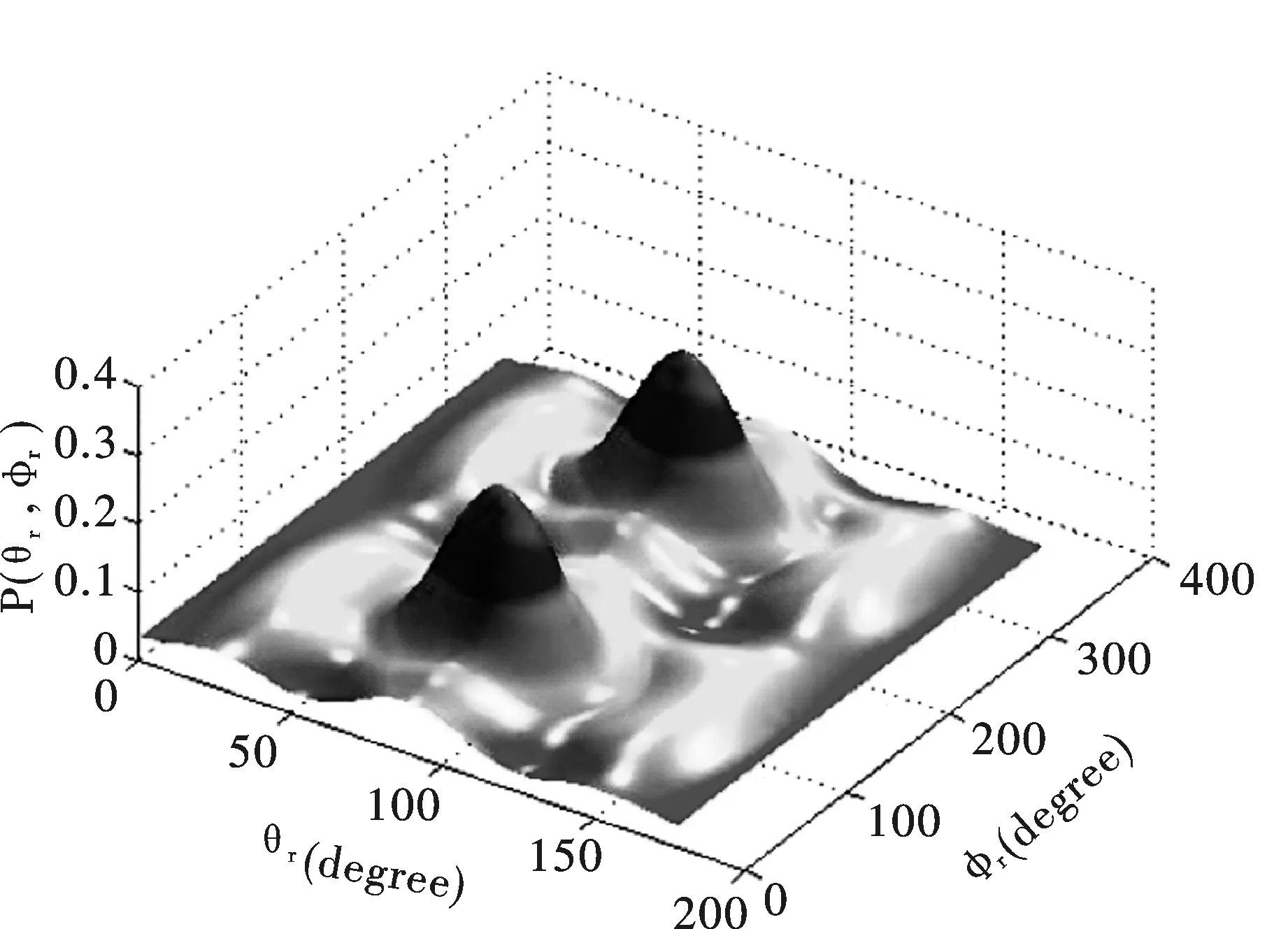

图 4 反应O(1D)+HBr p(θr,φr)分布随反应物振动量子数变化(a) v = 0; (b) v = 3 ; (c) v = 6Fig.4 Polar plots of the p(θr,φr) distributions with peaks and valleys at different vibrational levels. (a) v = 0; (b) v = 3 ; (c) v = 6
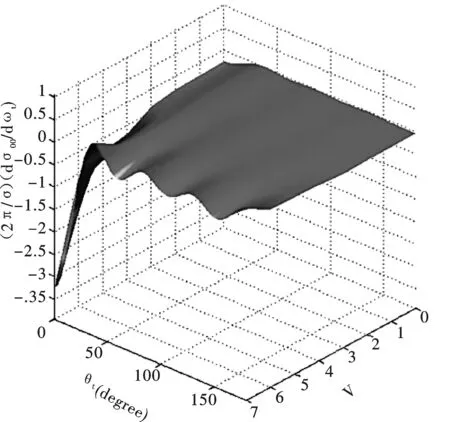
图 5 反应O(1D)+HBr在不同反应物振动量子数的微分散射截面 (a) (k , q)=(0,0); (b) (k , q)= (2, 0)Fig.5 Two polarization-dependent generalized differential cross sectionsof the reagent vibrational quantum numbers with (a) (k , q)=(0,0); (b) (k , q)= (2, 0), respectively
4 结 论



VibrationLevelv=0v=1v=2v=3v=4v=5v=6v=7
[1] Yagi K, Williams J, Wang N Y, Cicerone R J. Atmospheric methyl bromide (CH3Br) from agricultural soil fumigations [J].Science, 1995, 267(5206): 1979.
[2] Tie X X, Brasseur G G. The importance of heterogeneous bromine chemistry in the lower stratosphere [J].Res.Lett., 1996, 23(18): 2505.
[3] Mahmud K, Kim J S, Fontijn A. A high-temperature photochemical kinetics study of the oxygen atom + hydrogen chloride reaction from 350 to 1480 K [J].J.Phys.Chem., 1990, 94(7): 2994.
[4] Balucani N, Beneventi L, Casavecchia P,etal. The dynamics of the reaction of O(1D) with HBr studied by crossed molecular beams and time-resolved Fourier transform spectroscopy [J].Can.J.Chem., 1994, 72(3): 888.
[5] McRae G A, Cohen E A. Theν2band of HOBr [J].J.Mol.Spectrosc., 1990, 139(2): 369.
[6] Koga Y, Takeo H, Kondo S,etal. The rotational spectra, molecular structure, dipole moment, and hyperfine constants of HOBr and DOBr [J].J.Mol.Spectrosc., 1989, 138(2): 467.
[7] Cohen E A, McRae G A, Tan T L,etal. The ν1band of HOBr [J].J.Mol.Spectrosc., 1995, 173(1): 55.
[8] Wine P H, Wells J R, Ravishankara A R. Channel specific rate constants for reactions of O(1D) with HCl and HBr [J].J.Chem.Phys., 1986, 84(3): 1349.
[9] Cronkhite J M, Wine P H. Branching ratios for BrO production from reactions of O(1D) with HBr, CF3Br, CH3Br, CF2ClBr, and CF2HBr [J].Int.J.Chem.Kinet., 1998, 30(8): 555.
[10] McGrath M P, Rowland F S. Ideal gas thermodynamic properties of HOBr [J].J.Phys.Chem., 1994, 98(18): 4773.
[11] Tang B Y, Tang Q K, Chen M D,etal. Quantum scattering calculation of theO(1D)+HBr reaction [J].J.Chem.Phys., 2004, 120(18): 8537.
[12] Wang M L, Han K L, Zhan J P,etal. Reaction dynamics of the Sr(3RJ)+ RI → SrI*+ R (R=CH3, CH3CH2) systems: rotational alignment, electronic state branching ratio and vibrational state population of products [J].Chem.Phys.Lett., 1997, 278(4): 307.
[13] Han K L, He G Z, Lou N Q. Effect of location of energy barrier on the product alignment of reactionA+BC[J].J.Chem.Phys., 1996, 105(19): 8699.
[14] Zhu T, Hu G D, Zhang Q G. Quasi-classical trajectory study of the reaction O(3P) + HCl → OH + Cl and O(3P) + DCl → OD + Cl: Vector and scalar properties [J].J.Mol.Stru.Theo., 2010, 948(3): 36.
[15] Cai M Q, Zhang L, Tang B Y,etal. Erratum to “Quasiclassical calculation of the chemical reaction Sr+HF” [Chemical Physics 255 (2000) 283-289] [J].Chem.Phys., 2000, 260(1-2): 281.
[16] Tang P Y, Li D L, Wu M M,etal. Rotational alignment of product molecules from the reaction Ca + HCl→CaCl + H [J].J.Theor.Comput.Chem., 2011, 10(1): 19.
[17] Chu T S, Zhang Y, Han K L. The time-dependent quantum wave packet approach to the electronically nonadiabatic processes in chemical reactions [J].Int.Rev.Phys.Chem., 2006, 25(1-2): 201.
[18] Peterson K A. An accurate globalabinitiopotential energy surface for theX1A′ electronic state of HOBr [J].J.Chem.Phys., 2000, 113(11): 4598.
[19] Jiang Z J, Wang M S,Yang C L. Stereodynamics study of the reaction H/D/T+LiH [J].J.At.Mol.Phys., 2013, 30(2): 229(in Chinese) [姜志军, 王美山, 杨传路. H/D/T+LiH 反应的立体动力学研究[J]. 原子与分子物理学报, 2013, 30(2): 229]
[20] Miranda M P D, Aoiz F J, Brouard M,etal. Spatial distributions of angular momenta in quantum and quasiclassical stereodynamics [J].J.Chem.Phys., 2004, 121(20): 9830.
[21] Chen M D, Han K L, Lou N Q. Vector correlation in the H+D2reaction and its isotopic variants: isotope effect on stereodynamics [J].Chem.Phys.Lett., 2002, 357(5-6): 483.
[22] Miranda M P D, Clary D C. Quantum dynamical stereochemistry of atom-diatom reactions [J].J.Chem.Phys., 1997, 106(11): 4509.
[23] Chu T S. Quantum mechanics and quasiclassical study of the H/D+FO → OH/OD + F, HF/DF + O reactions: Chemical stereodynamics [J].J.Comp.Chem., 2010, 31(7): 1385.
[24] Chen M D, Han K L, Lou N Q. Theoretical study of stereodynamics for the reactions Cl+H2/HD/D2[J].J.Chem.Phys., 2003, 118(10): 4463.
[25] Zhao J, Xu Y, Yue D G,etal. Quasi-classical trajectory study of the reaction H + FO → OH + F [J].Chem.Phys.Lett., 2009, 471(1-3): 160.
[26] Li W L, Wang M S, Dong Y M,etal. A comparison of the stereodynamics between the reactions H + HH(D, T) and the reactions H-+ HH(D, T) [J].Chem.Phys., 2008, 348(1-3): 97.
[27] Han K L, Zhang L, Xu D L,etal. Experimental and theoretical studies of the reactions of excited calcium atoms with ethyl andn-Propyl bromides [J].J.Phys.Chem. A, 2001, 105(13): 2956.
[28] Zhang W Q, Li Y Z, Xu X S,etal. Isotope effects on the dynamics in the ion-diatomic collisions of D+, H+with H2and D2molecules [J].Chem.Phys., 2010, 367(2-3): 115.
[29] Wang M L, Han K L, He G Z. Product rotational polarization in the photoinitiated bimolecular reaction A+BC→AB+C on attractive, mixed and repulsive surfaces [J].J.Chem.Phys., 1998, 109(13): 5446.
[31] Zhao J, Xu Y, Meng Q T. Influence of collision energy on the axial polarization of product molecule for reaction F + HO → HF + O [J].Can.J.Phys., 2009, 87(12): 1247.
[31] Yao L, Zhong H Y, Liu Y L,etal. Quasi-classical trajectory study of the reaction dynamics of Ca(1S0,3P) atoms with CHCl3[J].Chem.Phys., 2009, 359(1-3): 151.
[32] Liu S L, Shi Y. Theoretical investigation of the effect of rotational excitation on the stereodynamics of the O(3P)+H2→OH+H reaction [J].Chem.Phys.Lett., 2011, 501(4-6): 197.[33] Xu W W, Liu X G, Luan S H,etal. An ab initio potential energy surface of the He+HH+→HeH++H reaction [J].Chem.Phys.Lett., 2008, 464(1-3): 92.
[34] Zhang W Q, Cong S L, Zhang C H,etal. Theoretical study of dynamics for the abstraction reaction H′ + HBr(v=0,j=0) → H′H + Br [J].J.Phys.Chem. A, 2009, 113(16): 4192.
[35] Zhang W, Liu Y F, He X H. Effect of reagent rotation on the integral cross-sections and isotopic branching of the reactions H-+ HD and D-+ HD [J].Chem.Phys.Lett., 2010, 489(4-6): 237.
[36] Zhang W Q, Li Y Z, Xu X S,etal. Isotope effects on the dynamics in the ion-diatomic collisions of D+, H+with H2and D2molecules [J].Chem.Phys., 2010, 367(2-3): 115.
[37] Yu Y J, Xu Q, Xu X W. Influence of rotational excitation and collision energy on the stereo dynamics of the reaction: N(4S) + H2(v= 0, j= 0, 2, 5, 10) → NH(X3.Σ-) + H [J].Chin.Phys. B, 2011, 20(12): 123402.
[38] Liang J J, Yang C L, Wang L Z. Collision energy effect on the H′ + BrH (ν= 0,j= 0) → H′Br + H reaction: A quasi-classical trajectory study [J].Chem.Phys., 2012, 392(1): 180.
[39] Aldegunde J, Aoiz F J, Gonzalez-Sanchez L. Orientation effects in Cl + H2inelastic collisions: characterization of the mechanisms [J].Phys.Chem.Chem.Phys., 2012, 14(8): 2911.
[40] Chen J W, Liu X G, Sun H Z. Effect of collision energy on the reactivity O++T2→OT++T by the quasiclassical trajectory method [J].Chin.Phys.Lett., 2011, 28(9): 093101.
[41] Liu S L, Shi Y. Theoretical study of isotopic effect of oxygen atom on the stereodynamics for the O(3P)+ D2→ OD+D reaction [J].Chin.Phys.Lett., 2010, 27(12): 123103.
[42] Han K L, Zheng X G, Sun B F,etal. Chemical reaction dynamics of barium atom with alkyl bromides [J].Chem.Phys.Lett., 1991, 181(5) : 47.
[43] Wei Q, Zhou B, Li X,etal. Theoretical study of stereodynamics for the O(3P)+H2→OH+H reaction [J].J.At.Mol.Phys., 2010, 27(3): 469(in Chinese) [魏强, 周波, 李兴, 等. O(3P)+H2→OH+H reaction反应的立体动力学研究 [J]. 原子与分子物理学报, 2010, 27(3): 469]
[44] Xue L L, Miao X Y. Effects of collision energy on stereodynamics for the reaction S(D1)+H2→SH+H [J].J.At.Mol.Phys., 2011, 28(5): 889 (in Chinese) [薛丽丽, 苗向阳. 碰撞能对S(D1)+H2→SH+H发应立体动力学性质的影响[J]. 原子与分子物理学报, 2011, 28(5): 889]
Influence of reagen vibration on the stereodynamics of theO(1D)+HBr→OH+Brreaction
XU Shen-De
(The first middle school of Juxian, Juxian 276500, China)

Stereodynamic; Quasi-classical trajectory; Product rotational alignment
103969/j.issn.1000-0364.2015.02.013
2013-12-20
许慎德(1970—), 男, 山东省莒县人, 本科, 主要从事计算数学研究. E-mail: xushende9@163.com
O
A

