一维链状氰基桥联配合物Mn(bpac)2(H2O)2M(CN)4(bpac=bis(4-pyridyl) acetylene;M=Pt,Pd)的合成、结构与热稳定性
田小燕,胡爱云,袁爱华
(江苏科技大学环境与化学工程学院,江苏镇江212003)
一维链状氰基桥联配合物Mn(bpac)2(H2O)2M(CN)4(bpac=bis(4-pyridyl) acetylene;M=Pt,Pd)的合成、结构与热稳定性
田小燕,胡爱云,袁爱华*
(江苏科技大学环境与化学工程学院,江苏镇江212003)
将过渡金属离子Mn2+与柱状配体bis(4-pyridyl)acetylene(bpac)和[M(CN)4]2-(M=Pt,Pd)通过缓慢扩散法进行组装反应,得到了两个一维链状氰基桥联配合物Mn(bpac)2(H2O)2M(CN)4(M=Pt(1),Pd(2)).单晶X射线衍射分析表明:配合物1和2为同构体,均属三斜晶系,空间群P1.其非对称单元由1个[Mn(bpac)2(H2O)2]2+阳离子和1个[M (CN)4]2-阴离子组成,Mn和M中心分别处于六配位的八面体和四配位的平面四方构型之中,两者通过氰基交替连接形成无限延伸的一维直线链.同时,文中研究了配合物1的热稳定性.
氰基桥联;配合物;合成;结构;热稳定性
近年来,设计与合成具有特定拓扑结构和性质的配合物引起了科学家们的广泛关注[1-3].氰基合金属阴离子(如[M(CN)4]2-(M=Ni,Pt,Pd)、[M (CN)6]3-(M=Cr,Mn,Fe)和[M(CN)8]3/4-(M=Mo,W))常被用作构筑单元,与过渡金属(或稀土金属)离子和有机配体通过组装反应,制备结构多样的氰基桥联配合物[4-6],这些配合物在磁性[7]、吸附[8]和负热膨胀[9]等领域具有潜在的应用[10-12].四氰合金属基配合物作为一个重要分支,具有拓扑结构多样、磁性和吸附性能优异等优点,备受人们关注[13-19].为了丰富四氰合金属基配合物的研究内容,本文采用[M(CN)4]2-(M=Pt,Pd)为构筑基元与过渡金属离子Mn2+和柱状刚性有机配体bis(4-pyridyl)acetylene(bpac)进行组装反应,得到了两个新型一维直线链状配合物Mn(bpac)2(H2O)2M (CN)4(M=Pt(1),Pd(2)).文中重点介绍了配合物1和2的合成、结构和热稳定性.
1 实验
1.1 试剂与仪器
合成所用药品及试剂均为市售分析纯,使用时均未进一步提纯,所用水为去离子水.
粉末X射线衍射测试采用日本岛津XRD-6000型衍射仪,管压40 kV,管流30 mA,Cu-Kα辐射,测定范围5°~50°,速率5°/min;红外光谱测定采用Nicolet FT-1703X红外光谱仪(KBr压片),测试范围为400~4 000 cm-1;热重分析采用PE公司的Pyris Diamond热分析仪,氮气保护,室温~800oC,升温速率为15oC/min.
1.2 单晶结构测定
配合物的单晶衍射数据在Bruker Smart APEX II X射线单晶衍射仪上收集,采用石墨单色器单色化的Mo-Kα射线(λ=0.710 73Å)作为入射线,用Bruker XSCANS程序测定.所有衍射数据均用SMART、SAINT和 XPREP程序[20]进行分析和还原,并用SADABS方法[21]来校正洛伦兹因子、极化因子和吸收因子.初结构由SHELXS-97程序的直接法解出,并用SHELXL-97程序精修[22].所有非氢原子的坐标用全矩阵最小二乘法各向异性温度因子修正,氢原子坐标按理想几何位置插入.这些氢原子的坐标和各向同性温度因子参加结构计算,但不参加修正,全矩阵最小二乘法精修基于F2.
1.3 单晶培养
分别称取MnSO4·H2O(0.05 mmol)和配体dpac(0.05 mmol)于2 mL小瓶中,称取 K2[Pt (CN)4]·3H2O(0.05 mmol)于20 mL大瓶中,将小瓶放入大瓶内.大小瓶中加入水直到没过小瓶口1~2 cm,拧紧瓶盖,在室温下静置三周,得到无色棒状晶体1.配合物2的合成方法与1类似,只需将K2[Pt(CN)4]·3H2O替换成K2[Pd(CN)4]·3H2O.
2 结果与讨论
2.1 晶体结构
单晶X射线衍射分析表明,配合物1和2为同构体,均属三斜晶系,空间群P1.配合物1和2的晶体学数据列于表1,部分键长和键角列于表2.
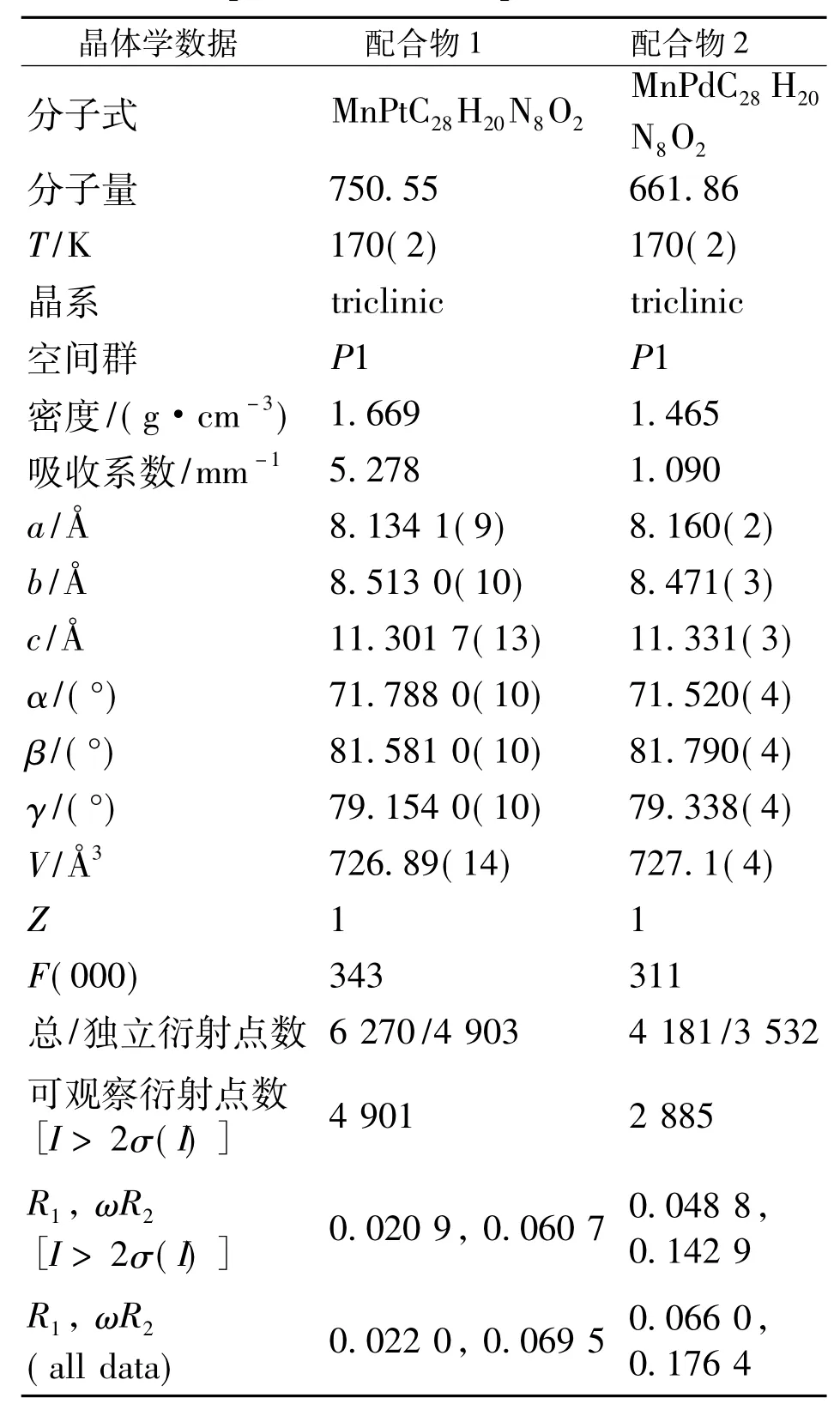
表1 配合物1和2的晶体学数据Table 1 Crystal data collection and refinement parameters for complexes 1 and 2
文中以配合物1为例进行了详细的晶体结构描述.图1为配合物1的ORTEP图,其非对称单元由1个[Mn(bpac)2(H2O)2]2+阳离子和1个[Pt (CN)4]2-阴离子组成.[Pt(CN)4]2-中的Pt原子处于四配位的平面四方构型之中,其中两个氰基(C2N2,C4N4)为端基,其他(C1N1,C3N3)为桥联氰基.在[Mn(bpac)2(H2O)2]2+结构中,Mn原子为六配位的畸变八面体构型,赤道位置被两个氰基氮原子和两个dpac配体中的两个氮原子占据,Mn1—N1和Mn1—N5键长分别为2.28 Å和2.34 Å.而轴向位置则被两个配位水占据,Mn1—O1和Mn1—O2键长分别为 2.26 Å和2.17 Å,O1—Mn1—O2的键角为 177.5°.如图 2所示,[Mn (bpac)2(H2O)2]2+和[Pt(CN)4]2-之间通过氰基交替连接形成无限伸展的一维链状结构,其中C1—N1—Mn1和N1—C1—Pt1的键角分别为170°和174.3°,弯曲角度较小,可近似为直线链.
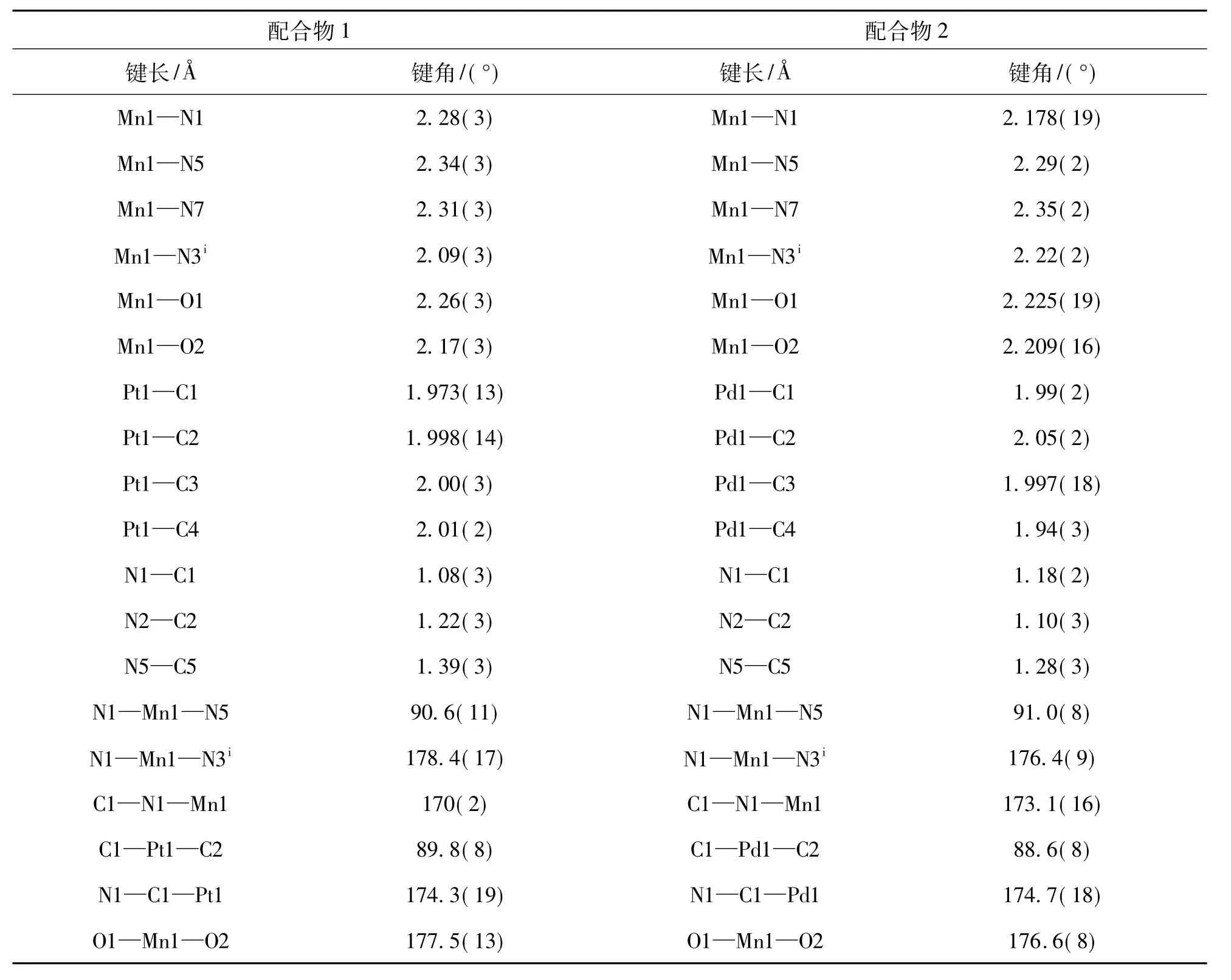
表2 配合物1和2的部分键长和键角Table 2 Selected bond distances and bond angles for complexes 1 and 2

图1 配合物1的ORTEP图(省略了H原子,椭球度30%)Fig.1 ORTEP diagram of complex 1(Hydrogen atoms are omitted for clarity and thermal ellipsoids are presented at the 30%probability level)
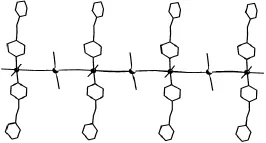
图2 配合物1的一维直线链结构Fig.2 1D linear chain structure of complex 1
2.2 红外光谱分析
图3为配合物1的红外光谱谱图.2 164 cm-1和2 142 cm-1处为氰基的特征吸收峰,其中2 164 cm-1处为桥联氰基吸收峰,2 142 cm-1则为端基氰基吸收峰.与K2[Pt(CN)4]·H2O中的氰基振动吸收峰(2 121 cm-1)相比,氰基吸收峰向高频方向移动,表明氰基与第二金属中心桥联.3 417 cm-1为游离水分子中O—H的伸缩振动吸收峰,可能由于样品吸附了部分水导致,3 228 cm-1为结构中配位水的O-H伸缩振动吸收峰,1 606 cm-1为吡啶环上的C=C伸缩振动峰,1 415 cm-1处为吡啶环上C-N的伸缩振动吸收峰.

图3 配合物1的红外光谱图Fig.3 FT-IR spectrum of complex 1
2.3 热重分析

图4 配合物1的TG曲线Fig.4 TG curve of complex 1
图4为配合物1的热重曲线.从图中可看出,室温到70℃区间配合物结构稳定.温度升高至90℃时,开始失去两个配位水分子,其失重率为5.1%,接近理论值4.8%.90~140℃之间出现短的平台,表明脱水配合物在该温度区域内保持稳定.随着温度继续升高,热重曲线出现多个转折点,说明配合物主体骨架的分解过程是逐步进行的.550℃左右时分解基本完全,残余率约为26.99%.
3 结论
以[M(CN)4]2-(M=Pt,Pd)为构筑模块与过渡金属离子Mn2+和刚性有机配体bpac通过扩散反应,得到了两个一维链状氰基桥联配合物.晶体结构分析表明:这两个配合物为同构体,Mn和M金属中心之间通过氰基连接形成一维直线链结构,加入的bpac柱状配体并未起到桥联作用.对于失去配位水后的配合物,其主体骨架随着温度的升高而开始分解.
References)
[1]Du M,Li C P,Liu C S,et al.Design and construction of coordination polymers with mixed-ligand synthetic strategy[J].Coordination Chemistry Reviews,2013,257(7-8):1282-1305.
[2]Wu T,You X Z,Petr B.Applications of chiroptical spectroscopy to coordination compounds[J].Coordination Chemistry Reviews,2015,284:1-18.
[3]Franz A M,Michael S,Christian B,et al.Synthesis,characterization and luminescence properties of zinc (II)complexes of pseudohalides and nitrite derived from 4-azidopyridine[J].Inorganica Chimica Acta,2015,425:46-51.
[4]Wang X Y,Andrey V P,Kim R D.A docosanuclear {Mo8Mn14}cluster based on[Mo(CN)7]4-[J].Angewandte ChemieInternationalEdition,2010,49 (30):5081-5084.
[5]Liu G N,Zhu W J,Chu Y N,et al.Three d10metal coordination compounds based on pyrazole-3-carboxylic acid showing mixed-ligand characteristic:syntheses,crystal structures,and photoluminescentproperties[J].Inorganica Chimica Acta,2015,425:28-35.
[6]Kou H Z,Gao S,Jin X L.Synthesis,crystal structure and magnetic properties of two cynao-bridged bimetallic 4f-3d arrays with one-dimensional chain and two-dimensional brick wall molecular structures[J].Inorganic Chemistry,2001,40(24):6295-6300.
[7]Fatima S,Catherine C,Sylvie H,et al.Spin crossover (SCO)iron(II)coordination polymer chain:Synthe-sis,structural and magnetic characterizations of[Fe (abpt)2(μ-M(CN)4)](M=PtIIand NiII)[J].Polyhedron,2013,61:242-247.
[8]Yuan A H,Zhou H,Cameron J K,et al.Gas and vapor adsorption in octacyanometallate-based frameworks Mn2[M(CN)8](M=W,Mo)with exposed Mn2+sites[J].International Journal of Hydrogen Energy,2014,39(2):884-889.
[9]Simon J H,Alex H,Matt G T,et al.Structures and negative thermal expansion properties of the one-dimensional cyanides,CuCN,AgCN and AuCN[J].Zeitschrift Für Kristallographie,2010,225(11):457-462.
[10]Juraj Cˇ,Martin O,Ivan P,et al.Cyanocomplexes with one-dimensional structures:preparations,crystal structures and magnetic properties[J].Coordination Chemistry Reviews,2002,224(1-2):51-66.
[11]Sourav A,Luke L D,Monika H,et al.Thermal expansion in 3d-metal Prussian Blue Analogs[J].Journal of Solid State Chemistry,2011,184(11):2854-2861.
[12]Natasha F S,Florence R,Cameron J K,et al.Spin crossover intermediate plateau stabilization in a flexible 2-D hofmann-type coordination polymer[J].Chemical Communication,2014,50(29):3838-3840.
[13]Mehran A,Okan Z Y,Orhan B,et al.One-dimensional heteropolynuclear tetracyanopalladate(II)complexes:Syntheses,crystal structures,thermal analyses and spectroscopic investigations[J].Journal of Molecular Structure,2014,1059:101-107.
[14]Günes S K,Okan Z Y,Orhan B,et al.A novel cyano-bridged heteronuclear complex exhibiting C-H…Ni close interaction:[Cd(C4H7N)2Ni(μ-CN)4]n[J].Journal of Molecular Structure,2011,994(1-3): 39-43.
[15]Anangamohan P.A cyanide-bridged 1-D helical chain involving both four-and six-coordinate nickel(II)[J].Journal of Coordination Chemistry,2011,64(6): 987-995.
[16]Dursun K,Günes S K,Okan Z Y,et al.Two dimensional heteronuclear complexes with cyanide and 4-aminomethylpyridine ligands[J].Journal of Molecular Structure,2014,1074:339-348.
[17]Gonzá lez M,Ana A L S,Knobel M,et al.π-π Interactions and magnetic properties in a series of hybrid inorganic-organic crystals[J].Journal of Solid State Chemistry,2013,197:317-322.
[18]Carlos B M,Lionel S,Amal A,et al.Synergetic effect of host-guest chemistry and spin crossover in 3D hofmann-like metal-organic frameworks[Fe(bpac)M (CN)4](M=Pt,Pd,Ni)[J].Chemistry-A European Journal,2012,18(2):507-516.
[19]Li Y,Liu Y,Wang Y T,et al.Hydrogen storage properties of[M(Py){Ni(CN)4}](M=Fe,Co,Ni)[J].International Journal of Hydrogen Energy,2007,32(15):3411-3415.
[20]Sheldrick G M.SMART,SAINT and XPREP[Z]∥Area detector and data integration and reduction software.Madison,WI:Bruker Analytical Instruments Inc.,1995.
[21]Sheldrick G M.SADABS.Empirical adsorption correction program for area detector data[Z].Göttingen,Germany:University of Göttingen,1996.
[22]Sheldrick G M.SHELXS-97 and SHELXL-97[Z]∥Program for crystal structural solution and refinement.Madison,WI:Bruker Analytical Instruments Inc.,1997.
(责任编辑:缪文桦)
Synthesis,structures and thermal stabilities of two cyano-bridged one-dimensional complexes Mn(bpac)2(H2O)2M(CN)4(bpac=bis(4-pyridyl)acetylene;M=Pt,Pd)
Tian Xiaoyan,Hu Aiyun,Yuan Aihua*
(School of Environment and Chemical Engineering,Jiangsu University of Science and Technology,Zhenjiang Jiangsu 212003,China)
Two new one-dimensional(1D)complexes Mn(bpac)2(H2O)2M(CN)4bpac=(bis(4-pyridyl)acetylene;M=Pt(1),Pd(2))were synthesized by the self-assembly reactions of Mn2+ion,pillar ligand bpac and[M(CN)4]2-(M=Pt,Pd).Single-crystal X-ray diffraction analysis revealed that complexes 1 and 2 are isostructural and they belong to triclinic space group P1.The asymmetric unit of both complexes consist of one[Mn (bpac)2(H2O)2]2+cation and one[M(CN)4]2-anion.The Mn and M centers adopt six-coordinated octahedral and four-coordinated planar square geometries,respectively.[Mn(bpac)2(H2O)2]2+and[M(CN)4]2-units are linked alternatively to generate a 1D linear chain.In addition,the thermal stability of complex 1 was also investigated.
cyano-bridged;complex;synthesis;structure; thermal stability
O614.4
A
1673-4807(2015)06-0602-05
10.3969/j.issn.1673-4807.2015.06.017
2015-05-11
国家自然科学基金资助项目(51272095)
田小燕(1988—),女,硕士.*
袁爱华(1965—),女,博士,教授,研究方向为功能配合物和纳米材料.E-mail:aihuaayuan@163.com
田小燕,胡爱云,袁爱华.一维链状氰基桥联配合物Mn(bpac)2(H2O)2M(CN)4(bpac=bis(4-pyridyl)acetylene;M=pt,pd)的合成、结构与热稳定性.江苏科技大学学报(自然科学版),2015,29(6):602-606.

