Evaluation of metalnanoparticles for drug delivery systems
Oluyomi S.Adeyemi,Faoziyat A.Sulaiman
1Department of Biological Sciences,Landmark University,Omu-Aran,Kwara State,Nigeria;
2Department of Biochemistry,University of Ilorin,Ilorin,Nigeria.
Evaluation of metalnanoparticles for drug delivery systems
Oluyomi S.Adeyemi1,✉,Faoziyat A.Sulaiman2
1Department of Biological Sciences,Landmark University,Omu-Aran,Kwara State,Nigeria;
2Department of Biochemistry,University of Ilorin,Ilorin,Nigeria.
Diminazene aceturate is a trypanocide with unwanted toxicity and limited efficacy.Itwas reasoned thatconjugating diminazene aceturate to functionalized nanoparticle would loweruntoward toxicity while improving selectivity and therapeutic efficacy.Silverand gold nanoparticles were evaluated fortheir capacities to serve as carriers fordiminazene aceturate.The silverand gold nanoparticles were synthesized,functionalized and coupled to diminazene aceturate following established protocols.The nanoparticle conjugates were characterized.The free diminazene aceturate and drug conjugated nanoparticles were subsequently evaluated for cytotoxicity in vitro.The characterizations by transmission electron microscopy or UV/Vis spectroscopy revealed thatconjugation ofdiminazene aceturate to silverorgold nanoparticleswas successful.Evaluation forcytotoxic actions in vitro demonstrated no significance difference between free diminazene aceturate and the conjugates.Our data suggest thatsurface modified metalnanoparticles could be optimized for drug delivery systems.
drugs,trypanocides,nanoparticles,toxicity,targeting
Introduction
One major challenge confronting the chemotherapeutic controlof trypanosomiasis is the issue of toxicity[1,2].Several trypanocides including melarsoprol and suramin,amongstothers,have poor efficacy and limited solubility and majorside effects[3,4].Animaltrypanosomiasis is also notleftoff this quagmire,as the major veterinary trypanocides,diminazene aceturate and homidium chloride,have their share of the undaunting toxicity challenges[5].These factors,besides masking the efficacy of the drugs,have also prompted research thatcould unravelnoveltreatmentstrategies for trypanosomiasis.While new drug developmentfor trypanosomiasis are being encouraged,modification of,orinvestigationson existing trypanocidesto increase theirtherapeutic index is a welcome idea.
Diminazene aceturate is a drug used to treatanimal trypanosomiasis.Previously,we have demonstrated the toxicity and limited efficacy ofthis drug and othertrypanocides in experimental infection using rats[6].It is interesting to note thatmostof the adverse effects have been attributed to the non-specificity of the drug. Furthermore,Asadishad etal.[7]proposed the conjugation of drugs to polymers in order to reduce toxicity while sustaining therapeutic efficacy.In order to circumvent drug toxicity challenges,conjugation to polymer-functionalized nanoparticles have been further explored in a parallel study[8].Nanotechnology is an emerging field with potentialapplications in diagnostic and therapeutic developments.Nanomaterials are defined as having at leastone dimension<100 nm in size,whereas the term nanoparticlesappliesto substanceswith allthree external dimensions in the nanoscale[9].Worthy of considerationis the fact that silver or gold nanoparticles(Ag or Au-NPs)are increasingly being applied for biomedical purposes either because of their respective broad antimicrobial or visible light extinction behavior[7,10]. Nanoparticles,because of their remarkable properties, have become attractive for use in drug delivery and/or targeting[11].Nanoparticles have attracted considerable attention worldwide because of the unique functional characters such as small particle size,high stability,tunable hydrophilic-hydrophobic balance and the ability to surface features for target specific localization amongst others[12,13].Hence,nanoparticles offerversatile opportunities fordrug delivery system as wellas modulation of drug toxicity.
In this study,diminazene acturate was conjugated to folate-modified silver(Ag)or gold(Au)nanoparticles supported in polyphenolic solution.Folic acid was selected for surafce modification targeting because it has high affinity for cells overexpressing folate receptors[14,15].The conjugated complex,folate modified nanoparticle and diminazene aceturate were evaluated for their cytotoxicity and compared.
Materials and methods
Synthesis of silver or gold nanoparticles
Silver or gold nanoparticles(Ag or Au-NPs)were synthesized based on established protocols with little modification.Briefly,Ag or Au-Nps were prepared separately by adding 50 mM silver nitrate or 50 mM chloroauric acid to a 1%(w/v)tannic acid solution (pH adjusted to 8 with 150 mM potassium carbonate) of polyvinylpyrrolidone(PVP)with stirring[16,17].The solution turned pale yellow(Ag-NPs)or brown red (Au-NPs).The Ag or Au-Nps were filtered using the 0.22μM filter and characterized by UV/Vis-spectrophotometry(Biotek Epoch,USA),inductively coupled plasma optical emission spectrometry(ICP-OES, Cambridge,UK),and transmission electron microscopy(TEM,Brno,Czech Republic).
PEG-SH-functionalized and folate modification of nanoparticle
The procedure previously repo rted by Lee and Low[18]wasadopted forthe preparation offunctionalized nanoparticles.Briefly,amino-PEG-SH was prepared by reacting molarequivalents of Trautˊs reagent(8.2 mg in 200μL of water)and PEG bis-amine(200 mg)in 1 mmol/L EDTA,10 0 mmol/L ph osphate buffer (1 mL,pH 8.0).Before it was attached to the PEGSH,functionalized folic acid was first activated.NHydroxysuccinimide ester of folic acid(NHS-folate) was prepared by dissolving 5 mg of folic acid in 100 mL dry dimethyl sulfoxide plus 2.5 mL triethylamine and reacted with N-hydroxysuccinimde(2.6 g)in the presence of dicyclohexycarbodiimide(4.7 g)overnightatroom temperature.The by-product,dicyclohexyurea,was removed by filtration.The dimethylsulfoxide solution was concentrated under reduced pressure and heating.NHS-folate was precipitated in diethylether. The product,NHS-folate,was washed several times with anhydrous ether under vacuum,and stored as a yellow powder.
Conjugation of diminazene aceturate to functionalized silver or gold nanoparticles
Diminazene aceturate(100 mmol/L)in Tris-HCl, pH 7 was added to folate modified PEG-SH functionalized nanoparticles in aqueous phase and stirred for 24 hours atroom temperature.The mixture was characterized and stored atroom temperature.
Characterisation of synthesized conjugates
Synthesized nanoparticles,PEG-SH functionalized, folate-modified and conjugates of the nanoparticles were subjected to characterization using UV/Visspectrophotometry(Biotek Epoch),FTIR(Perkin Elmer,USA),and transmission electron microscope.
In vitro cytotoxicity
MDA-MB-231 cells were used to evaluate the cytotoxicity of free diminazene aceturate,folate-modified nanoparticles and diminazene aceturate-conjugated nanoparticles.MTT assay was performed on MDAMB-231 cells using a commercial kit(Roche Diagnostics,South Africa).MDA-MB-231 cells were maintained in DMEM containing fetalbovine serum at37°C in 5%CO2.A 96 well plate was seeded at 6×104cells/mL.After 24 hours of incubation,the cells were treated with various concentrations of free diminazene aceturate,folate-modified nanoparticles, or diminazene aceturate-conjugated nanoparticles. Two times(2x)ofthe desired concentrations(the concentration ranged from 20,50 and 100μmol/L-all concentrations were normalized in order to attain unbiased comparison)were prepared in the culture media and 50μL added to the wells appropriately. The control was treated with 50μL culture media. The treated cells were incubated and after 96 hours, 10μL MTT dye was added to each well.The plate was further incubated and after 4 hours,100μL of the solubilizing reagent was added to each well and incubated overnight.The percentage of cell viability was determined at 595 nm relative to non-treated cellsusing a microplate reader(Biotek Epoch).The assay was repeated three times in triplicates[19].Data analysis
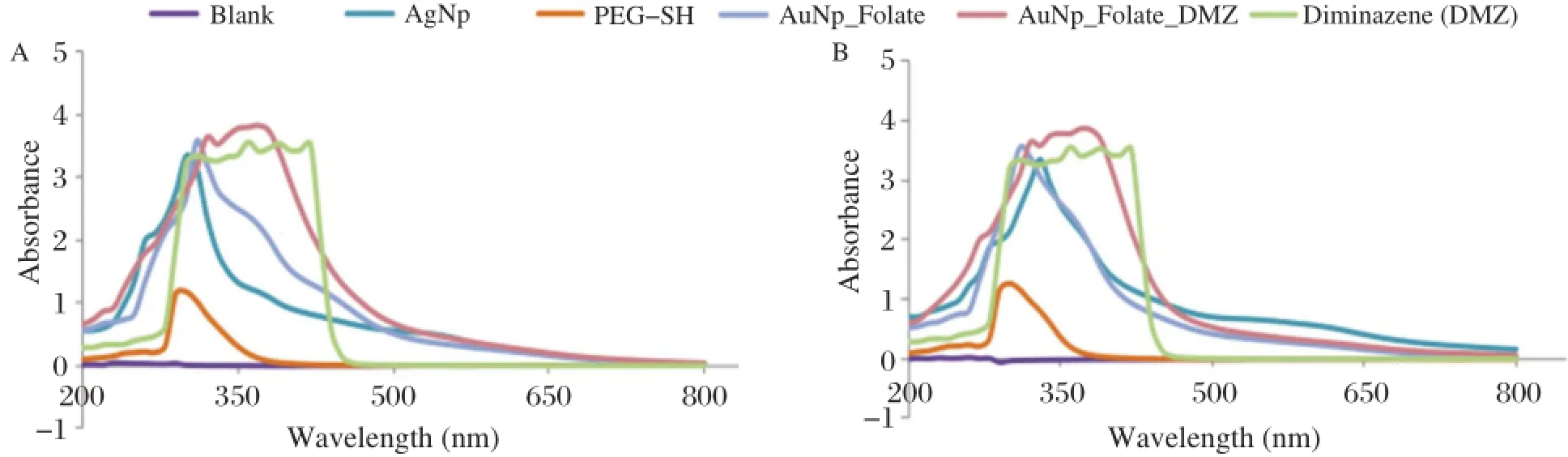
Fig.1 UV/Vis spectrum for silver nanoparticle-folate-diminazene aceturate(AgNp-Folate-Diminazene)conjugate(A)and gold nanoparticle-folate diminazene aceturate(AuNp-folate-diminazene)conjugate(B).
The data on cellviability were analysed using oneway analysis of variance(ANOVA)on GraphPad Prism 3(GraphPad Prism 3 Software Inc.USA).The results were presented as mean±SEM(n=3). Mean values at P<0.05 were considered significant.
Results
The present study attempted to determine the potential of metal nanoparticles for drug delivery systems. Fig.1 present the UV/Vis-spectra revealing peaks at the 370 nm range.This corresponds to the presence of diminazene aceturate in the conjugates.The TEM images are shown in Fig.2for silver and gold nanoparticle-diminazene aceturate conjugates,respectively.The nanoparticles had diameter sizes between 6 to 10 nm.The TEM images showed thatconjugation ofdiminazene aceturate to the nanoparticles did notalter nanoparticle morphology.Consequently,the free diminazene aceturate and nanoparticle conjugates were screened for comparative cytotoxicity(Table 1). When compared,the cytotoxicity data showed no appreciable(P>0.05)differences in the activities of free diminazene aceturate or nanoparticles-diminazene aceturate conjugates.Although,the free diminazene aceturate increased in cytotoxicity with increasing concentration,the nanoparticles-diminazene aceturate conjugates showed reduced cellular toxicity at similar concentrations(Table 1).
Discussion
The UV/Vis spectra suggest that the diminazene aceturate conjugation to the modified nanoparticles was successful.In addition,TEM shows nanoparticles of diameter sizes ranging between 6 and 10 nm.The absence ofsignificantchanges in the TEM images furtherrevealsthe successfulattachmentofthe diminazene aceturate to the nanoparticles and thatthe surface modification may nothave compromised the morphological integrity of the metalnanoparticles.
The free diminazene aceturate and the conjugates were screened for comparative cytotoxic actions in order to determine whether conjugating drugs to surface modified nanoparticles could optimize drug delivery to targetsite while minimizing collateraldamage. The free diminazene aceturate and the conjugates exhibited similar toxic potentials in the MDA-MB cell culture.There were non-significant reductions in the toxicity of corresponding dosages of the conjugatesrelative to the free diminazene aceturate.In constrast, the nanoparticle conjugates demonstrated consistent toxicity with cell viability higher than 50%.Dose dependenttoxicity was observed fordiminazene aceturate.On the other hand,increasing concentration of the nanoparticle conjugates did notresultin highertoxicity as opposed to increasing toxicity by free diminazene aceturate.Interestingly,a previous report had demonstrated successfulconjugation of diminazene aceturate to lipid nanoparticles[20].However,to our knowledge this is the firstattemptthat demonstrated a successful conjugation ofdiminazene aceturate to metalnanoparticles and as well provided preliminary evidence that metalnanoparticles could be explored fordrug targeting or delivery.Furthermore,in a parallel study[21],lipid nanoparticle-diminazene aceturate conjugate showed reduced cytotoxicity.This result is consistent with our observation for cytotoxicity of diminazene aceturatenanoparticle conjugates as obtained in the currentstudy.
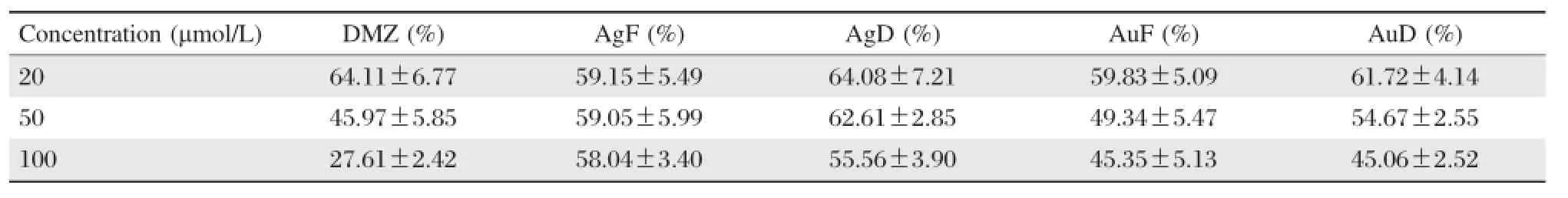
Table 1 Cytotoxic activities of free diminazene aceturate and nanoparticle-diminazene aceturate conjugates in cell culture meaniSEM
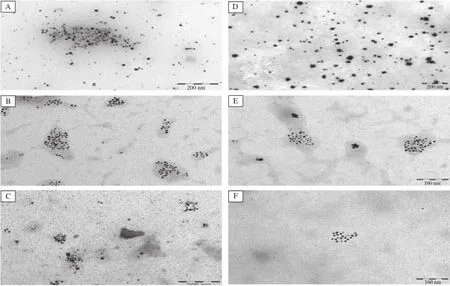
Fig.2 TEM images for AgNp-folate-diminazene conjugates(A-C)and AuNp-folate-diminazene conjugates(D-F).A:Ag nanoparticles(AgNp);B:AgNp-folate modified;C:AgNp-folate-diminazene conjugates;D:Au nanoparticles(AuNp);E:AuNp-folate modified;F: AuNp-folate-diminazene conjugates.
In conclusion,we have presented preliminary evidence to show thatmodification ofmetalnanoparticles could be exploited for drug delivery and/or used to modulate toxic actions of drug.As the concentration increased,non-significant reduction in the cytotoxic actions for the nanoparticle conjugates were observed relative to the cytotoxicity ofthe free diminazene aceturate in MDA-MB-231 cells.Further works on the in vitro kinetic release and cytotoxicity studies in Trypanosoma brucei cell culture are ongoing.
Acknowledgements
Department of Biochemistry,Rhodes University, South Africa.
[1]Adeyemi OS,Akanji MA,Oguntoye SA.Ethanolic leaf extract of Psidium guajava:Phytochemical and trypanocidal activity in rats infected with Trypanosoma brucei brucei.J Med Plants Res 2009;3(5):420-423.
[2]Sulaiman FA,Adeyemi OS.Changes in haematological indices and protein concentrations in Trypanosoma infected-rats treated with homidium chloride and diminazene aceturate.EXCLI Journal 2010;9:39-45.
[3]World Health Organisation.Control and surveillance of African trypanosomiasis:report of WHO expertcommittee.WHO Tech Rep Ser 1998;1-114.
[4]World Health Organisation.African trypanosomiasis or sleeping sickness:Fact Sh eet,20 01;2 59.(http:// www.who.int/mediacentre/factsheets/fs259/en/).
[5]Akpa PO,Ezeokonkwo RC,Eze CA,etal.Comparative efficacy assessmentof pentamidine isethionate and diminazene aceturate in the chemotherapy of Trypanosoma bruceibrucei infection in dogs.Vet.Parasitol 2008;151(2-4):139-149.
[6]Adeyemi OS,Sulaiman FA.Biochemical and morphologicalchanges in Trypanosoma bruceibrucei-infected rats treated with homidium chloride and diminazene aceturate. J Basic Clin Physiol Pharmacol 2012;23(4):179-183.
[7]Asadishad B,Vossoughi M,Alamzadeh I.In vitro release behavior and cytotoxicity of doxorubicin-loaded gold nanoparticles in cancerous cells.Biotechnol Lett 2010; 32(5):649-654.
[8]Patra CR,Bhattacharya R,Mukhopadhyay D,et al. Application of gold nanoparticles for targeted therapy in cancer.J Biomed Nantechnol 2008;4(2):99-132.
[9]Hudecov a´A,Kusznierewicz B,Runden-Pran E,et al. Silver nanoparticles induce premutagenic DNA oxidation that can be prevented by phytochemicals from Gentiana asclepiadea.Mutagenesis 2012;27(6):759-769.
[10]Kasthuri J,Veerapandian S,Rajendiran N.Biological synthesis of silver and gold nanoparticles using apiin as reducing agent.Colloids Surf B 2009;68(1):55-60.
[11]Maya K,Hamadi K,Didier B.Drug delivery systems in the treatment of African trypanosomiasis infections. Expt Opin Drug Deliv 2011;8(6):735-747.
[12]Frank G,Langer R,Farokhzad OC.Precise engineering of targeted nanoparticles by using self-assembled biointegrated block coploymers.PNAS 2008;105(7):2586.
[13]Chouhan R,Bajpai AK.Real time in vitro studies of doxorubicin release from PHEMA nanoparticles.J Nanobiotechnology 2009;7(5):7.
[14]Weitman SD,Lark RH,Coney LR,et al.Distribution of the folate receptor,GP38,in normal and malignant cell lines and tissues.Cancer Res 1992;52(12):3396-3401.
[15]Zhang Z,Huey Lee S,Feng SS.Folate-decorated poly(-lactide-co-glycolide)-vitamin ETPGS nanoparticles fortargeted drug delivery.Biomaterials 2007;28(10):1889-1899. [16]Sivaraman SK,Elango I,Kumar S,etal.A green protocol for room temperature synthesis of silver nanoparticles in seconds.Curr Sci 2009;97(7):1055-1059.
[17]Sivaraman SK,Kumar S,Santhanam Vl.Room-temperature synthesis ofgold nanoparticles-Size-controlby slow addition.Gold Bull 2010;43(4):275-286.
[18]Lee RJ,Low PS.Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis.J Biol Chem 1994;269(5):3198-3204.
[19]De la Mare JA,Lawson JC,Ch wakata MT,et al. Quinones and halogenated monoterpenes of algal origin show anti-proliferative effects against breast cancer cells in vitro.Invest New Drug 2012;30(6):2187-200.
[20]Olbrich C,Gessner A,Kayser O,etal.Lipid-drug-conjugate(LDC)nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazene diaceturate.J Drug Target 2002;10(5):387-96.
[21]Olbrich C,Gessner A,Schroder W,et al.Lipid-drug conjugate nanoparticles of the hydrophilic drug diminazene-cytotoxicity testing and mouse serum adsorption. J Control Release 2004;96(3):425-435.
✉Corresponding author:OluyomiS.Adeyemi,Departmentof Biological Sciences,Landmark University,Omu-Aran,Kwara State,Nigeria.Tel: +234 7034507902,E-mail:yomibowa@yahoo.com.
Received 24 June 2013,Revised 15 July 2013,Accepted 10 March 2014, Epub 10 December 2014
The authors reported no conflictof interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.28.20130096
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Recent advances in bariatric/metabolic surgery:appraisalof clinical evidence
- Metabolic bariatric surgery and type 2 diabetes mellitus:an endocrinologistˊs perspective
- Roux-en-Y gastric bypass for Chinese type 2 diabetes mellitus patients with a BMI<28 kg/m2:a multi-institutionalstudy
- Bariatric surgery in old age:a comparative study of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in an Asia centre ofexcellence
- Maternalinheritance of severe hypertriglyceridemia impairs glucose metabolism in offspring
- Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis
