Metabolic bariatric surgery and type 2 diabetes mellitus:an endocrinologistˊs perspective
Sonali Ganguly,Hong Chang Tan,Phong Ching Lee,Kwang Wei Tham
The Obesity&Metabolic Unit,Department of Endocrinology,LIFE Centre,Singapore General Hospital,Singapore
Metabolic bariatric surgery and type 2 diabetes mellitus:an endocrinologistˊs perspective
Sonali Ganguly,Hong Chang Tan,Phong Ching Lee,Kwang Wei Tham✉
The Obesity&Metabolic Unit,Department of Endocrinology,LIFE Centre,Singapore General Hospital,Singapore
Traditionaltreatmentof T2DMconsisting ofmodification ofdiet,an exercise regimen,and pharmacotherapy has problems ofpoorlifestyle modifications and failtend oftreatmentovertime,now bariatric surgery is recommended for treatmentof obese patients with T2DM because its greatimprovements on weightloss and metabolic.In this article,effects of bariatric surgery on diabetes and diabetes-related complications are reviewed.
type 2 diabetes mellitus,metabolic bariatric surgery,diabetes remission
Introduction
Obesity is a worldwide epidemic which contributes to numerous medicalcomplications including type 2 diabetes mellitus(T2DM).T2DM is a progressive disorder resulting in micro-and macro-vascular disease which confer significant morbidity and mortality. Thus,treatment of a T2DM patient aims to reduce these associated complications.Traditionaltreatment of T2DM consists of modification of diet,an exercise regimen,and pharmacotherapy.However,overall patientcompliance with lifestyle modifications is often poorand treatmentwith pharmacotherapy tends to fail over time[1].In addition,many medications are associated with hypoglycaemia and weight gain,especially during intensification of treatment.The resultant hypoglycaemia is associated with a higher incidence of cardiovascular deaths in intensive treatment and the weight gain may lead to further deterioration in glycemic control[1,2].
In recent years,bariatric surgery has emerged as a potential treatment for obese patients suffering from T2DM.Both the American Diabetes Association (ADA)and the International Diabetes Federation (IDF)now recommend bariatric surgery in their treatment guidelines for obese patients with T2DM[3,4]. Improvementin glycemic control following gastrointestinalsurgery was firstnoted in the firsthalf of the 20thcentury.Patients undergoing gastric resection for peptic ulcer disease or gastric cancer were found to have improvementin diabetes control[5].Early observationalstudies noted the benefitbariatric surgery had on glycemic control[6].In more recentyears,several randomized controlled trials(RCTs)have compared medical management of T2DM with surgical managementofT2DM.These studiesconfirm thatbariatric surgery is indeed more effective than medicalmanagement of T2DM[7-9].
We hereby discuss in detail the effects of bariatric surgery on diabetes and diabetes-related complications.

Table 1 Summary of the various criteria for diabetes mellitus remission used in trials.
The role of the endocrinologist in the short-and longterm management of the diabetic patient undergoing bariatric surgery is also discussed.
T2DM remission
Apart from substantial weight loss,the positive impact on T2DM control is the greatest metabolic improvement after bariatric surgery.Diabetes remission,defined as normoglycemia without the use of any hypoglycaemic medications or therapeutic procedures,is achieved in a substantial number of patients with T2DM following bariatric surgery.
However,reports of DM remission are highly variable,ranging anywhere from 24%to 95%[7-10].The reason for this wide variation is mostly due to use of differentdefinitions of DM remission,differentkinds of procedures studied and variable duration of postoperative follow-up,with most studies reporting outcomes within 2 years.In addition,moststudies were retrospective,observational or prospective observations with high drop-out rates.
In Buchwaldˊs 2004 meta-analysis,the overallrate of complete DM remission for all procedures was 76.8%[11].The remission rates forgastric banding,gastroplasty,gastric bypass(GB),and biliopancreatic diversion(BPD)or duodenal switch(DS)procedures were 48%,72%,84%and 99%,respectively.The magnitude of the DM remission correlated to the amount of weight loss after surgery.The definition of resolution of T2DM in this meta-analysis was‘‘the ability to stop all diabetes-related medications and maintain blood glucose levels within the normal range.’’[11]
In an effortto standardize the definition of diabetes remission,a consensus by a group ofspecialists(Buse et al.)defined complete resolution of T2DM as a totally normal glycemic state(HbA1C<5.7%with fasting plasma glucose<5.6 mmol/L)and partial remission as glucose controlin the non-diabetic range (Hb A1C<6.4%with fasting plasma glucose<6.0 mmol/L)without the need for DM medications for at least 1 year[12].
In recent years,there have been at least 5 RCTs comparing conventional or intensive medical therapy with bariatric surgery on the effect of glycemic control in T2DMpatients.Results continue to vary due to differentmethodologies employed in the studies.Several variables including type of procedure,presurgical duration of DM,severity of DM,study endpoints of DMremission and duration of follow-up existamongst these studies.
Itis importantto note thatthe higherremission rates occurred in the patients with the shortest duration of diabetes.Remission rates decreased in the studies which included patients with longer duration of diabetes.Additionally,the majority of reported DM remission rates are based on 1-2 year postoperative outcomes.Due to the relatively shortduration of follow-up in these newer RCTs,the concerning issues of long-term remission(lasting>5 years)and the recurrence oftype 2 diabetesare notyetfully addressed.
Durability of DM remission and relapse of DM
On medium term follow-up ofpatients post-bariatric surgery(over 3-5 years),a substantial percentage of patients(60%-91%)can achieve DM remission[14-17]. Jimenez etal.observed that66%oftheirpatients were in remission throughouttheentirefollow-up period(mean 35 months)after RYGB with a handful(17%)achieving remission only after 12 months postoperatively[15].
In prospective follow-up studies of medium-term follow-up,relapse into the diabetic state can occur in 12%-25%ofthe patients who have attained remission 1 year postoperatively[15,18].The only RCT with medium-term follow-up is the STAMPEDE trialin which patients with T2DMwere followed up forup to 3 years after randomization[10].A significantly greater proportion ofpatients who underwentSG(29%)or GB(46%) were stillable to achieve any DMremission(HbA1c<6.5%withoutmedications)compared to those in the intensive medical treatment arm(0%).In addition, the mean Hb A1c attained in the surgical group was significantly lower than that in the intensive medical arm.However,after 3 years,25%in the GB group and 50%in the SG group who attained DM remission at1 year had relapsed into the diabetic state[10].
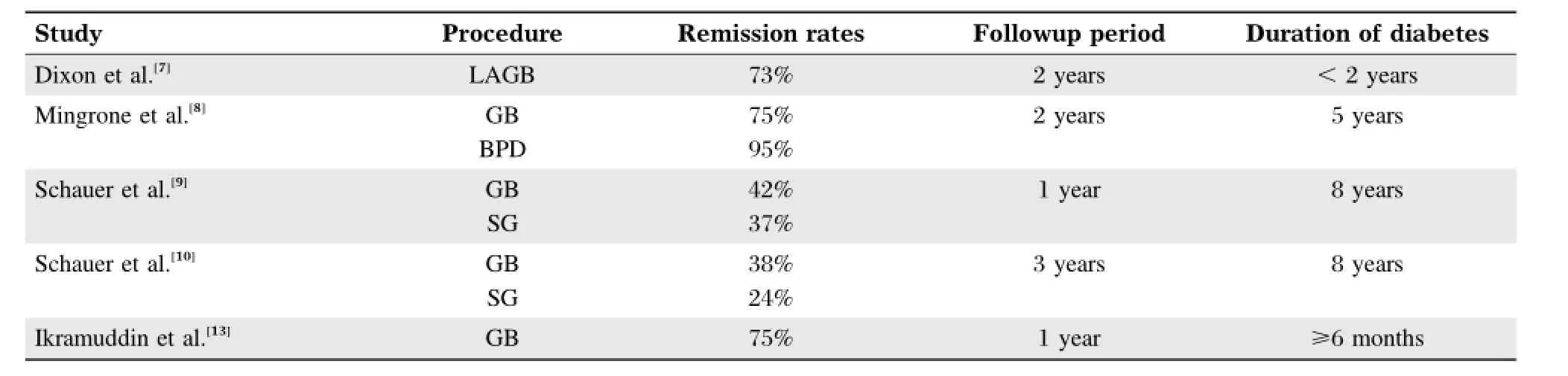
Table 2 Summary of remission rates amongst the various studies.
In long-term studies of 5 years and beyond,reported rates of complete DM remission range from 24%to 88%,with higher rates reported in studies using a higher HbA1c cut-off and for bypass type procedures[19].The Swedish Obese Subjects(SOS)study,a prospective case-matched study of various bariatric surgery procedures,reported that 36%of patients had recovered from their DM 10 years after surgery,indicating that 50%of patients who had initial DM remission suffered a recurrence of diabetes[20].
In a follow-up of 217 patients over 6 years, Brethauer et al.[19]reported that 24%of all patients achieved long-term complete remission with an additional 26%with partial remission(i.e.50%of all patients can achieve long-term DM remission)with betterglycemic outcomes noted after RYGB compared to SG or LAGB,and 27%ofthose in complete remission maintained this throughoutthe 6 years.However, 19%ofthose who achieved an initialcomplete remission suffered a relapse of DM over this period.
Patients who tended to experience relapse had a longer duration of DM(>5 years),had regained weight,were of older age,and required insulin before surgery.Nonetheless,even with recurrence of DM, fewer patients required insulin associated with a significant reduction in the number of glucose-lowering medications.They were also able to attain a lower HbA1c,blood pressure and lipid targets better than compared to baseline[16,19].
DM remission after bariatric surgery can be durable beyond 5 years after surgery.However,itis prudentto continue follow-up especially in patients who are at risk for DM recurrence.We now await further longterm results from the recent RCTs.
What predicts DM remission?
There are several variables to consider in determining what helps predict diabetes remission.As mentioned earlier,it does appear that duration of diabetes plays a pivotal role.A 2012 retrospective study of 88 Asian patients found thatshorter duration of diabetes was indeed a predictor of DM remission post surgery[21].In the STAMPEDE trial and in long-term follow-up studies in Asian and Caucasian populations, patients with the shortest duration of diabetes were those most likely to achieve complete resolution of their diabetes after surgery[9,14,19,22].
Other variables to consider are preoperative body mass index(BMI),type of procedure,amount of weight loss,and preoperative glycemic control. Buchwald et al.reported more frequent diabetes remission with the procedures thathave a malabsorptive component versus those that are solely restrictive[11].Dixon et al.found that the degree of weight loss had major impact on glycemic improvement,not the type of procedure[7].However,itis important to note that the procedure used in the Dixon study was a purely restrictive one-LAGB.Mingrone et al.compared 2 procedures with malabsorptive component (GB and BPD)and found no relationship between improved glycemic control and weight loss or preoperative BMI[8].
Kashyap et al.demonstrated that 2 years post-SG and RYGB,there were substantially more patients in the GB group than the SG group being able to attain HbA1c<6%.In addition,there was a more durable effecton DMremission after GB compared to SG with an overall reduction in medication use[23].In the longterm,there is a tendency fora lowerrate ofrelapse into the glycemic state from DM remission after GB compared to SG[19].This was despite a similar weightloss between the 2 groups.This implies thatmalabsorptive procedures may improve diabetes control independent of weightloss.
Preoperative predictors of remission reflect surrogate factors predicting‘‘healthier’’pancreatic betacells,a better residual pancreatic beta-cell function preoperatively and a greater degree of insulin resistance.These included younger age,higher preoperative BMI,greater levels of fasting C-peptide levels,a higher homeostatic model assessment of betacellfunction(HOMA%B)and a shorter duration of diabetes[23-25].
Therefore,selection ofappropriate patients based on these predictors,coupled with selecting the most appropriate procedure willhelp patients getthe maximalbenefitof metabolic bariatric surgery.
Mechanism of DMremission-weight loss effect versus bypass/hormonal changes effect or both?
Several acute and chronic physiological changes occur as a resultof bariatric surgery,which contribute to improved glycemic control or remission of type 2 diabetes.The mostpotentmechanism of DM remission is no doubt weight loss,in particular loss of truncal fat[23].Weight loss causes decreased peripheralinsulin resistance,thereby improving glycemic control.
However,very often-especially in the case of post-GB,glycemic controlimproves quite acutely,before weightloss has even occurred.The underlying mechanisms for this are manifold.First,acute caloric restriction contributes to improved glycemic control through rapid improvement in insulin sensitivity with reduction in hepatic insulin resistance.Even in the absence of surgery,improved beta cellfunction with increased post-prandialinsulin release,can be seen as early as 1 week after acute caloric restriction[26-28].
Many studies have also demonstrated thatafter GB, there is an earlier and enhanced glucagon-like peptide 1(GLP-1)secretion in response to nutrients[23-26].This can occur as early as a few days after GB and persist beyond 2-3 years after surgery.However,this may not be an importantmechanism in glucose controlin the immediate postoperative period[26,29].
Kashyap etal.demonstrated that2 years post SG and RYGB,the GB group had greater relative reduction in truncalthan subcutaneous fat,greaterimprovement in insulin sensitivity and pancreatic beta-cell function compared to SG group[23].There is also a tendency fora lowerrate ofrelapse into the glycemic state from DM remission after GB compared to SG[19].
Severalstudies have reported thatan enhanced GLP-1 secretion during oral glucose tolerance test confers benefits of DM remission and is durable up to 3 years after surgery(SG or RYGB)[15,24,28,30].All these imply thatafter metabolic surgery,in addition to weightloss, factors related to intestinalbypass and involving incretin changes play a major role in DM remission and improvement of the metabolic state.Salehi et al. demonstrated that enteral factors account for 80%of insulin secretion in response to a meal after RYGB as opposed to 53%in non-surgical obese controls[29]. However,there is growing evidence thata concomitant improvement in beta-cell function after surgery plays a key role in DM remission,especially in the long term[23,25,29,30].
Lastly,bile acids have been noted to be elevated after RYGB and SG.Bile acids are known to reduce food intake,gluconeogenesis,and insulin resistance. They are also known to increase guthormone production and increase energy expenditure[28].
Coupled with clinicalobservations of DM remission outcomes,there are clearly multiple mechanisms of DM remission after bariatric surgery-acute caloric restriction,intestinalbypass or rapid transit after SG causing enhanced GLP-1 secretion in response to food, bile acid effectamongstothers.These ultimately exert theirimpacton glycemic controlvia reduction in insulin resistance and increase in insulin secretion in response to nutrients-basic pathophysiologic defects ofin T2DM development.Perhapsin differentpatients,the predominantfactorinducing remission orthe lack thereofmay be different.Regardless ofwhich mechanism is the predominantfactor,itisimperative thata certain levelofpancreatic beta-cell function exist presurgically,with subsequentpostoperative improvementin its function, forany meaningfulclinicalimprovements in glycemia, especially DM remission,to occuraftersurgery.
Consideration in special populations with DM:the lower BMI patient and Asian population
In Asia,the burden of T2DMis rapidly increasing.It is predicted that China,India,Pakistan,Indonesia and Bangladesh are among the top 10 countries predicted to have the highest number of DM by 2030[31]. Distinctively in Asia,T2DMdevelops atleasta decade earlier and ata lower BMI than in white populations. Asians are more insulin resistantthan people of many other races;they are metabolically obese,displaying signs ofinsulin resistance even in the absence of obesity[31,32].This is related to a greaterdegree ofadiposity, especially visceraladiposity,characteristically seen in Asians even for the same waist circumference and BMI.Hence,for any given BMI level and waistcircumference measurement,the risk for T2DM developmentishigherin Asians than people of Europid origin, especially Asian Indians[33,34].Lower BMI cut-offs compared to Europid populations for any clinical intervention including bariatric surgery is taken in this context.The IDF recommends that MBS be considered in Asian patients with DM and BMI 27-30 kg/m2ifHbA1c remains suboptimal despite maximal medical therapy[4].
Bariatric surgery in Asian patients with lower BMI (classically defined as<35 kg/m2)has seen lower rates of metabolic improvements compared to those with BMI>35 kg/m2[35].One would wonder then that if Asians experience a larger burden of disease and insulin resistance ata lower BMI,would bariatric surgery benefit diabetes and related metabolic disorders when intervened ata lower BMI?
Dixon et al.studied a group of 103 Chinese and Korean patients with T2DM and BMI<30 kg/m2(mean 26.0;range 18.9-30.0 kg/m2)after GB.DM remission(HbA1c≤6%)at 1 year post-surgery occurred in 30%of patients[36].In a prospective study of 29 non-obese Chinese patients with T2DM and BMI of 20.9-26.9 kg/m2who had undergone RYGB, Malapan et al.reported that 38%of patients attained DM remission(HbA1c<6.5%withoutany glucoselowering medications)at1 year[37].These remission rates are clearly below previously reported rates ofup to 93% in Asians after RYGB[38].
Predictorsof DMremission at1 yearpostoperatively in Asian patients are no different from other populations.A shorter presurgical duration of DM,higher baseline C-peptide levels(surrogate of inter-play of presurgical pancreatic beta-cell function and insulin resistance),higherpreoperative BMI(which influences insulin resistance),a younger age and greater postoperative weight loss are strong predictors of DM remission 1 year after RYGB[22,38].
While metabolic improvements after bariatric surgery are seen in lower BMI patients,these outcomes are not as favourable as those with higher BMI.Perhaps the selection of T2DM Asian patients for metabolic surgery should take into consideration assessment and measurement of visceral obesity appropriate for ethnicity,insulin resistance and baseline pancreatic beta-cellfunction apartfrom BMI alone,for maximal benefits and long-term risk reduction from metabolic surgery.However,more long-term studies in this group of patients are needed before a generalrecommendation can be made in T2DM patients with lower BMIfor consideration of metabolic surgery.
Reducing outcomes of DM
Clearly,there iscopious data forthe beneficialeffect bariatric surgery has on glycemic control.However, diabetes patients often suffer additionalcomorbidities such as hypertension and dyslipidemia which collectively confer significant risk for microvascular and macrovascular complications such as renal failure, retinopathy,stroke and coronary artery disease[40]. Multi-factorial intervention targeting euglycemia, blood pressure and optimallipid levels have been proven to reduce the risk of cardiovascular disease and various microvascular complications[41].However, there remains a paucity of data on micro-and macrovascular disease outcomes following bariatric surgery.
The Swedish Obese Subjects(SOS)study reported reduced rates of myocardial infarction in obese type 2 diabetes patients who underwent bariatric surgery. This occurred despite a 50%recurrence rate ofdiabetes in these patients.An extended follow-up of 16 years revealed decreased overall mortality in obese patients with type 2 diabetes who had undergone bariatric surgery[42,43].
In the 3 year follow-up of the STAMPEDE trial, there were no significantdifferences in blood pressure and LDL levels among the 3 study groups.However the numberofmedicationsneeded to treathyperlipidemia and hypertension was significantly reduced in the surgicalgroups[10].
In the Diabetes Surgery Study by Ikramuddin etal., the primary endpoint specifically looked atachieving the ABC targets(i.e.the American Diabetes Association recommended targets:A1c<7%,LDL<100 mg/d L,and SBP<130 mmHg)in obese type 2 diabetes patients who were either treated medically or with RYGB surgery.At 12 months post intervention, 49%of RYGB patients achieved this composite endpoint,compared to only 19%of patients in the medically treated group[13].
In addition,improvements in renal function and microalbuminuria have been reported after bariatric surgery[19,44].In the 3-yearfollow-up ofthe STAMPEDE trial,albuminuria improved in the surgicalgroups even with reduced usage ofrenin-angiotensin system blockers. This suggests thatbariatric surgery can help delay the progression ofkidney disease in T2DM[10].
Other studies have looked at outcomes other than micro-and macro-vascular endpoints.Neff etal.like others,reported thatbariatric surgery can benefitmetabolic and cardiovascular diseases but also found improvements in functional levels and socioeconomic factors.This was studied using the modified Kingˊs Obesity Staging System which takes into accountphysical,psychological,socioeconomic,and functional domains[45].Hewittetal.found that pulmonary function improved significantly 5 years after bariatric surgery. They also reported significant improvements in symptomatic asthma and obstructive sleep apnea[46].
Although there is some data to support the role of bariatric surgery in reducing micro-and macro-vascular outcomes in obese patients with T2DM[47],furtherlong-term studies are required.Earlier medical intervention studies demonstrated that even shortperiods of tight glycemic control translate into micro-and macro-vascular benefits later[48,49].This occurs despite loss of the initialtightglycemic controland has been referred to as the‘legacy effect’[49].Itwill be especially interesting to see the effectof bariatric surgery on end organ complications in those patients with recurrence of T2DM after remission.
Conclusions
Indeed,there is now a plethora of sound evidence for the use of metabolic surgery in the treatment of obese type 2 diabetes patients.Early glycemic improvements are often seen postoperatively with a durable effect of remission beyond 5 years.About 20-25%ofthese patients willrelapse in the long term. Metabolic improvements with reduction in microvascular and macrovascular DM complications long term have also been reported after metabolic surgery.
T2DM is a chronic disorder characterized by progressive pancreatic beta-cellloss and dysfunction that can begin up to 5-10 years prior to its clinicalmanifestation and diagnosis[50].In follow-up ofT2DMpatientsafter bariatric surgery,the‘‘reversal’’ofthispathophysiologicalstate should be regarded in the same manner as its development.While there is a dramatic improvement of glycemic control involving even remission in the shortand medium-term,outcomes of glycemic control after bariatric surgery should be viewed in the longterm,as with allchronic diseases.The potentialfor lack of remission and relapse afterremission is a clear concern and allphysicians involved in the management of the T2DM patient undergoing bariatric surgery should bear this in mind.
Hence,Buse et al.recommended that there be at leasta 5-year continuous state of complete remission before a person with known T2DM can be considered potentially‘‘cured’’[12].Therefore,patients with DM who have undergone bariatric surgery should be treated holistically like any other T2DM patient prior to that. They should be followed up regularly foratleast5 years after DMremission.Appropriate hypoglycaemic agents should be instituted(orreinstituted)if there is residual diabetes or any sign of relapse into the diabetic range even though the optimalhypoglycaemic agentin this group of patients has not been well studied[51].In addition,blood pressure and lipid-lowering medications to achieve the appropriate targets for T2DM patients should be wellmanaged.Regularscreening for microand macro-vascular DM complications should continue and appropriate preventive and treatment measures taken[12].Hence,the multi-disciplinary team involved in the care of the patientundergoing metabolic surgery should consistofa physician experienced in the managementof T2DM patients.
As physicians looking after patients with diabetes, we mustfocus on glycemic control.However,itwould be remiss to not address all of the medical problems that often accompany T2DM.More randomized controlled studies are needed to examine the long-term effects ofbariatric surgery on allthe comorbidities that patients with T2DM suffer,especially hypertension and hyperlipidemia.In addition,the long-term outcomes in these patients in terms of macrovascular and microvascular disease need to be studied,as the ultimate goal of treatment is to reduce the burden of morbidities associated with T2DM.Long-term quality oflife and socio-economic benefits along with healthcare utilization and costs for various healthcare systems involved should also be carefully studied.Only then will we be able to know the true long-term benefits of metabolic surgery.
[1]UK Prospective Diabetes Study(UKPDS)Gro up. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of comp licatio ns in p atien ts with typ e 2 diab etes (UKPDS 33).Lancet 1998;352(9131):837-853.
[2]ACCORD Study Group,Gerstein HC,Miller ME,et al. Long-term effects ofintensive glucose lowering on cardiovascular outcomes.N Engl J Med 2011;364(9):818-828.
[3]American Diabetes Association.Standards of medical care in diabetes-2013.Diabetes Care 2013;36(Suppl 1): S11-S66.
[4]Dixon JB,Zimmet P,Alberti KG,etal.Bariatric Surgery: An IDF statementfor obese Type 2 diabetes.Diabet Med 2011;28(6):628-642.
[5]Rubino F.Is type 2 diabetes an operable intestinal disease?A provocative yet reasonable hypothesis.Diabetes Care 2008;31(Suppl 2):S290-S296.
[6]Pories WJ,Swanson MS,MacDonald KG,et al.Who would have thought it?An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222(3):339-350.
[7]Dixon JB,OˊBrien PE,Playfair J,etal.Adjustable Gastric Banding and Conventional Therapy for Type 2 Diabetes. JAMA 2008;299(3):316-322.
[8]Mingrone G,Panunzi S,De Gaetano A,et al.Bariatric Surgery versus Conventional Medical Therapy for Type 2 Diabetes.N Engl J Med 2012;366(17):1577-85.
[9]Schauer PR,Kashyap SR,Wolski K,et al.Bariatric Surgery versus Intensive Medical Therapy in Obese Patients with Diabetes.N Engl J Med 2012;366(17): 1567-76.
[10]Schauer PR,Bhatt DL,Kirwan JP,etal.Bariatric Surgery versus Intensive Medical Therapy for Diabetes-3 Year Outcomes.N Engl J Med 2014;370(21):2002-2013.
[11]Buchwald H,Avidor Y,Braunwald E,et al.Bariatric Surgery-A Systemic Rev iew an d Analy sis.JAMA 2004;292(14):1724-1737.
[12]Buse JB,Caprio S,Cefalu WT,et al.How do we define cure of diabetes?Diabetes Care 2009;32(11):2133-2135.
[13]Ikramuddin S,Korner J,Lee WJ,etal.Roux-en-YGastric Bypass vs Intensive Medical Managementfor the Control of Type 2 Diabetes,Hypertension,and Hyperlipidemia-The Diabetes Surgery Study Randomized Clinical Trial. JAMA 2013;309(21):2240-2249.
[14]Abbatini F,Capoccia D,Casella G,et al.Long-term remission of type 2 diabetes in morbidy obese patients after sleeve gastrectomy.Surg Obes Relat Dis 2013;9 (4):498-502.
[15]Jimenez A,Casamitjana R,Flores L,et al.Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects.Ann Surg 2012;256(6):1023-1029.
[16]Boza C,Gamboa C,Salinas J,et al.Laparoscopic Rouxen-Y gastric bypass versus laparoscopic sleeve gastrectomy:a case-control study and 3 years of follow-up. Surg Obes Relat Dis 2012;8(3):243-249.
[17]Zachariah SK,Chang P,Ooi ASE,et al.Laparascopic Sleeve Gastrecto my fo r Morbid Ob esity:5 Years Experience from an Asian Center of Excellence.Obes Surg 2013;23(7):939-946.
[18]DiGiorgi M,Rosen DJ,Choi JJ,et al.Re-emergence of diabetes after gastric bypass in patients with mid-to long-term follow-up.Surg Obes Relat Dis 2010;6(3): 249-253.
[19]Brethauer SA,Aminian A,Romero-Talamas H,etal.Can Diabetes Be Surgically Cured?Longterm Metabolic Effects of Bariatric Surgery in Obese Patients with Type 2 Diabetes Mellitus.Ann Surg 2013;258(4):628-636; discussion 636-637.
[20]Sjostrom L,Lindroos AK,Peltonen M,et al.Lifestyle, diabetes,and cardiovascularrisk factors 10 years afterbariatric surgery.N Engl J Med 2004;351(26):2683-2693.
[21]Lee WJ,Chong K,Chen JC,et al.Predictors of diabetes remission after bariatric surgery in Asia.Asian J Surg 2012;35(2):67-73.
[22]Lee WJ,Hur WY,Lakdawala M,et al.Diabetes surgery score for metabolic su rgery.Surg Ob es Rela t Dis 2013;9(3):379-384.
[23]Kashyap SR,Bhatt DL,Wolski K,etal.Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes.Diabetes Care 2013;36(8):2175-2182.
[24]Dutia R,Brakoniecki K,Bunker P,etal.Limited recovery ofβ-cellfunction after gastric bypass despite clinicaldiabetes remission.Diabetes 2014;63(4):1214-1223.
[25]Nannipieri M,Mari A,Anselmino M,et al.The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery.J Clin Endocrinol Metab 2011;96(9):E1372-E1379.
[26]Isbell JM,TamboliRA,Hansen EN,etal.The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33(7):1438-1442.
[27]Lim EL,Hollingsworth KG,Arbisala BS,et al.Reversal of type 2 diabetes:normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol.Diabetologia 2011;54(10):2506-2514.
[28]Miras AD,le Roux CW.Can medical therapy mimic the clinical efficacy or physiological effects of bariatric surgery?Int J Obes(Lond)2014;38(3):325-333.
[29]Salehi M,Prigeon RL,DˊAlessio DA.Gastric bypass surgery enhances glucagon-like peptide 1 stimulated postprandial insulin secretion in humans.Diabetes2011;60(9):2308-2314.
[30]Jimenez A,Casamitjana R,Flores L,et al.GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Ygastric bypass surgery in morbidly obese subjects.Ann Surg 2013;257(5):894-899
[31]Shaw JE,Sicree RA,Zimmet PZ.Global estimates of the prevalence of diabetes for 2010 and 2030.Diabetes Res Clin Pract 2010;87(1):4-14.
[32]Ramachandran A,Ching RWM,Snehalatha C.Diabetes in Asia.Lancet 2010;375(9712):408-418.
[33]Chen L,Magliano DJ,Zimmet PZ.The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives.Nat Rev Endocrinol 2012;8(4):228-236.
[34]Nyamdorj R,Pitk a¨niemi J,Tuomilehto J,et al.Ethnic comparison of the association of undiagnosed diabetes with obesity.Int J Ob 2010;34(2):332-339.
[35]Lee WJ,Wang W,Lee YC,et al.Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus:comparison of BMI>35 and<35 kg/m2.J Gastrointest Surg 2008;12(5):945-952.
[36]Dixon JB,Hur K-Y,Lee W-J,etal.Gastric bypass in type 2 diabetes with BMI<30:weightand weightloss have a major influence on outcomes.Diabet Med 2013;30(4): e127-e134.
[37]Malapan K,Goel R,TaiC,etal.Laparoscopic Roux-en-Y gastric bypass for nonobese type II diabetes mellitus in Asian patients.Surg Obes Relat Dis 2014;10(5):834-840. doi:10.1016/j.soard.2014.01.018.
[38]Lee WJ,Chong K,Ser KH,etal.Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus:a randomized controlled trial.Arch Surg 2011;146(2):143-148.
[39]Dixon JB,Chuang L,Chong K,etal.Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes.Diabetes Care 2013;36(1):20-26.
[40]Nathan DM.Long-term complications of diabetes mellitus.N Engl J Med 1993;328(23):1676-1685.
[41]Gaede P,Vedel P,Larsen N,et al.Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes.N Engl J Med 2003;348(5):383-393.
[42]Romeo S,Maglio C,Burza MA,et al.Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes.Diabetes Care 2012;35(12):2613-2617.
[43]Sjostrom L,Narbro K,Sjostrom CD,etal.Effects ofbariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357(8):741-52.
[44]Hou CC,Shyu RS,Lee WJ,etal.Improved renalfunction 12 months after bariatric surgery.Surg Obes Relat Dis 2013;9(2);202-206.
[45]Neff KJ,Chuah LL,Aashiem ET,et al.Beyond Weight Loss:Evaluating the multiple benefits of bariatric surgery after Roux-en-Y gastric bypass and adjustable gastric band.Obese Surg 2014;24(5):684-691.
[46]Hewitt S,Humerfelt S,Sovik TT,et al.Long-term improvements in pulmonary function 5 years after bariatric surgery.Obes Surg 2014;24(5):705-711.
[47]Johnson BL,BlackhurstDW,Latham BB,etal.Bariatric surgery is associated with a reduction in major macrovascularand microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus.J Am Coll Surg 2013;216(4):545-555.
[48]Holman RR,Paul SK,Bethel MA,et al.10-year followup of intensive glucose controlin type 2 diabetes.N Engl J Med 2008;359(15):1577-1589.
[49]Nathan DM,Cleary PA,Backlund J-AC,et al.Intensive diabetes treatment and cardiovascular disease in patients iwith type 1 diabetes.N Eng J Med 2005;353(25):2643-2653.
[50]UKPDS Group.UK Prospective Diabetes Study 16: Overview of six yearsˊtherapy of type 2 diabetes-a progressive disease.Diabetes 1995;44(11):1249-1258.
[51]Kashyap SR,Schauer P.Clinical considerations for the management of residual diabetes following bariatric surgery.Diabetes Obes Metab 2012;14(9):773-779.
✉Corresponding author:Kwang Wei Tham,the Obesity&Metabolic Unit,Department of Endocrinology,LIFE Centre,Singapore General Hospital,Singapore,e-mail:tham.kwang.wei@sgh.com.sg.
Received 09 September 2013,Accepted 22 November 2014,Epub 03 March 2015
The authors reported no conflictof interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140127
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Recent advances in bariatric/metabolic surgery:appraisalof clinical evidence
- Roux-en-Y gastric bypass for Chinese type 2 diabetes mellitus patients with a BMI<28 kg/m2:a multi-institutionalstudy
- Bariatric surgery in old age:a comparative study of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in an Asia centre ofexcellence
- Maternalinheritance of severe hypertriglyceridemia impairs glucose metabolism in offspring
- Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis
- Quercetin attenuates the development of 7,12-dimethylbenz(a) anthracene(DMBA)and croton oil-induced skin cancer in mice
