Quercetin attenuates the development of 7,12-dimethylbenz(a) anthracene(DMBA)and croton oil-induced skin cancer in mice
Huma Ali,Savita Dixit
Department of Chemistry,MANIT,Bhopal,M.P.462051,India.
Quercetin attenuates the development of 7,12-dimethylbenz(a) anthracene(DMBA)and croton oil-induced skin cancer in mice
Huma Ali,Savita Dixit✉
Department of Chemistry,MANIT,Bhopal,M.P.462051,India.
To evaluate the chemopreventive potential of quercetin in an experimentalskin carcinogenesis mouse model. Skin tumor was induced by topical application of 7,12-dimethyl Benz(a)anthracene(DMBA)and Croton oil in Swiss albino mouse.Quercetin was orally administered at a concentration of 200 mg/kg and 400 mg/kg body weight daily for 16 weeks in mouse to evaluate chemopreventive potential.Skin cancer was assessed by histopathological analysis.We found that quercetin reduced the tumor size and the cumulative number of papillomas.The mean latent period was significantly increased as compared to carcinogen treated controls.Quercetin significantly decreased the serum levels of glutamate oxalate transaminase,glutamate pyruvate transaminase,alkaline phosphatase and bilirubin.It significantly increased the levels of glutathione,superoxide dismutase and catalase.The elevated level of lipid peroxides in the controlgroup was significantly inhibited by quercetin.Futhermore, DNA damage was significantly decreased in quercetin treated mice as compared to DMBA and croton oil treated mice.The results suggestthatquercetin exerts chemopreventive effecton DMBA and croton oilinduced skin cancer in mice by increasing antioxidantactivities.
quercetin,papilloma,skin carcinogenesis,chemoprevention
Introduction
Ocimum sanctum Linn,commonly known as Tulsi, belongs to the Lamiaceae family.It has a variety of constituents e.g.saponins,flavonoids,triterpenoids, tannins,eugenol,vicenin-2,dimethylbenzene,myrecene, ethylbenzene,limocene,linoleic acid,aesculin,and others[1].Hence,it is an essential plant used in the preparation ofseveralayurvedic pharmacologicalproducts. A wide range of beneficial effects have been reported such as anti-cancer,analgesia and anti-inflammation. Leaves of Ocimum sanctum were investigated for its effect on male reproductive function(sperm count and reproductive hormones)in male albino rabbits.Ithas a good wound healing property[2].
In vitro and in vivo experiments have demonstrated that flavanoids produce a wide range of biological effects in severalmammalian cell systems.Quercetin is a polyphenolic flavanoid extensively present in plants and plantfood sources,such as Ocimum sanctum.This flavanoid has a broad range of biological activities,including anti-inflammatory,antioxidant, neuroprotective and antiviral activity.Quercetin has antioxidant properties and therefore may play an important role in preventing cancer.Some researchers reported that q uercetin acts as an oxygen radicalscavenger and an inhibitor of xanthine oxidase and lipid peroxidation in vitro.Independently and with ascorbic acid,quercetin reduced the occurrence ofskin oxidative damage and inhibited neuron damage caused by glutathione depletion.Earlier studies has investigated quercetin in animalmodels and cancercelllines, including cancerofthe breast,the colon,and the ovary, and reported that ithas antiproliferative effects[3].The ability of quercetin to inhibit inflammatory leukotriene production may be a key to its beneficial impact on anti-inflammatory activity.The anti-inflammatory activity ofquercetin is due to its antioxidantand inhibitory effects on inflammation-producing enzymes, such as cyclooxygenase and lipoxygenase and subsequentinhibition of inflammatory mediators,including leukotrienes and prostaglandins.Inhibition of histamine release by mast cells and basophils also contributes to the anti-inflammatory activity of quercetin[4]. Quercetin is a strong inhibitor of human lens aldose reductase[5].
The current study was aimed to understand the chemopreventive potential of quercetin isolated from Ocimum sanctum using a mouse modelofskin cancer induced by 7,12-dimethylbenz(a)anthracene(DMBA) and croton oil.
Materials and methods
Plant material and preparation of the methanolic extract
Leaves of Ocimum sanctum were collected from Sanjeevni,Bhopal,M.P.,India.Plant material was taxonomically identified by Dr.Zia-UL-Hassan, Department of Botany,Saifia College of Science, Bhopal.Voucher specimen(351/Bot/Saifia/12)was preserved in the above herbarium for future reference. The leaves were dried undershade and pulverized using electric grinder.Firstly,dried sample was extracted with differentsolvents,such as petroleum ether,benzene, acetone,ethanol,methanol and hot water by using soxhletapparatus.Then,the methanolic extractofleaves was fractionated by column chromatography.Slurry of silica gelwith 60-120 mesh size prepared in petroleum ether was added to the column.Methanolic extract (15.0 gm)was loaded on the column and then eluted with various solvents,including petroleum ether,benzene,chloroform,chloroform:acetone(1:1),acetone, acetone:ethanol(1:1),ethanol,methanol and distilled water in the order of their increasing polarity.The methanolic fraction was preserved in refrigerator to obtain a yellow powder(2.2 gm)[6].
Characterization of quercetin
Isolated compound was analyzed using infrared, gas chromatography mass spectrometry and nuclear magnetic resonance spectroscopy.IR spectralanalysis showed a broad peak for O-H stretch hydrogen bonded from 3412-2712 cm-1.The 1664,1649,1560 and 1450 cm-1showed C=C stretch of benzene and 1242 cm-1for C-O stretch.The 941,864,823,794 and 702 cm-1showed peaks for substituted benzene.1H NMR spectrum ofthe isolated compound exhibited proton signals atδ7.23(1H,H-6),δ7.28(1H,H-8), δ7.02(1H,H-2ˊ),δ7.33(1H,H-5ˊ),δ7.54(1H,H-6ˊ); δ3.56(1,OH-5),δ3.51(1,OH-7),δ2.19(1,OH-3), δ2.03(1,OH-3ˊ)andδ1.56(1,OH-4ˊ).The negative-ion electrospray ionization(ESI)mass spectrum of the isolated compound showed a quasi molecular ion [M-H]-at m/e 303,indicating a relative molecular weight of 302.The data of spectroscopic analysis revealed the presence ofa high contentofthe flavonoid 3,3ˊ,4ˊ,5,7-pentahydroxyflavone(quercetin).This material was further purified by recrystalization with methanol to obtain quercetin(99%purity).
Chemicals
DMBA and croton oilwere purchased from Sigma Aldrich(StLouis,MO,USA).Otherchemicalreagents were commercially available and of analytical grade.Animals
Swissalbino miceofeithersex were randomly selected from the animal house of the Pinnacle Biomedical Research Institute(PBRI),Bhopal.Animals were housed in polypropylene cages with sterile husk and provided standard pellet(Golden Feeds,New Delhi,India)and water with ad libitum access.The animals were maintained with a 12 hour light/dark cycle at 22±2°C at controlled condition.The study protocol was approved by the local institutional review board at the authorsˊ affiliated insituttion.Animalwelfare and the experimental procedures were carried outstrictly in accordance with the Guide for Care and Use of Laboratory Animals of the Institutional Animal Ethics Committee(IAEC)of PBRI,Bhopal(Reg No.1283/c/09/CPCSEA).
Determination ofeffectofquercetin on DMBA/ croton oilinduced skin carcinogenesis
Four groups(6 animals per group)of Swiss albino mice were used forthe study.Mice were dorsally shaved with hair clipper.Group Imice were given MiliQ water (10 mL/kg body weight),a normal diet and tap water with ad libitum access daily.After 16 weeks,mice wereautopsied and the skin of the dorsal area was taken for histopathologicalstudies and blood for biochemical analysis.Group IImice were treated with a single dose of DMBA(100μg/100μL ofacetone)overthe shaven area of the skin ofthe mice;thereafter,1%croton oil was applied to skin 3 times a week up to 16 weeks. After the single dose of DMBA,group IIImice were treated with quercetin(200 mg/kg)orally each day untill completion of the experiment and 1%croton oil was applied on the skin 3 timesa week 1 hourafterquercetin was administered.Group IV mice were treated with the same procedure as that of group III,butthe dose of quercetin was 400 mg/kg[7].After 14 days of DMBA application,mice were observed each week forincidence and size ofskin tumors,and the body weightand average latent period were recorded for 16 weeks[8].
Biochemical measurements
Mice were sacrificed by cervicaldislocation on the last day of the experiment.The skin was removed and washed in ice-cold saline(0.9%NaCl).Then,it wasweighed and blotted dry.A 10%tissue homogenate wasprepared from the skin in 0.15 mol/L Tris-KCL(pH 7.4)and was centrifuged at 12000 rpm for 15 minutes for clarification.The levels of lipid peroxidase(LPO), GSH(Moron MA),SOD(Marklund)and catalases (Clainborn)were determined as described[9-12].
Furthermore,blood was collected from allmice via the retro-orbitalplexus.Atroom temperature,the blood sampleswere clotted for45 minutes.Serum wasseparated by centrifugation at2500 rpm at30°Cfor15 minutesand utilized for the determination of various biochemical parameters including serum glutamate oxalate transaminase(GOT),glutamate pyruvate transaminase (GPT),alkaline phosphatase(ALP)and bilirubin by using the Span Diagnostic Kit.
Histopathology
Paraffin blocks were routinely prepared for processing formalin fixed tissue samples.Tissue sections(3-4μm)were performed for hematoxylin-eosin(H&E) staining and epidermal thickness was determined by microscopic examination of H&E-stained tissue samples.
Alkaline single cell gel electrophoresis(SCGE)was performed as a 3-layer procedure[13]with slight modification[14].The lymphocytes were separated from blood using histopaque density gradientcentrifugation and were diluted 20-fold forthe Cometassay.Viability of the lymphocytes was evaluated by trypan blue exclusion[15].Cells with viability>84%were further processed for Comet assay.In brief,15μL cell suspension(approx.20,000 cells)was mixed with 85μL 0.5%low melting-point agarose and layered on one end of a frosted glass slide,coated with a layer of 200μL of 1%normalagarose.Itwas covered with a third layer of 100μL low melting-pointagarose.After solidification of the gel,the slides were immersed in lysing solution(2.5 MNaCl,100 mmol/LNa2EDTA, 10 mmol/LTris,p H 10 with 10%DMSO and 1% Triton X-100 added fresh)overnightat4°C.The slides were then placed in a horizontal gel electrophoresis unit,immersed in fresh cold alkaline electrophoresis buffer(300 mmol/L NaOH,1 mmol/L Na2EDTA and 0.2%DMSO,pH>13.5),and left in solution for 20 minutes at 4°C for DNA unwinding and conversion of alkali-labile sites to single strand breaks. Electrophoresiswas carried outusing the same solution at 4°C for 20 minutes,using 15 V(0.8 V/cm)and 300 mA.The slides were neutralized gently with 0.4 mol/L Tris buffer at p H 7.5 and stained with 75μL ethidium bromide(20μg/mL).For positive control,lymphocytes cells were treated with 100μM H2O2for 10 minutes at 4°C.Two slides per mouse were prepared and 25 cells per slide(250 cells per group)were scored randomly and analyzed using an image analysis system(Komet-5.5,Kinetic Imaging) attached to a fluorescent microscope(Leica)equipped with appropriate filters.The parameter selected for quantification of DNA damage was percenttail DNA as determined by the software.Statistical analysis
Values were recorded as mean±SD.The data obtained from different groups was an alyzed by ANOVA.P<0.05 was considered statistically significantfor allexperiments.
Results
The normalcontrolmice showed normalstructure in the dermal layer(Fig.1A).In DMBA and croton oil treated mice(Fig.1B),the layers started differentiating in the form of papilloma with signs of abnormal architecture ofthe epidermallayerdue to irregularproliferation of stratum spinosum cells,with abnormal thickening of the stratum corneum and stratum spinosum.Displastic changes in the squamous layer,damage in the stroma,hyperkeratosis,acanthosis and cysts with horns were observed in the DMBA and croton oil treated controlgroup.In quercetin treated animals,histological observation revealed that signs of tumor were present,buthyperkeratosis and acanthosiswere present, which,however,were lessascompared to controltreatment(Fig.1C and D).
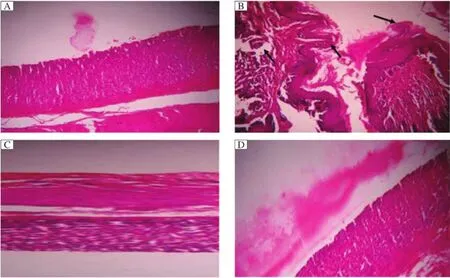
Fig.1H&E stained cross-sections of mouse skin.A:Group I(water),B:Group II(water+DMBA+croton oil),C:Group III(quercetin 200 mg/ kg+DMBA+croton oil),D:Group IV(quercetin 400 mg/kg+DMBA+croton oil).Arrow(→)shows damage in tissue.
The outcomes of the present study are shown in Table 1,2and 3.A gradualdecrease in body weight was found in allmice of the differentgroups.Mice of group III and IV,given a continuous treatment of differentdoses of quercetin orally as mentioned above along with the repeated applications of croton oil, showed a significantreduction in the cumulative number ofpapillomas and tumorsize(Table 1)ascompared to the controlgroup.The latency period was found to be 10.10±5.17 weeks in the carcinogen treated control group,whereasitwassignificantly longerin the quercetin treated groups.
A significant increase in GSH,SOD and catalase was found in the skin of quercetin administered mice (Group III and IV)than the control mice(Group II) (Table 2).On the contrary,lipid peroxide levelwas decreased significantly in quercetin administered mice as compared to the controlmice.A significantdecrease in serum glutamate oxalate transaminase and GPT,ALP and bilirubin levelwas found in quercetin administered mice(Group IIIand IV)than the controlmice(Group I) (Table 3).
DNA damage was measured as%tail DNA in the controland treated groups.Higher DNA damage was observed in lymphocytes of DMBA and croton oil treated mice than quercetin treated Group III and IV mice(Fig.2).
Discussion
Human epidemiological data indicate that regular use ofcertain medicinalplants suppresses carcinogenesis in various organs[16].Therefore,it is becoming increasingly importantto screen natural products which mightsuppressorreverse the processofcarcinogenesis[17]. The resultsofthe currentstudy and otherstudiesindicate severalbeneficialeffects ofquercetin.Skin carcinogenesis is based on the factthatinitiation with sequential application of a subthreshold dose of a carcinogen such as DMBA,followed by repetitive treatments with anoncarcinogenic promoterlike croton oilwilllead to the developmentof skin tumors.Among the initiation and promotion steps,animalstudies show thatthe promotion stage takes longer period to occur and itis reversible initially[18].Therefore,cancer prevention by inhibition of tumor promotion is expected to be a resourceful approach.In the presentstudy,quercetin administration could significantly inhibit DMBA induced papilloma formation by the incidence oftumor and the mean number ofpapillomas.
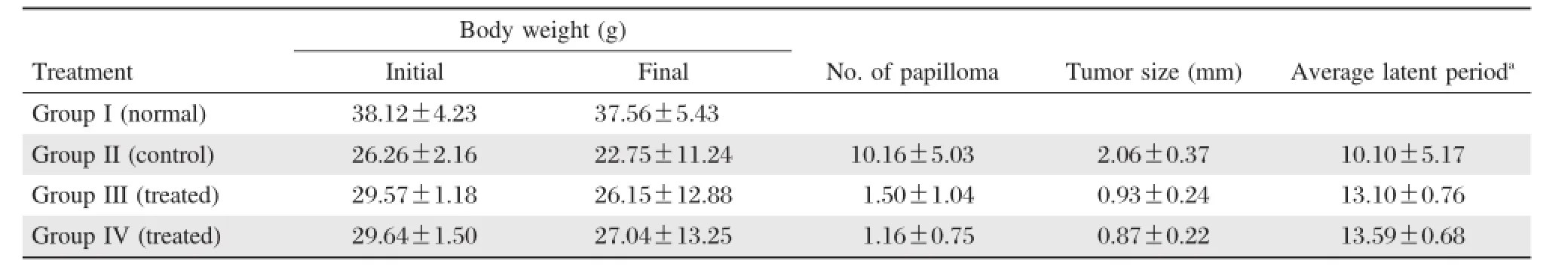
Table 1 Chemopreventive effect of quercetin on DMBA croton oil induced skin carcinogenesis in mice.

Table 2 Effect of quercetin on oxidative enzyme levels.
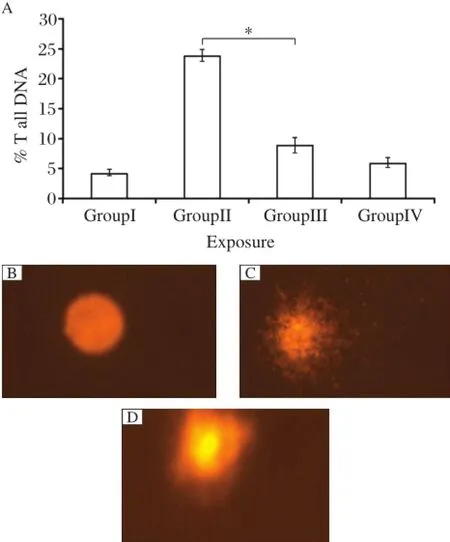
Fig.2 DNA damage in lymphocytes after exposure to quercetin.A:%tail DNA.B:Group I.C:Group II.D:Group III. Each value represents the mean±S.E.of 3 experiments.*P<0.05.
Lipid peroxidation is a free radical chain reaction and is known to cause 2 main steps of carcinogenesis i.e.initiation and propagation.Itis a highly destructive process.During the carcinogenic process,lipid peroxidation is increased and more complex and reactive compounds such as malondialdehyde(MDA)and 4-hydroxynonenal were obtained.These products of lipid peroxidation were observed to be mutagenic and carcinogenic.Therefore,agents that can reduce the production of free radicals in vivo may be considered to have the potential for chemoprevention[19].In the present study,administration of quercetin significantly reduced the level of LPO in mice exposed to DMBA and croton oil,and subsequently decreased the incidence of skin tumor.
GSH plays a crucial role in protecting cells against reactive oxygen species and free radicalproduced even in normalmetabolism.In the controlgroup,the activity of GSH was decreased while quercetin treatment increased the GSH level,which clearly suggests their antioxidant property.Antioxidants were reported to possess a chemopreventive property[20].Antioxidants are generally regarded as the first line of defense againstfree radicalstress and are usefulin eliminating the risk of oxidative damage induced during carcinogenesis.SOD and catalase are acting as mutually supportive antioxidative enzymes,which provide protective defense against reactive oxygen species(ROS)[21]. There was a significant enhancement in the levels of GSH,SOD,and catalases level in the quercetin treated group compared to the control group.
ROS plays an importantrole in the process ofapoptosis. ROS induces permeabilization of the outer mitochondrial membrane,which releases soluble proteins from the intermembrane space into the cytosol,where they promote caspase activation.ROS typically includes superoxide radical(O2-),H2O2,and the hydroxyl radical (OH˙),which cause damage to cellularcomponents,such as DNA,and ultimately lead to apoptotic celldeath.
DMBA treatments generate LPO and ROS in the affected area ofthe skin and ultimately lead to carcinogenesis.This oxidative stress was easily observed in the controlgroup as levelof LPO was higher and the levels of catalase,SOD and GSH were lower.The beneficial action of quercetin is probably due to its ability to stimulate antioxidantenzymes in the cells. This increase in enzyme activity effectively reduced the generation of ROS and LPO in the skin and thus might reduce the incidences of skin papillomas on the treated areas.

Table 3 Effect of quercetin on serum enzyme levels.
In conclusion,quercetin isolated from Ocimum sanctum could significantly enhance antioxidant enzyme levels,including GSH,SOD,and catalase,and inhibited lipid peroxides,hence showing itsrole in detoxification pathway.Both histology and enzyme activities suggestthatenvironmentaleffectsthatlead to skin carcinogenesiscan be inhibited by oralcombination ofquercetin in the daily dietto achieve some protection against skin cancer.The results from the presentstudy indicate thatquercetin can inhibitpapilloma growth.
Acknowledgement
The authors are gratefulto Dr.Gajendra Dixit,Dean R&C,MANIT,Bhopal,India(GrantNo.Dean R&C/ 2010/159)and Manvendra Karchuli,Research Officer, PBRI,Bhopal,India to support this research and that provided facilitiesto achievethedesired goalsofthe study.
[1]Joseph B,Nair VM.Ethanopharmacological and phytochemical aspects of Ocimum sanctum Linn-the elixir of life.BJPR 2013;3:273-292.
[2]Singh V,AmdekarS,Verma O.Ocimum sanctum(tulsi):biopharmacologicalactivities.Webmed Central Pharmacology 2010;1:1-7.
[3]Buer CS,Imin N,Djordjevic MA.Flavonoids:new roles for old molecules.JIPB 2010;52:98-111.
[4]JoshiUJ,Gadge AS,DˊMello P,etal.Anti-inflammatory, antioxidant and anticancer activity of quercetin and its analogues.IJRPBS 2011;2:1756-1766.
[5]Chaudry PS,Cabera J,Juliani HR,et al.Inhibition of human lens aldose reductase by flavonoids,sulindac, and indomethacin.Biochem Pharmacol 1983;32:1995-1998.
[6]Chand MM,Patni V.Isolation and Identification of flavonoid‘‘Quercetin’’from Citrullus colocynthis(Linn.) Schrad.Asian J.Exp.Sci 2008;22:137-142.
[7]Shoskes DA,Zeitlin SI,Shahed A,etal.Quercetin in men with category III chronic prostatitis:a preliminary prospective,double blind,placebo-controlled trial.Urology 1999;54:960-963.
[8]Chaudhary G,Parmar J,Verma P,et al.Inhibition of DMBA/Croton oil induced tumorigenesis in Swiss albino mice by Aloe vera treatment.IJBMR 2011;2:671-678.
[9]Das I,Saha T.Effect of garlic on lipid peroxidation and antioxidation enzymes in DMBA-induced skin carcinoma.Nutrition 2009;25:459-471.
[10]Pandey S,Agrawal RC.Effectof Bauhinia Variegate bark extract on DMBA-induced mouse skin arcinogenesis:a preliminary study.GJP 2009;3:158-162.
[11]Blessy D,Suresh K,Manoharan S,et al.Evaluation of chemopreventive potential of Zingiber Officinale Roscoe ethanolic root extract on 7,12-dimethyl Benz[a]anthracene induced oral carcinogenesis.Res.J.Agric.&Biol.Sci 2009;5:775-781.
[12]Das I,Das S,Saha T.Saffron suppresses oxidative stress in DMBA-induced skin carcinoma:a histopathological study.Acta histochem 2010;112:317-327.
[13]Singh NP,McCoy MT,Tice RR,etal.A simple technique for quantization of low levels of DNA damage in individual cells.Exp Cell Res 1988;175:184-191.
[14]Ali D,Ray RS,Hans RK.UVA-induced cyototoxicity and DNAdamaging potentialof Benz(e)acephenanthrylene in human skin cellline.Toxicol Lett 2010;199:193-200.
[15]Anderson D,Yu TW,Phillips BJ,etal.The effectof various antioxidants and other modifying agents on oxygenradical generated DNA damage inhuman lymphocytes in the comet assay.Mutat Res 1994;307:261-271.
[16]Mouli KC,Vijaya T,Rao SD.Phytoresources as potential therapeutic agents for cancer treatment and prevention. JGPT 2009;1:4-18.
[17]Roslida AH,Fezah O,Yeong LT.Suppression of DMBA/ Croton Oil-induced mouse skin tumor promotion by Ardisia Crispa root hexane extract.APJCP 2011;12: 665-669.
[18]Klaunig JE,Kamendulis LM,Hocevar BA.Oxidative stress and oxidative damage in carcinogenesis.J Toxicol Pathol 2010;38:96-109.
[19]Ekin S,Ozdemir H,Demir H,etal.Plantago major protective effects on antioxidantstatus after administration of 7,12-Dimethylbenz(a)anthracene in rats.APJCP 2011; 12:531-535.
[20]Blessy D,Suresh K,Manoharan S,et al.Evaluation of chemopreventive potential of Zingiber Officinale Roscoe ethanolic root extract on 7,12-dimethyl Benz[a]anthracene induced oral carcinogenesis.Res.J.Agric.&Biol.Sci 2009;5:775-781.
[21]Parmar J,Sharma P,Verma P,et al.Anti-tumor and antioxidative activity of R osmarinus officinalis in 7,12 dimethyl Benz(a)anthracene induced skin carcinogenesis in mice.Am.J.Biomed.Sci 2011;3:199-209.
✉Corresponding author:Dr.Savita Dixit,Professor,Department of Chemistry,MANIT Bhopal,M.P.462051,India.Tel/Fax: 00919425009287/0755-2670562,E-mail:savitadixit1@yahoo.com.
Received 04 March 2013,Revised 06 August 2013,Accepted 01 October 2013,Epub 15 December 2014
The authors reported no conflictof interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20130025
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Recent advances in bariatric/metabolic surgery:appraisalof clinical evidence
- Metabolic bariatric surgery and type 2 diabetes mellitus:an endocrinologistˊs perspective
- Roux-en-Y gastric bypass for Chinese type 2 diabetes mellitus patients with a BMI<28 kg/m2:a multi-institutionalstudy
- Bariatric surgery in old age:a comparative study of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in an Asia centre ofexcellence
- Maternalinheritance of severe hypertriglyceridemia impairs glucose metabolism in offspring
- Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis
