Maternalinheritance of severe hypertriglyceridemia impairs glucose metabolism in offspring
Ya-Hong Ma,Caiguo Yu,Abudurexiti Kayoumu,Xin Guo,Zhili Ji,George Liu,✉
1Department of Endocrinology,Beijing Puren Hospital,Beijing,China;
2Institute of Cardiovascular Sciences,Peking University and Key Laboratory of Cardiovascular Sciences,Administration of Education,Beijing,China;
3Department of Endocrinology,Luhe Teaching Hospital of Capital Medical University,Beijing;
4Department of General Surgery,Luhe Teaching Hospital of Capital Medical University,Beijin g.
Maternalinheritance of severe hypertriglyceridemia impairs glucose metabolism in offspring
Ya-Hong Ma1,△,Caiguo Yu2,3,△,Abudurexiti Kayoumu2,Xin Guo2,Zhili Ji4,✉,George Liu2,✉
1Department of Endocrinology,Beijing Puren Hospital,Beijing,China;
2Institute of Cardiovascular Sciences,Peking University and Key Laboratory of Cardiovascular Sciences,Administration of Education,Beijing,China;
3Department of Endocrinology,Luhe Teaching Hospital of Capital Medical University,Beijing;
4Department of General Surgery,Luhe Teaching Hospital of Capital Medical University,Beijin g.
Maternally inherited familialhypercholesterolemia(FH)impairs glucose metabolism and increases cardiovascular risks in the offspring to a greater degree than paternal inherited FH.However,it remains unknown whether hypertriglyceridemia affects glucose metabolism via inheritance.In this study,we soughtto compare the impact of maternally and paternally inherited hypertriglyceridemia on glucose and lipid metabolism in mice.ApoCIII transgenic mice with severe hypertriglyceridemia were mated with non-transgenic control mice to obtain 4 types of offspring:maternalnon-transgenic controland maternaltransgenic offspring,and paternalcontroland paternal transgenic offspring.Plasma triglycerides(TG),total cholesterol(TC),fasting plasma glucose(FPG)and fasting insulin(FINS)were measured.ApoCIII overexpression caused severe hypertriglyceridemia,but the transgenic female mice had unaltered fertility with normal pregnancy and birth of pups.The 4 groups of offspring had similar birth weightand growth rate.The plasma TG of maternaland paternal transgenic offspring were nearly 40-fold higher than maternal and paternal control mice,but there was no difference in plasma TG between maternal and paternal transgenic offspring.Although the FPG of the 4 groups of animals had no difference,the maternal transgenic mice showed impaired glucose tolerance,increased FINS levels and higher homeostasis model assessment insulin resistance index(HOMA-IR)than the other 3 groups.In conclusion,maternally inherited hypertriglyceridemia in ApoCIII transgenic mice displayed impaired glucose tolerance,hyperinsulinemia and increased HOMA-R, while paternally inherited hypertriglyceridemia did not have such impacts.
Apolipoprotein CIII,Transgenic mice,Hypertriglyceidemia,Insulin resistance.
Introduction
Large epidemiological studies have shown that hypertriglyceridemia(HTG)is an independentatherosclerosis risk factor[1],HTG is also associated with multiple organ damage,such as pancreatitis[2],renal dysfunction[3],impaired nervous system function[4]and impaired glucose regulation[5].The major causesfor the developmentof HTG are related to impaired metabolism of triglyceride-rich lipoproteins(TRLs) and is one ofmain components ofmetabolic syndrome. In general,environmental factors,such as high-fat, high-carbohydrate diet and lack of physicalactivities, play importantroles in HTG occurrence.Genetic factors like gene variants of the lipoprotein lipase(LPL) which hydrolyze triglycerides,and apolipoproteins (Apos)thatmodulate LPL activity and hepatic secretion of very low-density lipoprotein(VLDL),can all be operational through the interaction with environmental factors influencing the occurrence and extent of HTG.
Ithas been noticed thatHTG is also often accompanied with pregnancy because the metabolism of glucose and lipid will change significantly in the course ofpregnancy,with increasing concentrations ofestrogen and insulin resistance.In the midterm of pregnancy,fat storage starts to increase with concomitant reduction oflipolysis,and adipose tissue willnormally increase 1.5 to 2 fold in the third trimesterin comparison to non-pregnant women.Plasma triglycerides (TG)will also rise 2-3 times,up to 200-300 mg/dL. It then gradually decreases to pre-pregnant levels 6 weeks after term[6].If women have HTG before pregnancy,they tend to have severe HTG during pregnancy,which may induce acute pancreatitis with higher maternal and perinatal mortality,along with fetal malformation,miscarriage,premature birth and retarded growth rate.Other adverse pregnancy outcomes are also significantly higher[7,8].
As an inhibitor of LPL[9],high plasma levels of ApoCIII are closely related to severity of human HTG[10],while low plasma levels of ApoCIIIare associated with low plasma TG and even with decreased incidence ofcoronary artery disease(CAD)as defined by angiography[11].In addition,intracellular ApoCIII stimulates hepatic secretion of TG-rich lipoprotein via enhanced packaging of larger TG-rich lipoprotein, VLDL1.Such stimulatory effect has been shown to be through the 2 charged amino acid residues(Lys 58 and Lys 60 inα-helix 5)and potentially resulted in more TG-rich lipoproteins into plasma pool,hence aggravating HTG[12].
Therefore,the ApoCIII transgenic mice with plasma TG levels 5-10 times higher than non-transgenic littermates would serve as an ideal model to study HTG and its related abnormalities[13]because most other animals,when fed high fat diet,would only have plasma TG levels at 50%-2 times higher than that of chow diet controls[14].
Although both genetic and environmental factors play important roles in the development of HTG, transgenic ApoCIII mice were often used as genetic HTG model.Owing to the inherited nature of transgenesis,one should bear in mind that the severity of phenotypes in the offspring may not be identical.In human studies,it was found that maternally inherited familial hypercholesterolemia(FH)offspring would have higher cholesterol levels and cardiovascular risk than thatof paternally inherited offspring[15].The same results were also found in the hypercholesterolemic Apo E-deficient mice[16].However,it is unknown whether maternal or paternal inheritance would have differenteffects on the severity of HTG,which is closely related to hypercholesterolemia.Therefore,in the present study,severe HTG ApoCIII transgenic male and female mice were mated with the normal male and female mice,respectively,to observe whether the female HTG mice would have normalpregnancy and parturition.If either normal or ApoCIII transgenic females would be able to give birth,we willhave some normal and some HTG transgenic offspring,which would allow us to study the impactof maternaland paternalinheritance on severity of HTG and the glucose metabolism which is closely related to TG metabolism.
Materials and methods
Animals
ApoCIII transgenic mice in C57B6 background were from Jackson Laboratories,United States.They were bred with ICR mice to 5 generations to obtain ApoCIII transgenic mice with ICR background forlargerlittersize and easy reproduction.Genotyping ofthe parental mice was performed by PCR using genomic DNA obtained from the clipped tail.Primers used for the Apo CIII gene were 5ˊ-AGC TGG CAT AGC AGA GGT GT-3ˊ(forward),and 5ˊ-GCA GCC TCT CAT TTG GAA AG-3ˊ(reverse).Using a mixture of these primers,PCR was done with 35 cycles of 30 s of denaturation at 94°C,30 seconds of annealing at 65°C and 30 seconds of elongation at 72°C.The PCR product was 173 bp in size.
Totally 8 female HTG ApoCIII transgenic mice were mated with 8 normal ICR male mice,and 6 ApoCIII transgenic males with 6 normal ICR females to obtain 150 pups in 14 litters.Two litters from respective transgenic parents were taken randomly for assay of blood lipids,glucose and insulin.There were 13,10, 13 and 11 pups in each litter,respectively,and they were divided into 4 groups:maternal non-transgenic normal controls(MC)and transgenics(MT);paternal normal controls(PC)and transgenics(PT).Plasma TG and total cholesterol(TC)levels were measured after 2 hour fasting at 14 days after birth.
Measurement of plasma parameters
At 28 days after birth the blood from the above 4 groups ofpups were collected by retroorbitalbleeding after 4 hours fasting.TC,TG,glucose and insulin in plasma were measured with commercially available kits(Sigma,St.Louis,MO,USA for TC,TG and Glu;Linco Research Inc,MO,USA for insulin).In order to eliminate the influence of turbidity caused by severe HTG,plasma samples from PT and MT pups were ultracentrifugated at 20,000 rpm for 30 minutes at 4°C in a n Optima TM TLX Ultra ce ntrifuge (Beckman Coulter,Inc.,Brea,CA,USA)before glucose and insulin measurement.
Oral glucose tolerance tests(OGTT)
OGTT was performed by giving a glucose bolus(3 g/ kg)by gavage after16 hours fasting.Blood was collected by retroorbitalbleeding at0,30,60,90 and 120 minutes. Plasma glucose levelswere measured asdescribed above.
Western blot of human ApoCIII.
Plasma(1μL)was electrophoresed on 12%Bis-Tris PAGE gels.The size-separated proteins were transferred to nitrocellulose membranes(Millipore)and probed by a primary antibody of goat anti-human ApoCIII(#K74130G,Biodesign)at1:1000 dilution. A rabbitanti-goat HRP conjugated secondary antibody (#7074,CellSignaling Technology)at1:5000 dilution was chosen for color development and visualization.
Homeostasis model assessment insulin resistance index(HOMA-IR)
HOMA-IR was calculated as follows:HOMA-IR= (fasting insulin×fasting glucose)/22.5
Statistical analysis
The data showed a normal distribution and were expressed as means±standard deviations(SD). Significantdifferencesin parametersamong groupswere assessed by One-Way Analysis of Variance(One-Way ANOVA)and multiple comparison forpost-hocpairwise test.Significantdifferencesin comparison ofplasma triglycerides among 4 groups of mice 14 days were assessed by Kruskal-Wallis test.The statisticalanalysis was performed in SPSS 11.5(SPSS Inc,Chicago,IL, USA).Statisticalsignificance was defined as P<0.05.
Results
Normal fertility in both male and female ApoCIII transgenic mice with severe HTG.
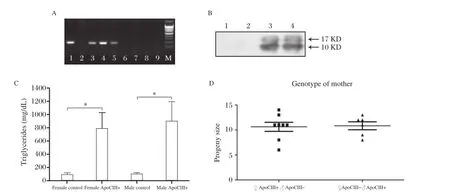
Fig.1Validation of parental ApoCIII transgenic mice and comparison of litter size.A.Genotyping of ApoCIIItransgenic mice.M.DNA marker;1,3,4 and 5,transgenic mice;2,6,7,8 and 9,none transgenic animals.B.Western blot of plasma probed by anti-human ApoCIII polyclonal antibody.1 and 2,none transgenic;3 and 4,transgenic.Arrows indicate molecular weight.C.Plasma TG levels in ApoCIII transgenic and nontransgenic control female and male mice.*P<0.01.D.Litter size of pups from female and male CIII transgenic parental mice.
Presence of human ApoCIII in parentaltransgenic mice were validated by both PCRgenotyping of genomic DNA,Western blotanalysis ofplasma and plasma TG levels,as shown in Fig.1 A,B and C.ApoCIII overexpression caused severe HTG butthe transgenic male mice had normalfertility and the transgenic female mice had normal fertility with normal pregnancy,birth of pups and lactation as well.Totally 8 female HTGApoCIII transgenic mice were mated with 8 normalmale mice to obtain 85 pups.At14 days afterbirth the blood of 85 pups were collected after 2 hours of fasting.TG were measured,47 pups TG levels<300 mg/dL,which were defined asthe MC group;38 exhibited TG levels>1500 mg/d L,which were defined as the MT group. Totally 6 male HTG Apo CIII transgenic mice were mated with 6 normalfemale mice to obtain 65 pups. Thirty-five pups had TG levels<300 mg/d L,which were defined as the PC group;30 pups exhibited TG levels>1500 mg/dL,which were defined as the PT group.There were no statisticaldifferences in the litter size(Fig.1D),offspring birth weightand growth rate (Fig.2)between female ApoCIII transgenic and male ApoCIII transgenic mice.
Unaltered plasma TG levels in ApoCIII transgenic offspring from maternal and paternal inheritance.
At 14 days after birth plasma TG of MT and PT were significantly increased than their respective MC, PC groups,3676.3±1921.3 vs 231.2±55.2 mg/dL (P<0.01)and 3601.9±1298.4 vs 195.4±56.3 mg/ dL(P<0.01),which were milky appearance in both MT and PT groups(Fig.3A and B).
At28 days afterbirth,the 4 groups of offspring had similar weight,TG of MT and PT were nearly 40-fold higherthan theirrespective MC,PC groups,3309.1± 676.54 vs 79.8±14.9 mg/dl(P<0.01)and 3024.6± 970.0 vs 78.6±25.9 mg/dl(P<0.01),butthere was no difference in plasma TG between MT and PT groups.There were about 3 times increase in plasma TC levels between MT/PT groups and MC/PC groups (Table 1).
Impaired glucose tolerance in maternal transgenic offspring
Although the FBG of the 4 groups of animals had no difference,but the MT group animalsˊBG of 15 minutes after oralglucose was significantly higherthan MC,PC,PT group(P<0.01),4 groups of animalsˊBG after oral glucose 30 minutes,60 minutes 120 minutes BG was no significant difference.The MT mice showed mild impaired glucose tolerance, than the otherthree groups.The PT mice showed normalglucose tolerance(Fig.4).
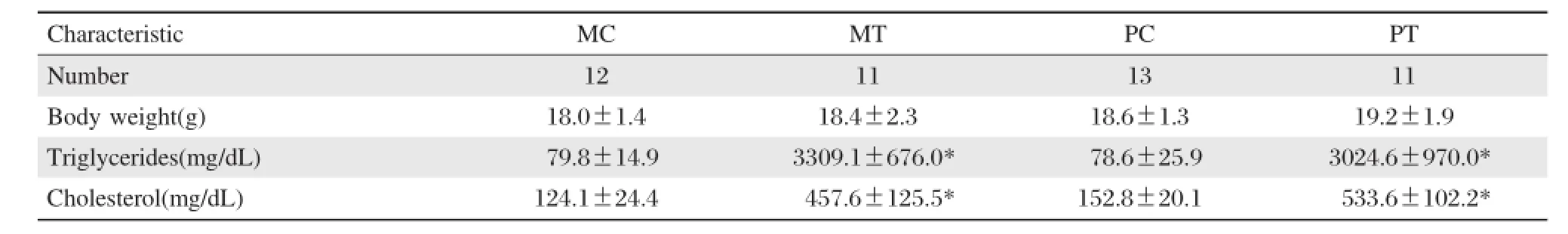
Table 1.Comparison of body weight and blood lipids among 4 groups of mice 28 days after birth(mean±SD)
Fig.2 The growth curve of offspring from female and male ApoCIII transgenic parental mice.MC,maternal non-transgenic normal controls;MT,transgenics;PC,paternal normal controls;PT transgenics.

Fig.3Comparison of plasma triglycerides among 4 groups of mice 14 days after birth.A.Milky appearance of plasma from MT and PT ApoCIII transgenic offsspring;B.Levels of plasma triglycerides. *P<0.01.MC,maternal non-transgenic normal controls;MT, transgenics;PC,paternal normal controls;PT transgenics.
Increased fasting insulin and HOMA-IR in maternal transgenic offspring
The MT mice showed significantly increased FINS levels and higher HOMA-IR than the other three groups.At28 days afterbirth,the MT group had insulin resistance(Fig.5).
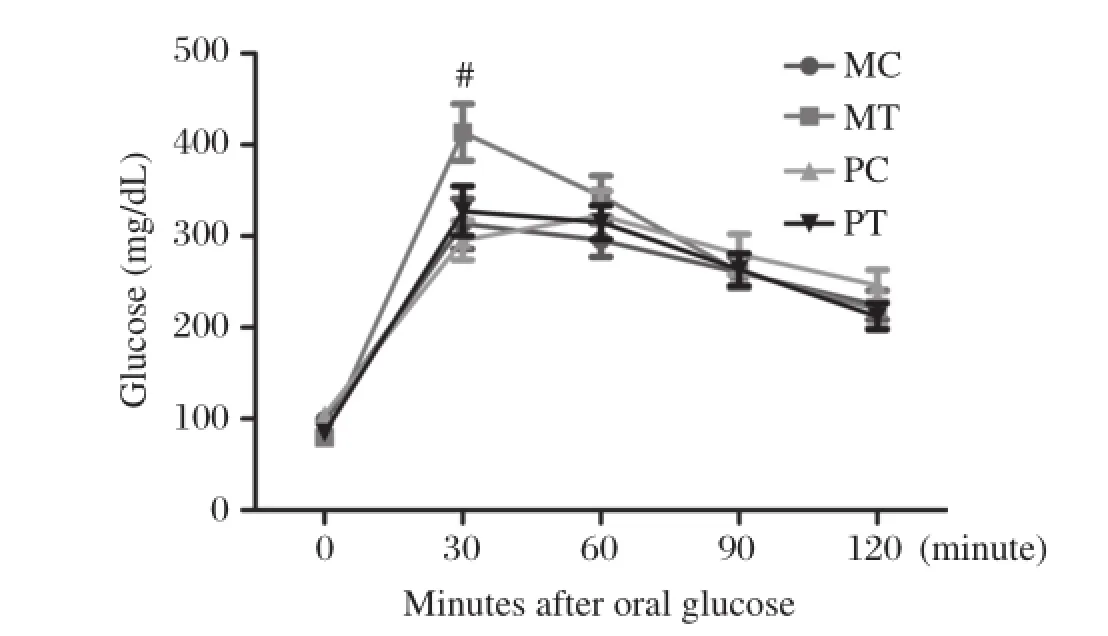
Fig.4Oral glucose tolerance tests among 4 groups of 28 dayold mice.MC,maternal non-transgenic normal controls;MT, transgenics;PC,paternal normal controls;PT transgenics.*P<0.01.
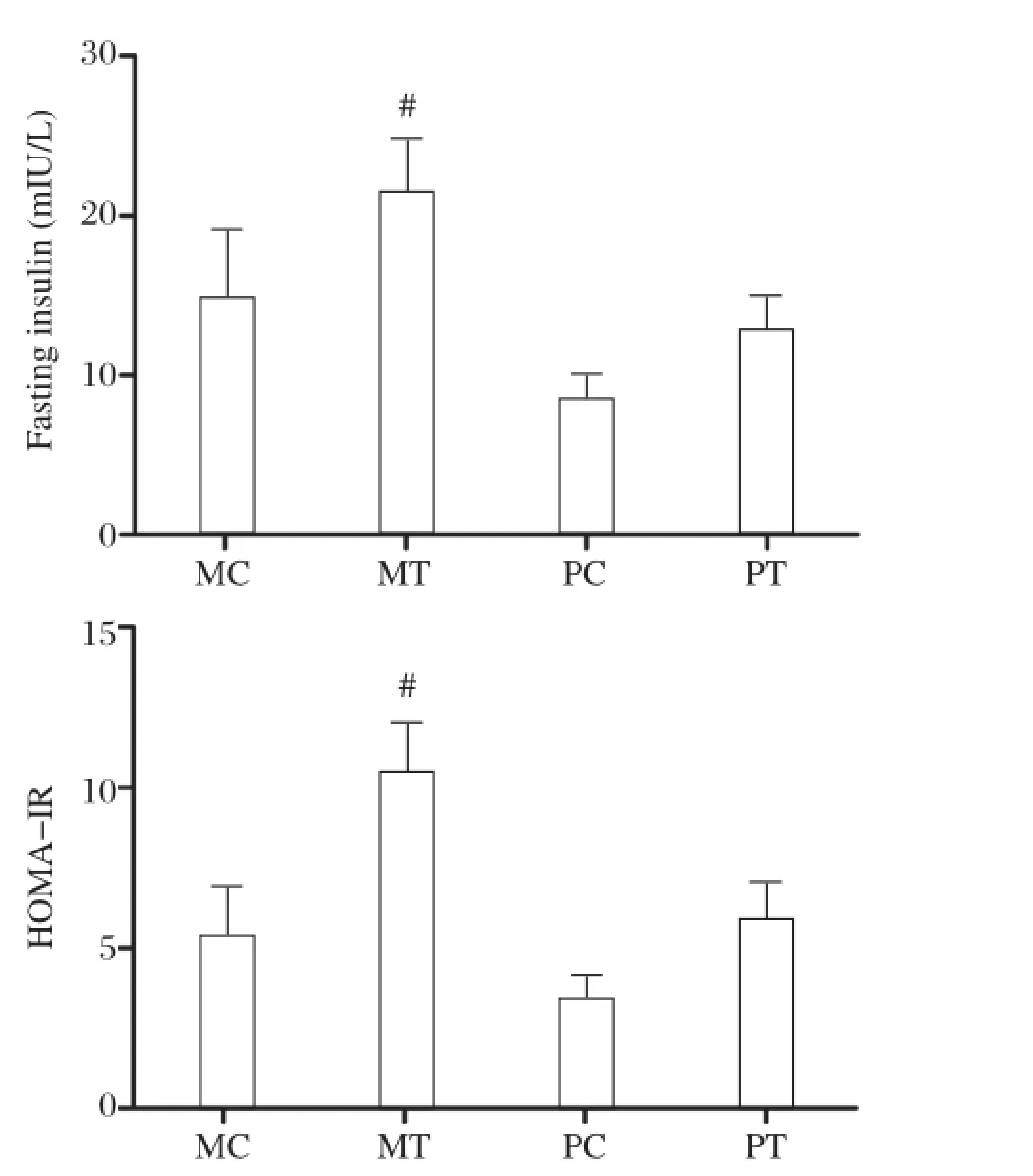
Fig.5 Comparison of fasting insulin and HOMA-IR among 4 groups of 28 day-old mice.MC,maternal non-transgenic normal controls;MT,transgenics;PC,paternalnormalcontrols;PT transgenics. (*P<0.05).
Discussion
In this study,we utilized male and female ApoCIII transgenic mice with severe HTG to mate respective non-transgenic mice,and obtained maternaland paternal inherited HTG offspring and their respective controls.ApoCIII transgenic male mice were fertile and the transgenic females also had normalpregnancy,birth of pupsand lactation.There were no statisticaldifferences in littersize,birth weightand growth rate oftransgenic and non-transgenic pups from eithermaternalorpaternal inheritance,indicating that parental severe HTG from ApoCIII transgene did notaffectthe development and growth rate of the offspring with different genotypes.Severe HTG accompanied with ApoCIII overexpression was inherited following Mendelian law of inheritance with half of the offspring being normal and half being transgenic either paternally or maternally.The plasma levels of triglycerides in the PT and MT group were both over 10 times higher than their non-transgenic littermate controls(PC and MC) during lactation and after weaning,but there was no difference between the MT and PT group,implying no influence of paternal or maternal inheritance of ApoCIII transgenic on severity of HTG.This is in contrast to inheritance of hypercholesterolemia in ApoE deficientmice which showed stronger maternalinfluence on the developmentof hypercholesterolemia in the offspring than paternalinheritance.
However,the present study found that mice from maternally inherited HTG displayed impaired glucose tolerance,in comparison to paternally inherited HTG mice.The fasting insulin levels of MT were significantly higher than MC,PC,and PT though the fasting plasma glucose levels of MT were not affected.The value of HOMA-RIderived from fasting plasma insulin and glucose levels was then the highestin MT mice among all4 offspring groups.The results suggestthat maternally inherited HTG due to ApoCIIIoverexpression mightaffectglucose metabolism.
It is well known that diabetic patients often have HTG accompanied with low HDL[17],because insulin resistance was shown to be a cause for enhanced synthesis and secretion of hepatic VLDL[18]Recently, Caron et al.showed that glucose might have a direct effect by up-regulating ApoCIII gene at the transcriptional level,resulting in HTG in h yperglycemic patients[19].However,the impact of primary HTG oninsulin and glucose metabolism has notbeen wellstudied.Lin etal.reported the severe HTG patients with non-diabetic microalbuminuria have glucose intolerance and hyperinsulinemia,demonstrating the potential impact of severe HTG on glucose metabolism[20].In anotherclinicalstudy,itis found thatin a HTG cohort with LPL gene mutation(LPL291),HTG was positively correlated with type 2 diabetes,also implying abnormal glucose metabolism resulting from HTG[21]. Our previous study showed that LPL-deficientmice with severe HTG had a significant reduction in the first-phase insulin secretion with late stage occurrence of insulin resistance and proliferation of pancreaticβ cells[5].
In an early study using similar ApoCIII transgenic mice,Chen et al.did not find significant differences in glucose metabolism from non-transgenic controls either in normal or in streptozotozin-induced diabetes[22],although ApoCIII deficient mice displayed increased adipose tissue and insulin resistance[23].In the present study,we found that only the severe HTG mice from maternalinheritance had higherinsulin levels and hence increased HOMA-RIafter weaning.Since the transgenic offsprings were usually obtained from paternal inheritance(routine breeding of transgenic males with non-transgenic females),or a mixture of paternal and maternal inheritance,it is hence possible to draw such conclusion that severe HTG resulting from ApoCIII transgenic had no effect on glucose metabolism.In addition,the age ofthe animals may also influence the results of the study.
Although we did not explore the mechanisms of increased insulin resistance in maternally inherited HTG in the current study,itshould be noted that the fetus in utero environment of the HTG dams may be an importantcontributorto later developmentofinsulin resistance.As Goharkhay et al.have shown hypercholesterolemia in ApoE-deficient female mice induced methylation and demethylation in certain regionsofthe genome,causing up-and down-regulation of genes involved in cholesterol homeostasis of embryos,thereby increasing cholesterol levels and hence the susceptibility to AS in maternally inherited offspring[16].The exact mechanisms of the finding that maternal inheritance of HTG through Apo CIII transgenic expression can affect glucose metabolism in offspring in the current study certainly warrant further investigation.
Our finding that maternal inheritance of HTG can affect the glucose metabolism in offspring would be of great in terest if such finding can be validated through the clinical studies that maternally inherited severe HTG could indeed affect glucose metabolism. More attention on potential impairment of glucose metabolism then will be placed to those who have mothers experiencing severe HTG during pregnancy and lactation period.
In conclusion,maternally inherited HTG in ApoCIII transgenic mice displayed impaired glucose tolerance, hyperinsulinemia and increased HOMA-R,while paternally inherited HTG did not have significant impacton the glucose metabolism.
Acknowledgements
This work was funded by Research Program Grants 20110001110017,Ministry of Education,the Peopleˊs Republic of China,and Science and Technology Project(2014-3-005)of Dongcheng District,Beijing.
[1]Sarwar N,Danesh J,Eiriksdottir G,et al.Triglycerides and the risk of coronary heart disease:10,158 incident cases among 262,525 participants in 29 Western prospective studies.Circulation 2007;115(4):450-458.
[2]Gan SI,EdwardsAL,SymondsCJ,etal.Hypertriglyceridemiainduced pancreatitis:A case-based review.World J Gastroentero 2006;12(44):7197-7202.
[3]Prinsen BH,de Sain-van der Velden MG,de Koning EJ, et al.Hypertriglyceridemia in patients with chronic renal failure:Possible mechanisms.Kidney Int 2003;May;(84): S121-S124.
[4]Xian XD,Liu TT,Yu J,et al.Presynaptic Defects Underlying Impaired Learning and Memory Function in Lipoprotein Lipase-Deficient Mice.J Neurosci 2009; 29(14):4681-4685.
[5]Ding YL,Wang YH,Huang W,etal.Glucose intolerance and decreased early insulin response in mice with severe h y p ertrig ly cerid emia.Exp Bio l Med(Ma ywood) 2010;235(1):40-46.
[6]Koukkou E,Watts GF,Lowy C.Serum lipid,lipoprotein and apolipoprotein changes in gestational diabetes mellitus:A cross-sectionaland prospective study.J Clin Pathol 1996;49(8):634-637.
[7]Swisher SG,Hunt KK,Schmit PJ,et al.Management Of Pancreatitis Complicating Pregnancy.Am Surg 1994;60(10): 759-762.
[8]Eddy JJ,Gideonsen MD,Song JY,et al.Pancreatitis in Pregnancy.Obstet Gynecol 2008;112(5):1075-1081.
[9]Brown WV,Baginsky ML.Inhibition Of Lipoprotein-Lipase by an Apoprotein Of Human Very Low-Density Lip oprotein.Biochem Biophys Res Commun 1972; 46(2):375-382.
[10]Wang CS,Mcconathy WJ,Kloer HU,etal.Modulation Of Lipoprotein-Lipase Activity by Apolipoproteins.Effect Of Apolipoprotein-C-III.J Clin Invest1985;75(2):384-390.
[11]Pollin TI,Damcott CM,Shen HQ,etal.A Null Mutation in Human APOC3 Confers a Favorable Plasma Lipid Profile an d Ap p arent Card iopro tectio n.Science 2008;322(5908):1702-1705.
[12]Qin W,Sundaram M,Wang YW,etal.Missense Mutation in APOC3 within the C-terminal Lipid Binding Domain of Human ApoC-III Results in Impaired Assembly andSecretion of Triacylglycerol-rich Very Low Density Lipoproteins:Evidence That Apo C-III Plays A Major Role In The Formation Of Lipid Precursors Within The Microsomal Lumen.J Biol Chem 2011;286(31):27769-27780.
[13]Ito Y,Azrolan N,Oconnell A,etal.Hypertriglyceridemia as a Result Of Human Apo-CIII Gene-Expression In Transgenic Mice.Science 1990;249(4970):790-793.
[14]Aaltosetala K,Fisher EA,Chen XL,et al.Mechanism Of Hypertriglyceridemia In Human Apolipoprotein-(Apo)-Ciii Transgenic Mice-Diminished Very Low-Density-Lipoprotein Fractional Catabolic Rate Associated with Increased Apo-CIIIAnd Reduced Apo-E on the Particles. J Clin Invest 1992;90(5):1889-1900.
[15]v an der Graaf A,Vissers MN,Gau d et D,et al. Dyslipidemia of Mothers With Familial Hypercholesterolemia Deteriorates Lipids in Adult Offspring. Arterioscl Throm Vas 2010;30(12):2673-2677.
[16]Goharkhay N,Tamayo EH,Yin HZ,et al.Maternal hypercholesterolemia leads to activation of endogenous cholesterol synthesis in the offspring.Am J Obstet Gynecol 2008;199(3).
[17]Howard BV,Mayer-Davis EJ,Goff D,etal.Relationships between insulin resistance and lipoproteins in nondiabetic African Americans,Hispanics,and non-Hispanic whites: The Insulin Resistance Atherosclerosis Study.Metabolism 1998;47(10):1174-1179.
[18]Pollex RL,Hegele RA.Genetic determinants ofthe metabolic syndrome.Nat Clin Pract Card 2006;3(9):482-489.
[19]Caron S,Verrijken A,Mertens I,et al.Transcriptional Activ atio n o f Apo lipop ro tein CIII Expression by Glucose May Contribute to Diabetic Dyslipidemia. Arterioscl Throm Vas 2011;31(3):513-U572.
[20]Lin CY,Chen MF,Lin LY,etal.Insulin Resistance is the Major Determinant for Microalbuminuria in Severe Hypertriglyceridemia:Implicatio n for Hig h-Risk Stratification.Internal Med 2008;47(12):1091-1097.
[21]Hu YM,Liu W,Huang R,etal.A systematic review and meta-analysis of the relationship between lipoprotein lipase Asn291Ser variant and diseases.J Lipid Res 2006;47(9):1908-1914.
[22]Reaven GM,Mondon CE,Chen YDI,et al.Hypertriglyceridemic Mice Transgenic for the Human Apolipoprotein C-Iii Gene Are Neither Insulin-Resistant nor Hyperinsulinemic.J Lipid Res 1994;35(5):820-824.
[23]Du ivenvoord en I,Teu sink B,Rensen PC,et al. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice.Diabetes 2005;54(3):664-671.
△These authors contribute equally to the work.
✉Corresponding author:George Liu,Institute of Cardiovascular Sciences,Peking University and Key Laboratory of Cardiovascular Sciences,Administration of Education,PRC.Tel/Fax:86-10-82802769/ 86-10-82802779,E-mail:georgeliu@bjmu.edu.cn;Zhili Ji,Department of General Surgery,Luhe Teaching Hospital of Capital MedicalUniversity,Xinhua Rd.82#,Tongzhou District,Beijing 101149, PRC.Tel/Fax:8 6-1 0-6 9 5 4 3 901ex t2 1 1 1/8 6-1 0-6 9 5 4 3 9 50, E-mail:jizhili@medmail.com.cn.
Received 14 October 2014,Revised 25 November 2014,Accepted 16 January 2015,Epub 13 March 2015
The authors reported no conflictof interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140139
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Recent advances in bariatric/metabolic surgery:appraisalof clinical evidence
- Metabolic bariatric surgery and type 2 diabetes mellitus:an endocrinologistˊs perspective
- Roux-en-Y gastric bypass for Chinese type 2 diabetes mellitus patients with a BMI<28 kg/m2:a multi-institutionalstudy
- Bariatric surgery in old age:a comparative study of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in an Asia centre ofexcellence
- Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis
- Quercetin attenuates the development of 7,12-dimethylbenz(a) anthracene(DMBA)and croton oil-induced skin cancer in mice
