紫外光介导合成1,2,4-三芳基 -1,4-丁二酮反应研究
黄宏丽,高国林,杨 超*,夏吾炯,陈晓明
(1.哈尔滨工业大学 基础与交叉科学研究院,黑龙江 哈尔滨150080;2.温州医科大学 检验医学院与生命科学学院,浙江 温州325035)
烯烃的双官能团化是一类非常重要的有机化学反应,它为构建化学结构的多样性和新颖性提供了可靠的保证[1-3]。一般来说,实现烯烃双官能团化的反应主要是由过渡金属催化实现的[4-7]。此外,多个课题组发展了以α,α-二芳基烯丙醇为底物,通过提供自由基加成/1,2-芳基迁移的串联过程,实现双键的官能团化的新方法,同时合成了α-芳基-β-取代的羰基化合物[8-16]。近年来,在绿色化学理念的推动下,以光作为介质,基于C-H活化直接实现烯烃双官能团化的合成方法引起了有机化学家的广泛关注[17-21]。
安息香是一种稳定且易于制备的α-羟基酮,在紫外光的照射下,易发生Norrish I型裂解光化学反应产生苯甲酰基自由基。2015年,本课题组报道了以安息香为自由基源,对α-芳基烯丙醇进行苯甲酰基自由基加成/1,2-芳基迁移的串联光化学反应的研究工作[22]。在此基础上,本文设计合成了多种不对称α,α-二芳基烯丙醇,在紫外光作用下与安息香衍生物进行反应,研究电子效应对反应选择性的影响。
1 实验部分
1.1 试剂与仪器
本论文中所用试剂均为分析纯,若非特殊说明均未进一步处理而直接使用。无水无氧反应中使用的四氢呋喃、苯、二氯甲烷、乙腈等溶剂均经过严格的除水脱氧处理。
1HNMR (400MHz)和13CNMR (100MHz):Bruker AVANCE III 400核磁共振谱仪,内标为TMS;HRMS(ESI):Agilent 1200-6520Q-TOF高 分 辨 液 质 联 用 仪;GC-MS:Agilent 7890A-5975C气质联用仪;紫外光源:RPR-200光反应器,350nm。
1.2 实验方法
1.2.1 化合物1a的合成[23]
无水无氧处理,氮气保护下,向50mL圆底烧瓶中,加入0.55g二苯甲酮的无水THF溶液 (20 mL)。将上述反应装置放入冰水浴,抽取2.3mL氯乙烯基镁溶液,逐滴滴加到烧瓶中。溶液由无色变为紫色,最后变为黄色。烧瓶在冰水浴中反应30min,后恢复至室温反应3h。TLC监测反应完全,饱和的氯化铵溶液淬灭反应,乙酸乙酯萃取3次,合并浓缩有机相,用柱层析手段分离纯化产物,洗脱剂为石油醚/乙酸乙酯 =18/1,得到1a(0.57g),产率90%。
1,1-Diphenylprop-2-en-1-ol(1a)[22]:无色油状物。1HNMR(400MHz,CDCl3):7.36(d,J=7.6Hz,4H),7.30(t,J=7.6Hz,4H),7.24(t,J=7.6Hz,2H),6.48(dd,J=16.8,10.8Hz,1H),5.30(d,J=16.8Hz,1H),5.29(d,J=10.8Hz,1H),2.34(s,1H,—OH)。
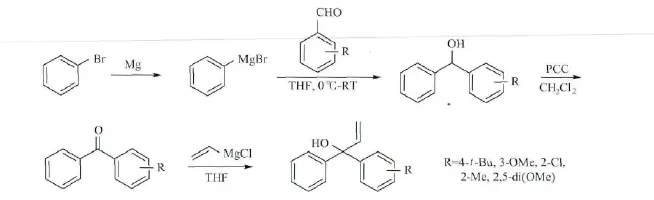
图1 不对称α,α-二芳基烯丙醇化合物的合成
1.2.2 α,α-二芳基烯丙醇化合物的合成[15]
化合物1b~f的合成路线,如图1所示。以化合物1b的合成为例:向50mL圆底烧瓶中,依次加入0.6g镁屑和半粒碘单质,冷凝,无水无氧处理,氮气保护。抽取6mL溴苯溶液 (1.4g溴苯,20mL无水THF)滴加到烧瓶中,吹风机加热引发反应。引发后,将剩余的溴苯溶液逐滴滴加到烧瓶中,滴加速度使反应液保持微沸状态。待反应瓶冷却至室温,可得到溴苯格氏试剂。
无水无氧处理,氮气保护下,向50mL圆底烧瓶中,加入0.6g对叔丁基苯甲醛的THF(25 mL)溶液。冰浴下,抽取6mL上述溴苯格氏试剂,逐滴滴加到反应瓶中。30min后,去掉冰浴,室温反应3h。TLC检测反应完全,氯化铵溶液淬灭反应,乙酸乙酯萃取。柱层析分离粗产物得到0.63g 4-叔丁基二苯甲醇,产率71%。将得到的无色油状物溶于18mL CH2Cl2中,经PCC氧化得到对叔丁基二苯基甲酮0.5g,产率80%。将4-叔丁基二苯基甲酮与氯乙烯基镁发生格氏反应,得到1b(0.48g),产率87%。
1-(3-tert-Butylphenyl)-1-phenylprop-2-en-1-ol (1b):无 色 油 状 物。1HNMR (400MHz,CDCl3):7.40~7.38(m,2H),7.34~7.33(m,2H),7.32~7.29(m,3H),7.29~7.23(m,2H),6.50(dd,J=16.8,10.4Hz,1H),5.32(dd,J=16.8,1.2Hz,1H),5.29(dd,J=10.4,1.2Hz,1H),2.28(s,1H,—OH),1.30(s,3×CH3,9H);13CNMR (100MHz,CDCl3):150.1,145.7,143.6,142.7,128.1,127.1,126.8,126.6,125.1,113.6,79.2,34.4,31.3;HRMS(ESI):[M-H2O+H]+calcd for C19H+21:249.1643,found:249.1637。
1-(3-Methoxyphenyl)-1-phenylprop-2-en-1-ol(1c)[16]:无 色 油 状 物。1HNMR (400MHz,CDCl3):7.38(d,J=7.6Hz,2H),7.29 (t,J=7.6Hz,2H),7.23 (d,J=7.6Hz,1H),7.19(d,J=7.6Hz,1H),6.99 (d,J=1.6 Hz,1H),6.93 (dd,J=7.6,0.8Hz,1H),6.77(dd,J=7.6,1.6Hz,1H),6.48(dd,J=17.2,10.8Hz,1H),5.29(dd,J=17.2,1.2 Hz,1H),5.27 (dd,J=10.8,1.2Hz,1H),3.75(s,OCH3,3H),2.43(s,—OH,1H)。
1-Phenyl-1-o-tolylprop-2-en-1-ol(1d)[16]:无色油状物。1HNMR (400MHz,CDCl3):7.57~7.55(m,1H),7.29 (d,J= 8.0Hz,4H),7.25~7.19(m,3H),7.13~7.11(m,1H),6.52(dd,J=17.2,10.8Hz,1H),5.26(d,J=10.8Hz,1H),5.24(d,J=17.2Hz,1H),2.72(s,—OH,1H),2.03(s,CH3,3H)。
1-(2-Chlorophenyl)-1-phenylprop-2-en-1-ol(1e)[16]: 无 色 油 状 物。1HNMR (400MHz,CDCl3):7.70(dd,J=7.6,2.0Hz,1H),7.35~7.25(m,8H),6.60(dd,J=17.2,10.8Hz,1H),5.34(dd,J =10.8,1.2Hz,1H),5.33(dd,J=17.2,1.2Hz,1H),3.23(s,—OH,1H)。
1-(2,5-Dimethoxyphenyl)-1-phenylprop-2-en-1-ol(1f):无色油状物。1HNMR (400MHz,CDCl3):7.32(dd,J=7.6,1.6Hz,2H),7.28(t,J=7.6Hz,2H),7.22 (t,J= 7.6Hz,1H),6.84(dd,J=8.8,1.2Hz,2H),6.79(dd,J=8.8,2.8Hz,1H),6.38(dd,J=17.2,10.8Hz,1H),5.28(dd,J=10.8,1.2Hz,1H),5.06(dd,J=17.2,1.2Hz,1H),4.80(s,—OH,1H),3.74 (s,OCH3,3H),3.57(s,OCH3,3H);13CNMR (100MHz,CDCl3):153.5,151.3,145.7,142.5,135.3,127.7,126.8,126.5,115.7,115.0,113.4,112.3,79.5,56.3,55.6;HRMS(ESI):[M-H2O + H]+calcd for C17H17O+2:253.1228,found:253.1231。
1.2.3 安息香衍生物2b~f的合成
安息香2b~f的合成与安息香2a类似[24]。在15mL封管中,加入0.45g维生素B1、1mL H2O和3.5mL乙醇,摇匀溶解后将烧瓶置于冰水浴中冷却。用10%的氢氧化钠溶液调pH=9~10。加入3mL对甲氧基苯甲醛,密封。水浴温度75℃,反应6h,冷却析出浅黄色固体。乙酸乙酯萃取,柱层析手段分离纯化产物,洗脱剂为石油醚/乙酸乙酯=6/1,得到2b(0.98g),产率61%。
2-Hydroxy-1,2-bis(4-methoxyphenyl)ethanone (2b): 无 色 固 体。1HNMR (400MHz,CDCl3):7.89(d,J=8.8Hz,2H),7.24(d,J=8.8Hz,2H),6.85 (d,J=8.8Hz,2H),6.84(d,J=8.8Hz,2H),5.85(d,J= 5.8 Hz,1H),4.57(d,J=5.8Hz,1H,—OH),3.81 (s,OCH3,3H),3.75 (s,OCH3,3H);13CNMR (100MHz,CDCl3):197.3,163.9,159.6,131.8,131.5,129.0,126.3,114.5,113.9,75.2,55.4,55.2;HRMS(ESI):[M+Na]+calcd for C16H16NaO+4:295.0946,found:295.0948。
1,2-Bis(4-tert-butylphenyl)-2-hydroxyethanone(2c):无 色 固 体。1HNMR (400MHz,CDCl3):7.89(d,J=8.4Hz,2H),7.42 (d,J=8.4 Hz,2H),7.35(d,J=8.4Hz,2H),7.27(d,J=8.4Hz,2H),5.91(d,J=6.2Hz,1H),4.51(d,J=6.2Hz,1H,—OH),1.29(s,3×CH3,9H),1.27 (s,3×CH3,9H);13CNMR(100MHz,CDCl3):198.4,157.8,151.5,136.3,130.9,129.2,127.4,126.1,125.7,75.7,35.2,34.6,31.2,31.0;HRMS(ESI):[M+Na]+calcd for C22H28NaO+2:347.1987,found:347.2017。
1,2-Bis(4-fluorophenyl)-2-hydroxyethanone(2d):无色 固体。1HNMR (400MHz,CDCl3):7.94(d,J=8.8Hz,1H),7.93 (d,J=8.8 Hz,1H),7.31(d,J=8.8Hz,1H),7.30(d,J=8.8Hz,1H),7.09(t,J=8.8Hz,2H),7.03(t,J= 8.8Hz,2H),5.90(d,J= 6.0 Hz,1H),4.51(d,J=6.0Hz,1H,—OH);13CNMR (100MHz,CDCl3):197.2,166.1 (d,J=255.7Hz),162.9(d,J= 246.7Hz),134.8(d,J=3.3Hz),131.8(d,J=9.5Hz),129.7(d,J=3.1Hz),129.5(d,J=8.3Hz),116.2(d,J=21.7Hz),116.1(d,J=22.0Hz),75.4;HRMS(ESI):[M+Na]+calcd for C14H10F2NaO+2:271.0547,found:271.0551。
2-Hydroxy-1,2-bis(3-methoxyphenyl)ethanone (2e):无 色 固 体。1HNMR (400MHz,CDCl3):7.49 (d,J=8.0Hz,1H),7.46 (s,1H),7.30(t,J=8.0Hz,1H),7.25(t,J=8.0Hz,1H),7.07 (dd,J= 8.0,2.4Hz,1H),6.94 (d,J= 8.0Hz,1H),6.87 (s,1H),6.82(dd,J=8.0,2.4Hz,1H),5.91(d,J=6.4Hz,1H),4.53(dd,J=6.4,1.6 Hz,1H,—OH),3.80(s,OCH3,3H),3.76(s,OCH3,3H);13CNMR (100MHz,CDCl3):198.7,160.1,159.7,140.4,134.7,130.1,129.6,121.7,120.4,120.1,114.1,113.3,113.1,76.2,55.4,55.2;HRMS(ESI):[M+Na]+calcd for C16H16NaO+4:295.0946,found:295.0951。
2-Hydroxy-1,2-bis(2-methoxyphenyl)ethanone (2f): 无 色 固 体。1HNMR (400MHz,CDCl3):7.69(dd,J=7.6,1.6Hz,1H),7.37(t,J=7.6Hz,1H),7.21~7.15(m,2H),6.93(t,J=7.6Hz,1H),6.84(t,J=7.6Hz,1H),6.77(t,J=7.6Hz,2H),6.12(d,J=5.6 Hz,1H),4.47(d,J=5.6Hz,1H,—OH),3.73(s,OCH3,3H),3.72 (s,OCH3,3H);13CNMR (100MHz,CDCl3):201.6,158.1,157.2,133.8,130.6,129.9,129.5,127.5,125.3,120.5,120.5,111.2,110.8,75.8,55.2,55.1;HRMS (ESI):[M + Na]+calcd for C16H16NaO+4:295.0946,found:295.0943。
1.2.4 烯丙醇衍生物1a~f的光化反应
向50mL的光照管中,依次加入1a(0.3 mmol,63mg),安息香2a(1.5mmol,318mg),15mL无水苯使其溶解,搅拌下通氮气30min后,将光照管密封。在350nm光源光照下,反应34h。硅胶柱层析(石油醚/乙酸乙酯=15/1)得到无色油状物3a61mg,产率为65%,转化率为100%。
1,2,4-Triphenylbutane-1,4-dione (3a)[22]:无色 固 体。1HNMR (400MHz,CDCl3):8.03(d,J=7.6Hz,2H),7.97(d,J= 7.6Hz,2H),7.54(t,J=7.6Hz,1H),7.47(t,J=7.6Hz,1H),7.43(t,J=7.6Hz,3H),7.37(t,J= 7.6Hz,3H),7.30 (t,J= 7.6Hz,2H),7.21(t,J=7.6Hz,1H),5.33(dd,J=10.4,3.6Hz,1H),4.21(dd,J=18.0,10.4 Hz,1H),3.30(dd,J=18.0,3.6Hz,1H)。
1-(4-tert-Butylphenyl)-2,4-diphenylbutane-1,4-dione (3b): 无 色 油 状 物。1HNMR (400 MHz,CDCl3):7.99(d,J=7.2Hz,2H),7.97(d,J=7.2Hz,2H),7.53(t,J=7.2Hz,1H),7.42(t,J=7.2Hz,4H),7.38(d,J=7.2Hz,2H),7.30(t,J=7.2Hz,2H),7.21(t,J=7.2Hz,1H),5.32(dd,J=10.0,4.0 Hz,1H),4.21(dd,J=18.0,10.0Hz,1H),3.29(dd,J=18.0,4.0Hz,1H),1.29(s,3×CH3,9H);13CNMR(100MHz,CDCl3):198.4,198.1,156.5,138.8,136.4,133.7,133.2,129.1,128.9,128.5,128.2,128.1,127.2,125.5,48.4,43.9,35.0,31.0;HRMS(ESI):[M + Na]+calcd for C26H26O2Na+:393.1830,found:393.1821。
1-(3-Methoxyphenyl)-2,4-diphenylbutane-1,4-dione(3c):无色油状物。1HNMR (400MHz,CDCl3):8.00(d,J=7.2Hz,2H),7.66(d,J=7.2Hz,1H),7.59~7.55(m,2H),7.46(t,J=8.0Hz,2H),7.38(d,J=7.2Hz,2H),7.34~7.30 (m,3H),7.24 (t,J= 7.2Hz,1H),7.05(dd,J=8.0,2.0Hz,1H),5.32(dd,J=10.0,3.6Hz,1H),4.22(dd,J=18.0,10.0Hz,1H),3.81 (s,OCH3,3H),3.32(dd,J=18.0,3.6Hz,1H);13CNMR (100 MHz,CDCl3):198.7,198.0,159.6,138.6,137.7,136.4,133.2,129.4,129.2,128.5,128.2,128.1,127.3,121.6,119.6,113.0,55.3,48.8,43.9;HRMS(ESI):[M+Na]+calcd for C23H20O3Na+:367.1310,found:367.1303。
2,4-Diphenyl-1-o-tolylbutane-1,4-dione (3d):无色油状物。1HNMR (400MHz,CDCl3):8.03(d,J=7.6Hz,2H),7.94 (d,J=7.6Hz,1H),7.58(t,J=7.6Hz,1H),7.47(t,J=7.6Hz,2H),7.32~7.29(m,5H),7.24(t,J=7.6Hz,2H),7.16(d,J=7.6Hz,1H),5.16(dd,J=10.4,3.6Hz,1H),4.28(dd,J=18.0,10.4Hz,1H),3.30 (dd,J=18.0,3.6Hz,1H),2.30(s,CH3,3H);13CNMR(100MHz,CDCl3):202.6,198.2,138.3,138.2,137.7,136.5,133.2,131.4,130.9,129.0,128.6,128.5,128.4,128.1,127.4,125.4,51.7,43.2,20.6;HRMS (ESI):[M + Na]+calcd for C23H20O2Na+:351.1361,found:351.1339。
1-(2-Chlorophenyl)-2,4-diphenylbutane-1,4-dione(3e):无 色 油 状 物。1HNMR (400MHz,CDCl3):8.02(d,J=7.6Hz,2H),7.79(dd,J=6.4,2.8Hz,1H),7.58(t,J=7.6Hz,1H),7.47(t,J=7.6Hz,2H),7.35~7.28(m,7H),7.26~7.22(m,1H),5.18(dd,J=9.6,4.0Hz,1H),4.23 (dd,J=18.0,9.6Hz,1H),3.34 (dd,J=18.0,4.0Hz,1H);13CNMR (100MHz,CDCl3):200.4,197.9,138.5,136.7,136.5,133.3,131.5,131.5,130.5,129.4,129.0,128.7,128.6,128.1,127.6,126.5,52.6,42.5;HRMS(ESI):[M+Na]+calcd for C22H17ClNaO+2:371.0824,found:371.0815。
1-(3,5-Dimethoxyphenyl)-2,4-diphenylbutane-1,4-dione(3f):无色油状物。1HNMR (400 MHz,CDCl3):8.04(d,J=7.2Hz,2H),8.00(d,J=7.2Hz,2H),7.56 (t,J= 7.2Hz,1H),7.50~7.43(m,3H),7.65(t,J=7.6 Hz,1H),7.38(t,J=7.2Hz,1H),6.84(d,J=8.0Hz,1H),6.75(s,1H),6.74(d,J=8.0Hz,1H),5.74 (dd,J= 10.4,3.2Hz,1H),4.12(dd,J=17.6,10.4Hz,1H),3.87(s,OCH3,3H),3.70 (s,OCH3,3H),3.20(dd,J=17.6,3.2Hz,1H);13CNMR (100 MHz,CDCl3):199.3,198.3,153.7,150.2,136.6,136.3,133.0,132.8,128.8,128.5,128.3,128.1,128.0,114.6,112.9,112.0,56.0,55.6,42.3,41.3;HRMS(ESI):[M +Na]+calcd for C24H22NaO+4:397.1416,found:397.1396。
1.2.5 安息香衍生物2b~f的光化反应
安息香3ab~f的合成与3a类似。4-(4-Methoxyphenyl)-1, 2-diphenylbutane-1, 4-dione(3ab): 无 色 油 状 物。1HNMR (400MHz,CDCl3):8.05(d,J=7.6Hz,2H),7.97(d,J=8.8Hz,2H),7.49 (t,J=7.6Hz,1H),7.41(t,J=7.6Hz,2H),7.38(d,J=7.6Hz,2H),7.32(t,J=7.6Hz,2H),7.23(t,J=7.6Hz,1H),6.92(d,J=8.8Hz,2H),5.33(dd,J=10.0,3.6Hz,1H),4.18(dd,J=18.0,10.0Hz,1H),3.86(s,OCH3,3H),3.28(dd,J=18.0,3.6Hz,1H);13CNMR (100MHz,CDCl3):199.0,196.5,163.6,138.8,136.5,132.8,130.4,129.6,129.1,128.9,128.4,128.2,127.3,113.7,55.4,48.7,43.5;HRMS(ESI):[M+Na]+calcd for C23H20NaO+3:367.1310,found:367.1308。
4-(4-tert-Butylphenyl)-1,2-diphenylbutane-1,4-dione (3ac): 无 色 油 状 物。1HNMR (400 MHz,CDCl3):8.05(d,J=8.6Hz,2H),7.94(d,J= 8.6Hz,2H),7.50(t,J= 8.0Hz,1H),7.47(d,J=8.0Hz,2H),7.41(t,J=8.0Hz,2H),7.38(d,J=7.2Hz,2H),7.32(t,J=7.2Hz,2H),7.24 (t,J=7.2Hz,1H),5.35(dd,J=10.0,4.0Hz,1H),4.21(dd,J=18.0,10.0Hz,1H),3.31(dd,J=18.0,4.0Hz,1H),1.35(s,3×CH3,9H);13CNMR (100MHz,CDCl3):199.0,197.7,157.0,138.8,136.5,133.9,132.8,129.1,128.9,128.5,128.2,128.1,127.3,125.5,48.7,43.7,35.1,31.1;HRMS(ESI):[M+Na]+calcd for C26H26NaO+2:393.1830,found:393.1831。
4-(4-Fluorophenyl)-1,2-diphenylbutane-1,4-dione(3ad):无 色 油 状 物。1HNMR (400MHz,CDCl3):8.03(m,4H),7.50(t,J=7.2Hz,1H),7.41(t,J=7.2Hz,2H),7.37(d,J=7.2Hz,2H),7.33(t,J=7.2Hz,2H),7.24(t,J=7.2Hz,1H),7.12 (t,J=7.2Hz,2H),5.33(dd,J=10.0,3.8Hz,1H),4.20(dd,J=18.0,10.0Hz,1H),3.27(dd,J=18.0,3.8Hz,1H);13CNMR(100MHz,CDCl3):198.8,196.5,165.8(d,J=253.3Hz),138.5,136.4,132.9,132.9,130.8(d,J=9.3Hz),129.2,128.9,128.5,128.2,127.4,115.6(d,J=21.8Hz),48.7,43.7;HRMS(ESI):[M+Na]+calcd for C22H17FNaO+2:355.1110,found:355.1117。4-(3-methoxyphenyl)-1,2-diphenylbutane-1,4-dione (3ae): 无 色 油 状 物。1HNMR (400MHz,CDCl3):8.05(d,J=7.2Hz,2H),7.60(d,J=7.6Hz,1H),7.50(s,1H),7.50(t,J=7.6 Hz,1H),7.41(t,J=8.0Hz,2H),7.40~7.36(m,3H),7.33(t,J=7.6Hz,2H),7.24(t,J=7.2Hz,1H),7.12 (dd,J=8.0,0.2Hz,1H),5.33(dd,J=10.0,4.0Hz,1H),4.21(dd,J=18.0,10.0Hz,1H),3.84(s,OCH3,3H),3.32 (dd,J=18.0,4.0Hz,1H);13CNMR (100MHz,CDCl3):198.9,197.9,159.8,138.6,137.8,136.5,132.9,129.5,129.2,128.9,128.5,128.2,127.3,120.9,120.0,112.1,55.4,48.7,44.0;HRMS(ESI):[M+Na]+calcd for C23H20NaO+3:367.1310,found:367.1321。
4-(2-Methoxyphenyl)-1,2-diphenylbutane-1,4-dione (3af): 无 色 油 状 物。1HNMR (400 MHz,CDCl3):8.04(d,J=7.2Hz,2H),7.75(dd,J=7.2,1.6Hz,1H),7.51~7.44(m,2H),7.40(t,J=7.2Hz,2H),7.36(d,J=7.2Hz,2H),7.31(t,J=7.2Hz,1H),7.22(t,J=7.2Hz,1H),7.00~6.95 (m,2H),5.32(dd,J=10.0,4.0Hz,1H),4.17(dd,J=18.8,10.0Hz,1H),3.88(s,OCH3,3H),3.41 (dd,J=18.8,4.0Hz,1H);13CNMR(100MHz,CDCl3):199.7,199.3,159.0,139.0,136.7,133.7,132.7,130.6,129.0,128.9,128.4,128.3,127.4,127.1,120.6,111.5,55.5,49.1,48.9;HRMS(ESI):[M+Na]+calcd for C23H20NaO+3:367.1310,found:367.1305。
2 结果与讨论
2.1 1a~f与2a的光化反应
2.1.1 实验条件优化
以1a(0.3mmol,63mg)和安息香2a(1.5 mmol,318mg)的反应为模板反应,对光化反应条件进行筛选优化。由表1可知,在测试的所有溶剂中,苯的效果最好,产率为56%。当改变苯的体积时,反应液浓度为0.02mol/L,光照34h后,1a被完全转化,3a的产率最高为65%。在300nm波长照射和2当量2a的条件下,3a的产率降低到38%和32%。因此,该光化反应的最优条件是350nm波长照射下,无水苯为溶剂,5当量的2a为自由基源,浓度为0.02mol/L时,3a的产率最高。
2.1.2 底物拓展
接下来的研究主要是对烯丙醇底物进行扩展:在最优光化反应条件下,1b~f分别与2a反应,其实验结果见表2。基团R的取代位置(对位、间位、邻位)不影响光化反应的发生。芳环上不管是连有吸电子基团还是供电子基团的α,α-二芳基烯丙醇都能够被完全转化,与2a发生反应,生成中等产率的1,2,4-三芳基-1,4-丁二酮化合物。产物中,主要得到的是以苯基迁移为主的酮化合物。
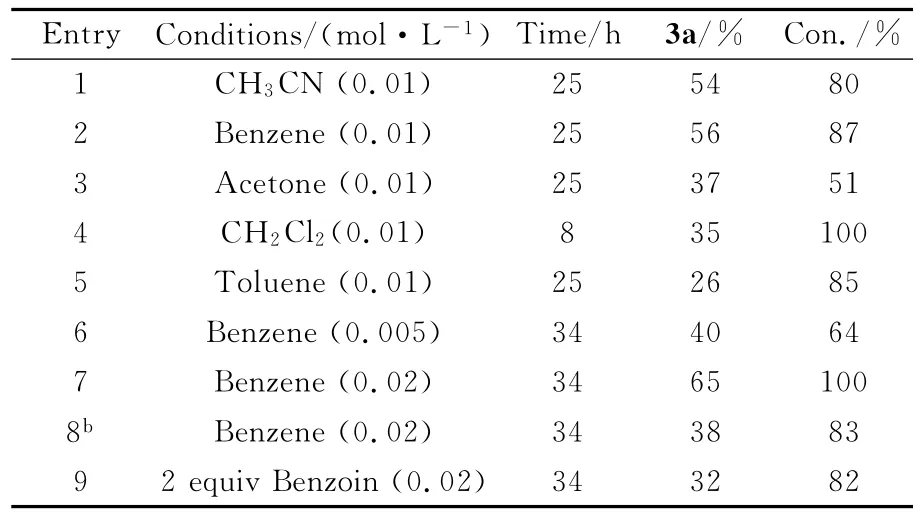
表1 光化反应的优化条件aOptimization of photochemical reaction conditions
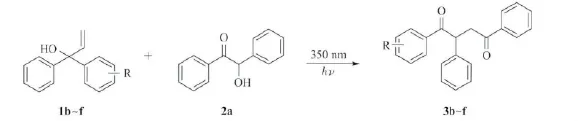
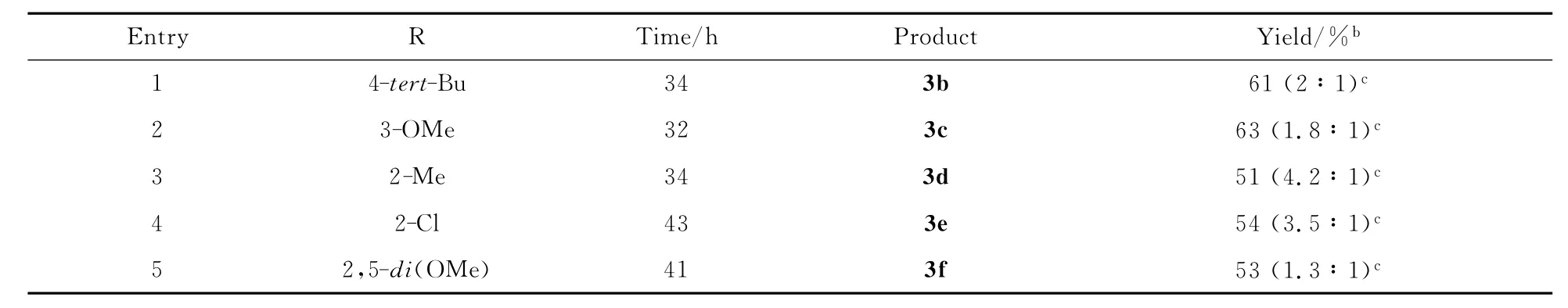
表2 光化反应的底物扩展aThe scope of photochemical reaction
2.2 1a与2b~f的光化反应
2.2.1 安息香底物拓展
研究中还对安息香底物进行了扩展:在最优光化反应条件下,1a分别与2b~f反应,其实验结果见表3。基团R的取代位置(对位、间位、邻位)和取代成分(吸电子基团或供电子基团)均不影响光化反应的发生,都能得到1,2,4-三芳基-1,4-丁二酮化合物。从反应时间和产率上来看:对位、邻位上为带有供电子基团的芳环时,反应速率加快,产率达到90%。当对位为带有吸电子基团的芳环或间位为带有供电子基团的芳环时,反应时间增长,得到中等产率的产物。上述结果表明,电子效应影响着芳基迁移的速率。
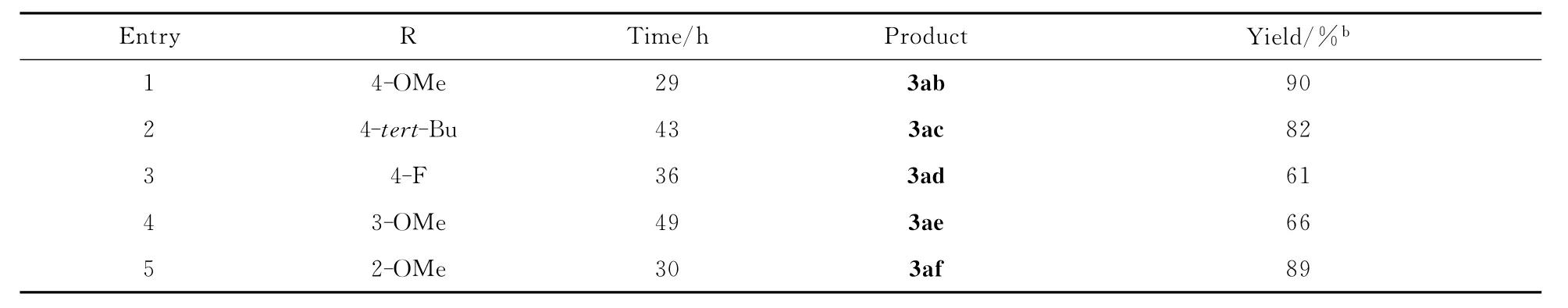
表3 安息香底物扩展aThe scope of benzoin
2.2.2 机理研究
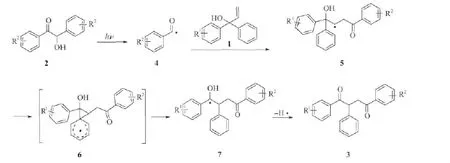
图2 可能的反应机理
根据我们组以前报道的工作[22],并结合观察到的实验现象,我们推测可能的实验机理如图2所示。在紫外光的照射下,安息香2发生Norrish I型裂解,生成苯甲酰基自由基4。自由基4与烯丙醇底物1发生分子间自由基加成,生成烷基自由基5。随后,5通过分子内自由基加成产生螺[2,5]辛二烯基自由基6[25,26]。自由基6发生1,2-芳基迁移,苯环恢复稳定结构,产生自由基7[27,28]。7失去 H·后,转化成1,2,4-三芳基-1,4-丁二酮化合物3。
3 结论
本文以α,α-二芳基烯丙醇和安息香为底物,通过苯甲酰基自由基加成/1,2-芳基迁移实现烯烃的双官能团化,提供了一种直接合成1,2,4-三芳基-1,4-丁二酮类衍生物的新颖的光化学方法。该反应具有条件温和,环境友好,底物官能团容忍性好的优点。同时,此光化学反应进一步拓展了Norrish I型反应在有机合成中的应用。
[1] Egami H,Sodeoka M.Trifluoromethylation of alkenes with concomitant introduction of additional functional groups[J].Angewandte Chemie International Edition,2014,53(32):8294-8308.
[2] Hari D P,Hering T,König B.The photoredox-catalyzed meerwein addition reaction:intermolecular amino-arylation of alkenes[J].Angewandte Chemie International Edition,2014,53(3):725-728.
[3] Merino E,Nevado C.Addition of CF3across unsaturated moieties:apowerful functionalization tool[J].Chemical Society Reviews,2014,43(18):6598-6608.
[4] Xie Y J,Hu J H,Xie P,Qian B,Huang H M.Palladiumcatalyzed difunctionalization of enol ethers to amino acetals with aminals and alcohols[J].Journal of the American Chemical Society,2013,135(49):18327-18330.
[5] Manna M K,Hossian A,Jana R.Merging C—H activation and alkene difunctionalization at room temperature:apalladium-catalyzed divergent synthesis of indoles and indolines[J].Organic Letters,2015,17(3):672-675.
[6] Shen T,Yuan Y Z,Song S,Jiao N.Iron-catalyzed aerobic difunctionalization of alkenes:a highly efficient approach to construct oxindoles by C—S and C—C bond formation[J].Chemical Communications,2014,50(31):4115-4118.
[7] Wei W,Wen J W,Yang D S,Guo M Y,Tian L J,You J M,Wang H.Copper-catalyzed cyanoalkylarylation of activated alkenes with AIBN:a convenient and efficient approach to cyano-containing oxindoles[J].RSC Advances,2014,4(89):48535-48538.
[8] Egami H,Shimizu R,Usuiac Y,Sodeoka M.Iron-catalyzed trifluoromethylation with concomitant C—C bond formation via 1,2-migration of an aryl group[J].Chemical Communications,2013,49(66):7346-7348.
[9] Chu X Q,Zi Y,Meng H,Xu X P,Ji S J.Radical phosphinylation ofα,α-diaryl allylic alcohols with concomitant 1,2-aryl migration[J].Chemical Communications,2014,50(57):7642-7645.
[10] Zhao J C,Fang H,Song R C,Zhou J,Han J L,Pan Y.Metal-free oxidative C(sp3)-H bond functionalization of alkanes and alkylation-initiated radical 1,2-aryl migration inα,αdiaryl allylic alcohols[J].Chemical Communic ations,2015,51(3):599-602.
[11] Li Y,Liu B,Li H B,Wang Q A,Li J H.Oxidative radical 1,2-alkylarylation of alkenes with a-C(sp3)-H bonds of acetonitriles involving 1,2-aryl migration[J].Chemical Communications,2015,51(6):1024-1026.
[12] Chu X Q,Meng H,Zi Y,Xu X P,Ji S J.Metal-free oxidative direct C(sp3)-H bond functionalization of ethers with α,α-diaryl allylic alcohols[J].Chemical Communications,2014,50(68):9718-9721.
[13] Song R J,Tu Y Q,Zhu D Y,Zhang F M,Wang S H.A nickel-mediated oxidative a-C(sp3)-H functionalization of amides with allylic alcohols terminated by radical 1,2-aryl migration[J].Chemical Communications,2015,51(4):749-752.
[14] Mi X,Wang C Y,Huang M M,Wu Y S,Wu Y J.Silvercatalyzed carbonphosphonation ofα,α-diaryl allylic alcohols:synthesis ofβ-aryl-γ-ketophosphonates[J].Organic&Biomolecular Chemistry,2014,12(42):8394-8397.
[15] Liu X W,Xiong F,Huang X P,Xu L,Li P F,Wu X X.Copper-catalyzed trifluoromethylation-initiated radical 1,2-aryl migration inα,α-diaryl allylic alcohols[J].Angewandte Chemie International Edition,2013,52(27):6962-6966.
[16] Huang H L,Yan H,Yang C,Xia W J.Visible light-mediated arylalkylation of allylic alcohols through concomitant 1,2-aryl migration[J].Chemical Communications,2015,51(23):4910-4913.
[17] Yasu Y,Koike T,Akita M.Three-component oxytrifluoromethylation of alkenes:highly efficient and regioselective difunctionalization of C C bonds mediated by photoredox catalysts[J].Angewandte Chemie International Edition,2012,51(38):9567-9571.
[18] Sahoo B,Hopkinson M N,Glorius F.Combining gold and photoredox catalysis:visible light-mediated oxy-and aminoarylation of alkenes[J].Journal of the American Chemical Society,2013,135(15):5505-5508.
[19] Oh S H,Malpani Y R,Ha N,Jung Y S,Han S B.Vicinal difunctionalization of alkenes:chlorotrifluoromethylation with CF3SO2Cl by photoredox catalysis[J].Organic Letters,2014,16(5):1310-1313.
[20] Carboni A,Dagousset G,Magnier E,Masson G.One pot and selective intermolecular aryl-and heteroaryl-trifluoromethylation of alkenes by photoredox catalysis[J].Chemical Communications,2014,50(91):14197-14200.
[21] Xu P,Xie J,Xue Q C,Pan C D,Cheng Y X,Zhu C J.Visible-light-induced trifluoromethylation of N-aryl acrylamides:a convenient and effective method to synthesize CF3-containing oxindoles bearing aquaternary carbon center[J].Chemistry-a European Journal,2013,19(42):14039-14042.
[22] Zheng L W,Huang H L,Yang C,Xia W J.UV light-mediated difunctionalization of alkenes through aroyl radical addition/1,4-/1,2-aryl shift cascade reactions[J].Organic Letters,2015,17(4):1034-1037.
[23] Zhang J J,Yan C S,Peng Y,Luo Z B,Xu X B,Wang Y W.Total synthesis of (± )-sacidumlignans D and A through Ueno-Stork radical cyclization reaction [J].Organic & Biomolecular Chemistry,2013,11(15):2498-2513.
[24] Gao G,Xiao R,Yuan Y,Zhou C H,You J S,Xie R G.Efficient imidazolium catalysts for the benzoin condensation[J].Journal of Chemical Research (Synopses),2002,2002(6):262-263.
[25] Rüchardt C,Hecht R.Radikalumlagerungen,VII:relative wanderungsgeschwindigkeiten substituierter phenylreste beim thermischen zerfall vonβ-aryl- perisovaleriansäuretert.-butylestern[J].Chemische Berichte,1965,98(8):2471-2477.
[26] Aureliano Antunes C S,Bietti M,Ercolani G,Lanzalunga O,Salamone M.The effect of ring substitution on the O-neophyl rearrangement of 1,1-diarylalkoxyl radicals.A product and time-resolved kinetic study[J].Journal of Organic Chemistry,2005,70(10):3884-3891.
[27] Gao P,Shen Y W,Fang R,Hao X H,Qiu Z H,Yang F,Yan X B,Wang Q,Gong X J,Liu X Y,Liang Y M.Coppercatalyzed one-pot trifluoromethylation/aryl migration/carbonyl formation with homopropargylic alcohols[J].Angewandte Chemie International Edition,2014,53(29):7629-7633.
[28] Kong W Q,Casimiro M,Merino E,Nevado C.Coppercatalyzed one-pot trifluoromethylation/aryl migration/desulfonylation and C(sp2)-N bond formation of conjugated tosyl amides[J].Journal of the American Chemical Society,2013,135(39):14480-14483.

