乳酸菌对猪肠道屏障功能的调节作用及其机制
谯仕彦 侯成立 曾祥芳
(中国农业大学动物科学技术学院,北京 100193)
乳酸菌(lactic acid bacteria,LAB)是一类能发酵碳水化合物产生大量乳酸的革兰氏阳性细菌的总称。乳酸菌广泛分布于人体和动物的消化道内,参与多种生理作用,影响宿主健康。目前,乳酸菌作为最主要的和最安全的益生菌来源已被广泛关注,而乳酸菌调控肠道屏障功能已成为研究的热点。乳酸菌发挥益生作用的主要机制是通过调节肠道屏障功能来实现的,这已被众多体内外试验所证实[1-2]。迄今为止,关于乳酸菌在猪上的应用研究报道很多,但相对于啮齿动物而言,关于乳酸菌对猪肠黏膜屏障作用的研究相对较少。本文拟就乳酸菌对猪肠道屏障功能的调节作用及其机制作一综述,以期能帮助对乳酸菌的深入认识和科学应用。
1 肠道屏障
肠道是机体营养物质消化吸收的主要场所,也是机体抵御异物的第一道防线。肠道屏障功能是指肠道将肠腔环境与机体内组织环境分隔开,防止肠道内致病性抗原、毒素以及致病微生物侵入的功能[3]。肠道屏障主要由肠道正常菌群、黏液层、肠上皮细胞层和肠道免疫系统组成(图 1)。
1.1 肠道正常菌群
肠道内寄居着1013~1014个微生物,构成了一个复杂的生态系统。随着近年来对机体微生物研究的深入,肠道微生物被认为是肠道防御屏障的重要组成部分。对于新生动物,肠道微生物在宿主肠道发育过程中发挥着重要作用[5]。正常情况下肠道微生态处于平衡状态,肠道内的有益菌如双歧杆菌、乳酸菌黏附于肠上皮细胞表面或分布于黏液层,形成菌膜屏障,抑制病原菌黏附定植[3]。乳酸菌分布于猪整个肠道的各个部分[6],可通过生物拮抗作用抑制病原微生物的生长繁殖[2,7]。与长白猪相比,金华猪对毒素源性大肠杆菌K88攻毒有更低的腹泻率,其原因是肠道内存在更多的乳酸菌数量[8]。肠道复杂的生态系统构建了肠道内的第一道屏障,不同种属微生物间的相互依赖和制衡,在有外界环境波动或遭受有害刺激时能更好地应对不良因素对机体造成的负面影响,从而起到保护肠道屏障的作用[9-10]。
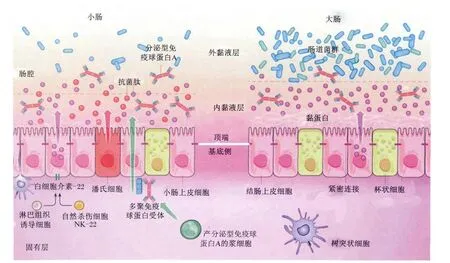
图1 肠道屏障功能Fig.1 The intestinal barrier function[4]
1.2 黏液层
肠道表面由单层上皮细胞排列而成,而肠上皮细胞表面覆盖着一层黏液。黏液主要由杯状细胞和肠上皮细胞分泌的黏蛋白(mucin)组成。黏蛋白是一种大分子的糖蛋白,主要分为肠上皮细胞顶端覆盖的跨膜黏蛋白以及杯状细胞分泌的凝胶状态的黏蛋白,其中膜结合蛋白主要有MUC1、MUC3、 MUC4、 MUC12、 MUC13、 MUC16、MUC17,分 泌 蛋 白 主 要 有 MUC2、MUC5AC、MUC5B、MUC6、MUC7[11]。黏蛋白有细菌黏附结合位点,可与肠上皮上的结合位点竞争,阻止病原微生物与肠上皮细胞结合。黏液层一方面可将肠上皮细胞与肠腔环境隔离开,另一方面为专性厌氧菌提供了良好的栖息环境,可促进双歧杆菌、乳酸菌等有益菌的生长繁殖[12]。
1.3 肠上皮细胞
肠上皮细胞是肠道黏膜屏障的重要组成部分,主要通过通透性来高效选择肠腔内的物质。一方面允许营养物质和其他可溶性的成分被吸收,另一方面阻止肠腔微生物、毒素等进入机体[13]。相邻肠上皮细胞间通过细胞连接毗邻,从上皮顶端到基底膜,连接复合体依次为紧密连接(tight junctions,TJs)、黏附连接、桥粒和缝隙连接[14],其中TJs与肠道营养物质的吸收和微生物的黏附关系最为密切,且TJs和黏附连接通过胞质衔接蛋白与肌动蛋白微丝相连,参与多种信号通路,比如调控细胞增殖、分化和极性[15]。TJs为多种蛋白相互作用下形成的复合结构,是上皮细胞选择性通透性的决定性因素,而Claudin-1、Occludin和ZO-1是TJs结构和功能单位中最为基础和重要的组成[16]。TJs是参与控制细胞旁渗透的关键分子[17],将上皮细胞之间的空隙密封,阻止微生物和其他抗原物质转运扩散穿过上皮细胞。
1.4 肠道免疫系统
肠黏膜免疫屏障主要由肠相关淋巴组织(gutassociated lymphoid tissue,GALT)构成,包括黏膜固有层及上皮细胞层内的淋巴细胞、孤立淋巴滤泡、肠系膜淋巴结、潘氏细胞等。肠道免疫系统受到刺激以后,可以分泌免疫球蛋白、白细胞介素和干扰素等蛋白和分子,通过免疫调节作用维持肠道上皮稳态。由B细胞转化为浆细胞产生,并穿过上皮细胞分泌至肠腔的分泌型免疫球蛋白A(sIgA)是肠道免疫屏障的重要方面,参与调控肠道菌群,维持肠道稳态。sIgA与非特异性免疫蛋白共同作用可阻止微生物黏附于上皮细胞,阻止损伤相关的炎症反应[18]。黏膜免疫球蛋白 A(IgA)一方面可以中和微生物毒素和病原微生物,另一方面可以调控肠道共生菌群[19]。IgA可维持不同肠段菌群的特异性[20]。另外,肠细胞分泌的内源抗菌肽是肠道先天性免疫的一部分。小肠内的柱状细胞能够产生再生胰岛素衍生蛋白3γ(REG3γ)和再生胰岛素衍生蛋白 3β(REG3β)[21],结肠柱状细胞主要表达β防御素和Cathelicidins,潘氏细胞能够表达多种抗菌肽,包括α防御素和血管生成素4(ANG4)等[22]。防御素可以破坏细菌细胞壁或细胞膜来发挥抑菌或杀菌作用[23]。
2 乳酸菌对猪肠道屏障功能的调节
2.1 乳酸菌对猪肠道菌群的调节
仔猪新生期是其个体发育的关键时期,由于肠道及机体免疫机能尚未发育完善,使得机体在受到外界环境应激的刺激下,仔猪极易遭受病原微生物侵袭,抵御能力低下,而新生期严重感染或应激对仔猪未来个体发育存在不可逆转的负面影响,从而影响个体发育的整个进程。在新生阶段给予乳酸菌干预,可以调控仔猪肠道微生物菌群的形成,帮助建立以有益菌为主体的肠道微生物菌群,介导机体肠道发育,促进机体消化生理成熟并增强抵御病原微生物感染的能力[24-25]。Liu等[26]研究发现,给新生仔猪灌服发酵乳酸杆菌(L.fermentum)I5007可以降低肠道潜在致病菌肠杆菌及梭菌属数量,有助于新生仔猪肠道菌群的建立。
肠道菌群建立后,乳酸菌可以影响肠道菌群结构,但这种影响作用是局部的[27]。乳酸菌可以降低仔猪肠道中潜在致病菌产气荚膜梭菌(Clostridium perfringens)的数量,并且乳酸菌干预的仔猪共生乳酸菌沿着绒毛-隐窝轴的分布与肠细胞关联更加密切[24]。Bezkorovainy[28]报道,饲粮中添加乳酸菌可提高肠道有益微生物的定植并降低肠道pH。Ohashi等[29]给断奶仔猪饲喂保加利亚乳杆菌(L.bulgaricus)2038发酵乳,能显著增加肠道中乳酸杆菌相对丰富度。肠道菌群结构的变化会改变肠道中短链脂肪酸的组成及数量[4],丁酸具有抵抗致病微生物侵袭并增强肠道屏障功能的作用[30],给新生仔猪灌服发酵乳酸杆菌I5007可增加后肠丁酸的数量[26]。
2.2 乳酸菌对猪肠绒毛结构的调节
小肠的绒毛高度、隐窝深度是衡量小肠消化吸收能力的重要指标。绒毛高度/隐窝深度综合反映小肠的功能状态,其比值的下降表明黏膜受损,消化吸收能力下降[8]。Yu 等[31]发现饲粮中添加发酵乳酸杆菌I5007能显著增加断奶仔猪回肠绒毛高度/隐窝深度。复合乳酸菌制剂可以增加肠黏膜比例、肠绒毛高度、RNA完整性以及刷状缘氨肽酶A和N活性,从而显著降低仔猪坏死性小肠炎评分[24]。Suo 等[32]研究表明,植物乳杆菌(L.plantarum)ZJ316可以显著提高断奶仔猪十二指肠、空肠以及回肠的绒毛高度。Wu等[33]研究发现,鼠李糖乳杆菌(L.rhamnosus)GG可以抑制病毒感染引起的猪回肠细胞凋亡,并可部分抑制病毒引起的组织损伤。
2.3 乳酸菌对猪肠道通透性的调节
肠道通透性是反映肠道黏膜屏障的重要指标,肠道通透性增大是屏障功能受损的主要表现之一,且易引起腹泻等疾病的发生。植物乳杆菌DSM 9843(2099v)和罗伊氏乳杆菌(L.reuteri)R2LC能明显改善氨甲喋呤诱导的鼠结肠炎的肠道通透性[34]。鼠李糖乳杆菌GG能减少乙醇诱导的肠黏膜高通透性及小肠和结肠中的氧化应激,显著减小结肠中嗜中性粒细胞的浸润和炎症[35]。此外,鼠李糖乳杆菌GG还能抑制肠出血性大肠杆菌引起的MDCK-1和T84细胞旁通透性的增加[36]。植物乳杆菌 DSM 2648可降低大肠杆菌O127∶H6(E2348/69)对跨上皮电阻的负作用和定植[37]。
TJs与肠上皮细胞的通透性密切相关。鼠李糖乳杆菌GG能够阻碍氧化应激对Caco-2细胞TJs和屏障功能的破坏[38]。L.sobrius DSM 16698(T)可以通过抑制产肠毒素大肠杆菌引起的闭锁小带ZO-1的移位,减少闭合蛋白的数量、F-肌动蛋白重排以及闭合蛋白的去磷酸化,维护黏膜屏障的完整性[39]。植物乳杆菌CGMCC 1258能阻止肠侵袭大肠杆菌(EIEC)引起的Caco-2单层细胞屏障功能的破坏和周边连接肌动蛋白微丝的改变[40]。用大肠杆菌Nissle 1917处理肠上皮细胞,可增加TJs蛋白ZO-2的表达,并且重新分配ZO-2从细胞质到细胞边界[41]。Yeung 等[42]研究表明,不同的乳酸菌对细胞表层完整性的调节不同,脂多糖(LPS)可以破坏上皮细胞屏障功能,乳酸菌则可维持TJs的完整性。
2.4 乳酸菌对猪黏液分泌的调节
在人和鼠上,有大量的研究证实乳酸菌可以促进肠道黏蛋白(MUC1、MUC2和 MUC3)的表达[2],但在猪上研究的相对较少。Yu 等[31]研究发现,断奶仔猪饲粮中添加5.8×107CFU/g的发酵乳酸杆菌I5007可提高肠道黏蛋白MUC2和MUC3的表达。Wang等[43]采用蛋白质组学方法比较研究了发酵乳酸杆菌I5007对新生仔猪空肠黏膜蛋白表达的影响,发现发酵乳酸杆菌I5007可提高空肠黏膜蛋白中与脂类代谢、细胞结构及活力相关的蛋白表达。
2.5 乳酸菌对猪肠道免疫屏障的调节
对仔猪新生期肠道微生物进行干预,可以影响宿主免疫机能朝向更加稳定的、不易受到侵害的方向发展[44]。罗伊氏乳杆菌可通过降低促炎因子的表达,提高调节性T细胞相关细胞因子的表达,介导肠道免疫耐性[45]。Liu 等[26]研究表明,间隔灌服发酵乳酸杆菌I5007可提高仔猪新生期血清中IgA的含量,相应提高了机体体液免疫能力。L.sobrius DSM 16698(T)可阻滞产肠毒素大肠杆菌诱发的白细胞介素-8(IL-8)分泌增加,同时上调白细胞介素 -10(IL-10)的表达[39]。植物乳杆菌ATCC 8014能抑制肿瘤坏死因子-α(TNF-α)引起的Caco-2细胞IL-8分泌的下降,维持上皮细胞屏障功能,并通过改变信号传导通路抑制炎症反应[46]。Liu 等[26]研究表明,发酵乳酸杆菌 I5007可以显著降低仔猪回肠炎性因子白细胞介素-1β(IL-1β)的表达。Vlasova 等[47]研究发现,乳酸菌可通过调节先天性免疫应答来促进新生仔猪的免疫稳态,缓解轮状病毒引起的腹泻。此外,选用乳酸菌作为免疫刺激要考虑剂量效应,同一种乳酸菌剂量的不同可以促进或抑制由干扰素-γ(IFN-γ)引起的T细胞或调节性T细胞免疫反应[48]。
3 乳酸菌对猪肠道屏障功能调节的机制
3.1 乳酸菌的代谢产物
乳酸菌产生的有机酸(主要是乳酸)是一种重要的抗菌物质,有机酸可以通过螯合金属离子和改变细菌细胞膜通透性来发挥作用,另外,有机酸还能降低肠道pH,抑制有害菌(如沙门氏菌、致病性大肠杆菌等)的生长繁殖[49]。许多乳酸菌的抑菌作用是通过乳酸来实现的,鼠李糖乳杆菌GG对鼠伤寒沙门菌的抑制作用是乳酸积累的结果[50]。乳酸菌产生的过氧化氢也是一种重要的抗菌物质,如约氏乳杆菌(L.johnsonii)NC533分泌的过氧化氢在体外可以有效杀死沙门氏菌[51]。格氏乳杆菌(L.gasseri)CRL 1421产生的乳酸和过氧化氢能使金黄色葡萄球菌细胞膜崩解,使其细胞内容物渗出,从而对其起到抑制作用[52]。
一些乳酸菌可以产生细菌素,这些细菌素可直接作用于病原菌,抑制病原菌在肠道内的生长繁殖[53]。乳酸乳球菌产生的细菌素乳链菌肽(nisin)、嗜酸乳杆菌产生的细菌素lactacin B、植物乳杆菌产生的细菌素plantaricin、罗伊氏乳杆菌的细菌素reuterin等,它们均可通过干扰细菌细胞膜通透性来杀死细菌[54-55]。唾液乳杆菌(L.salivarius)UCC118能保护鼠免受单核细胞增生李斯特氏菌的感染,其原因是唾液乳杆菌产生的细菌素发挥了作用[56]。乳酸乳球菌(Lactococcus lactis)产生的细菌素lacticin 3147能有效抑制艰难梭状芽胞杆菌的生长[57]。此外,研究发现细菌素及其产生系统可以直接发挥免疫调节作用[58]。
乳酸菌能够产生一些蛋白质而不经过直接的接触细胞发挥对肠道屏障的调节作用。例如鼠李糖乳杆菌GG的p40和p75蛋白可抑制细胞因子诱发的上皮细胞凋亡,降低TNF诱发的上皮细胞损伤并促进人和小鼠结肠上皮细胞生长[59]。格氏乳杆菌BL23也编码p40和p75同源蛋白,并且发挥类似的作用[60]。业已发现,鼠李糖乳杆菌GG编码的p40蛋白通过表皮生长因子受体介导的信号途径对肠道屏障功能发挥作用[61]。
3.2 乳酸菌竞争与排斥
乳酸菌一方面通过竞争营养物质来抑制病原菌的生长繁殖,另一方面,乳酸菌定植在肠上皮细胞,竞争性排斥肠道内源性及外源性潜在致病菌对肠上皮细胞的黏附、定植。德氏乳杆菌和嗜酸乳杆菌可以在其菌体表面螯合氢氧化铁,从而限制肠道内病原菌的利用[62]。大量的文献报道表明,乳酸菌可以抑制病原菌在肠上皮细胞上的定植[7,39]。在肠道定植和黏附是乳酸菌发挥作用的前提,乳酸菌可通过菌体表面的磷壁酸、多糖、S-层蛋白等与宿主细胞进行特异性黏附,这种特异性黏附在乳酸菌竞争与排斥功能中发挥了重要的作用[63]。
3.3 乳酸菌菌体表面成分
乳酸菌的细胞壁主要由肽聚糖、磷壁酸(lipoteichoic acid,LTA)、表面蛋白(surface layer protein,SLP)和胞外多糖(exopolysaccharides,EPS)组成(图2)。这些分子包含微生物相关分子模式(microorganism-associated molecular patterns,MAMPs),可以识别宿主肠黏膜上的特定模式识别 受 体 (pattern recognition receptors,PRRs)。PRRs在感染和先天性免疫过程中起着重要的作用,其中 Toll样受体(toll-like receptors,TLRs)和NOD样受体(nucleotide binding oligomerizationdomain-like receptors,NLRs)分别作为胞外和胞内模式识别受体。乳酸菌的菌体表面成分是乳酸菌发挥作用的重要因子,通过MAMPs与PRRs之间的作用产生一系列的免疫信号,调节肠道屏障功能(图3)。
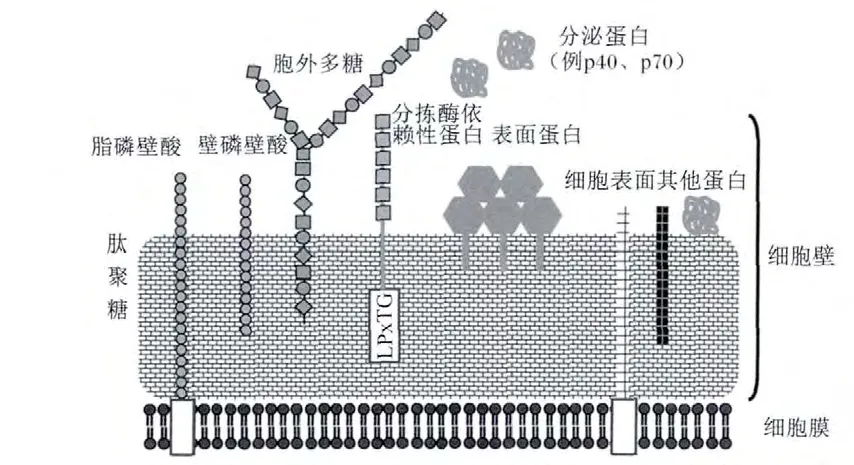
图2 乳酸菌菌体表面结构Fig.2 The surface structure of lactic acid bacteria[64]
肽聚糖及其衍生的胞壁肽是乳酸菌发挥益生功能的活性化合物。肽聚糖是乳酸菌细胞壁的主要成分,主要通过Toll样受体2(TLR2)的介导来发挥其在先天性免疫中的作用[58]。TLR2优先识别二氨基庚二酸型(Dap型)肽聚糖片段,并且可识别L-赖氨酸型(Lys型)肽聚糖片段,但亲和度较低[58]。然而肽聚糖和其他非酰化细胞壁成分诱导TLR2信号传导的能力是有争论的[65],一些研究表明,确认为肽聚糖沾染的脂蛋白是干扰因素[66]。在细胞内,肽聚糖通过 NOD样受体进行信号传导[65-67],NOD 样受体 1(NOD1)的配体是γ-D-谷氨酰基-内消旋二氨基庚二酸(γ-D-glu-meso-DAP,存在于包括乳酸菌在内的所有革兰氏阳性菌的肽聚糖结构中),NOD样受体2(NOD2)的配体是胞壁酸二肽(MurNAc-L-Ala-D-isoGln,MDP,存在于所有的肽聚糖中)[68]。然而一些研究认为,NLRs-肽聚糖相互作用仅发生在特定的益生菌中[69]。唾液乳杆菌 Ls33通过NOD2介导的信号途径诱导局部IL-10的产生,发挥抗炎作用,而嗜酸乳杆菌NCFM则没有这种效果,这是因为菌株Ls33肽聚糖结构中有一种胞壁肽(M-tri-Ly)的存在,这种配体以依赖 NOD2、不依赖髓样分化因子88(MyD88)的方式发挥作用[67]。
LTA存在于许多乳酸菌的细胞壁中,根据其在细胞表面上的固定方式,通常分为壁磷壁酸(wall-teichoic acid,WTA)和脂磷壁酸(lipoteichoic,LTA)。
WTA不深入质膜,通过磷酸二酯键共价锚定在肽聚糖的N-乙酰胞壁酸残基上,LTA则跨过肽聚糖层,其末端磷酸共价连接于质膜中糖脂的寡糖基部分[58]。LTA不仅可以作为黏附分子与肠上皮细胞结合,而且作为配体可以与宿主免疫细胞上的TLR2结合,调节肿瘤坏死因子的水平。在植物乳杆菌WCFS1和L-137上,TLR2受体的识别需要LTA骨架的D-丙氨酰化,而在植物乳杆菌KCTC10887BP和鼠李糖乳杆菌 GG71上,TLR2受体的识别要求并不严格[58]。由于试验设计的差异,关于LTA微妙结构的差异与免疫反应之间的关系并不明确。最近的研究表明,植物乳杆菌WCFS1的突变体(LTA不能D-丙氨酰化)诱导较少的TLR2依赖的促炎因子分泌,提高了对小鼠结肠炎模型的保护作用[70]。另外,在鼠李糖乳杆菌GG突变体上也得到了类似的结果[71]。这些研究表明,LTA骨架的修饰可以增加抗炎免疫调节。格氏乳杆菌Shirota和植物乳杆菌ATCC 14917的WTA在LTA通过TLR2途径介导IL-10产生过程中发挥协同作用[72]。但是关于WTA在TLR2信号通路中的作用还存在争议[58]。
EPS是乳酸菌在生长代谢过程中分泌到细胞壁外的一类糖类化合物,根据其分布位置可分为2种形式:一种紧密依附在细菌表面形成荚膜称为荚膜多糖(capsular polysaccharide,CPS),一种松散地分布在细菌表面称为黏液多糖(slime polysaccharides,SPS)。鼠李糖乳杆菌 GG突变株产生CPS的能力下降,反而提高了鼠李糖乳杆菌的黏附能力和生物膜形成能力[73]。相反,高水平的CPS可以保护鼠李糖乳杆菌GG抵抗肠道先天性免疫因子,如鼠李糖乳杆菌 LL-37[74]。格氏乳杆菌Shirota的胞外多糖抑制巨噬细胞促炎性免疫反应[75]。由于 CPS的糖原组成复杂,妨碍了CPS相关信号通路的研究。就目前而言,CPS在宿主免疫调节中的作用仍不明确。
SLP是乳酸菌细胞壁最外层的单分子亚结晶体排列蛋白,在乳酸菌黏附过程中起重要作用,一方面调节乳酸菌结合上皮细胞的能力,另一方面能够抑制病原菌的黏附。乳酸杆菌表面蛋白的同源性主要集中于C端,该区域主要负责蛋白对细胞外膜的锚定作用,而N端的区域主要与蛋白的自身组装及对细胞的黏附作用相关,变异较大。SLP是复杂的细胞壁结构的一部分,除了黏附功能以外,对SLP的其他功能了解得并不多,很多方面还处于假说阶段[58]。嗜酸乳杆菌NCFM 可以被树突状细胞表面特异性非整联蛋白,又称CD209(dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintergrin,DC-SIGN)识别(图3),而表面蛋白A(SlpA)缺陷株不能被识别,SlpA缺陷株反而引起人DCs92更多的促炎细胞因子谱[76]。其他乳酸菌的SLP是否具有同样的功能仍不清楚。分拣酶依赖性蛋白(sortase-dependent proteins,SDP)是镶嵌到细胞壁上带有LPxTG基序的表面蛋白,在乳酸菌与宿主相互作用之间发挥重要作用[77]。最新的研究表明,植物乳杆菌的SDP在宿主免疫调节中发挥重要作用[78]。
4 小结
大量研究证实,乳酸菌具有很好的改善猪肠道屏障功能的作用,这种作用与乳酸菌对肠道菌群结构、肠绒毛结构、上皮细胞通透性和TJs、黏液的分泌和免疫功能的调节有关。众多研究者从乳酸菌的表面分子组成中寻求微生物相关分子模式与宿主上皮细胞(包括树突状细胞)分子识别受体间的关系,进而解析乳酸菌调节肠道屏障功能的分子机制。但这种机制仍然具有很多的不确定性,且都是由体外细胞试验开展的。乳酸菌对肠道功能的调节具有很强的菌株特异性,且人和动物的肠道环境非常复杂。近年来,基因组学、蛋白质组学和代谢组学的发展,为从体内试验的角度探索乳酸菌的确切作用机制提供了新的认识和研究手段。
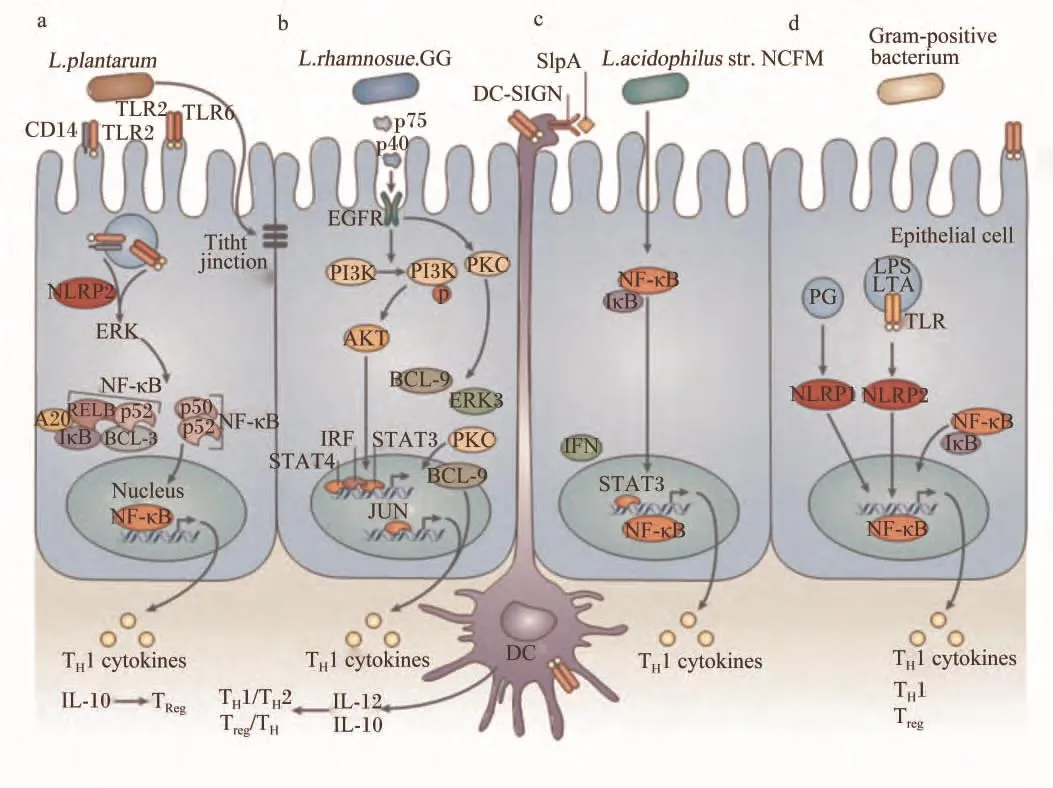
图3 乳酸菌与肠上皮细胞和树突细胞之间的相互作用机制Fig.3 The molecular interactions of lactic acid bacteria in general with intestinal epithelial cells and dendritic cells[58]
[1] MENNIGEN R,BRUEWER M.Effect of probiotics on intestinal barrier function[J].Annals of the New York Academy of Sciences,2009,1165(1):183-189.
[2] OHLAND C L,MACNAUGHTON W K.Probiotic bacteria and intestinal epithelial barrier function[J].A-merican Journal of Physiology:Gastrointestinal and Liver Physiology,2010,298(6):G807-G819.
[3] BAUMGART D C,DIGNASS A U.Intestinal barrier function[J].Current Opinion in Clinical Nutrition and Metabolic Care,2002,5(6):685-694.
[4] MAYNARD C L,ELSON C O,HATTON R D,et al.Reciprocal interactions of the intestinal microbiota and immune system[J].Nature,2012,489(7415):231-241.
[5] SOMMER F,BÄCKHED F.The gut microbiota-masters of host development and physiology[J].Nature Reviews Microbiology,2013,11(4):227-238.
[6] WALTER J.Ecological role of lactobacilli in the gastrointestinal tract:implications for fundamental and biomedical research[J].Applied and Environmental Microbiology,2008,74(16):4985-4996.
[7] LI X J,YUE L Y,GUAN X F,et al.The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus[J].Journal of Applied Microbiology,2008,104(4):1082-1091.
[8] GAO Y,HAN F,HUANG X,et al.Changes in gut microbial populations,intestinal morphology,expression of tight junction proteins,and cytokine production between two pig breeds after challenge with Escherichia coli K88:a comparative study[J].Journal of Animal Science,2013,91(12):5614-5625.
[9] HOOPER L V,MACPHERSON A J.Immune adaptations that maintain homeostasis with the intestinal microbiota[J].Nature Reviews Immunology,2010,10(3):159-169.
[10] SHANAHAN F.Probiotics in perspective[J].Gastroenterology,2010,139(6):1808-1812.
[11] JOHANSSON M E V,SJÖVALL H,HANSSON G C.The gastrointestinal mucus system in health and disease[J].Nature Reviews Gastroenterology and Hepatology,2013,10(6):352-361.
[12] TURNER J R.Intestinal mucosal barrier function in health and disease[J].Nature Reviews Immunology,2009,9(11):799-809.
[13] PODOLSKY D K.Mucosal immunity and inflammation.Ⅴ.Innate mechanisms of mucosal defense and repair:the best offense is a good defense[J].American Journal of Physiology,1999,277(3 Pt 1):G495-G499.
[14] GUTTMAN JA,FINLAY B B.Tight junctions as targets of infectious agents[J].Biochimica et Biophysica Acta(BBA):Biomembranes,2009,1788(4):832-841.
[15] CHIBA H,OSANAI M,MURATA M,et al.Transmembrane proteins of tight junctions[J].Biochimica et Biophysica Acta(BBA):Biomembranes,2008,1778(3):588-600.
[16] FURUSE M.Molecular basis of the core structure of tight junctions[J].Cold Spring Harbor Perspectives in Biology,2010,2(1):a002907.
[17] MIYAUCHI E,MORITA H,TANABE S.Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo[J].Journal of Dairy Science,2009,92(6):2400-2408.
[18] MACPHERSON A J,MCCOY K D,JOHANSEN F E,et al.The immune geography of IgA induction and function[J].Mucosal Immunology,2008,1(1):11-22.
[19] HAPFELMEIER S,LAWSON M A E,SLACK E,et al.Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses[J].Science,2010,328(5986):1705-1709.
[20] SUZUKI K,MEEK B,DOI Y,et al.Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut[J].Proceedings of the National Academy of Sciences of the United States of America,2004,101(7):1981-1986.
[21] VAISHNAVA S,YAMAMOTO M,SEVERSON K M,et al.The antibacterial lectin regⅢ gamma promotes the spatial segregation of microbiota and host in the intestine[J].Science,2011,334(6053):255-258.
[22] NIJNIK A,HANCOCK R E W.Host defence peptides:antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections[J].Emerging Health Threats Journal,2009,2:e1.
[23] MUNIZ L R,KNOSP C,YERETSSIAN G.Intestinal antimicrobial peptides during homeostasis,infection,and disease[J].Frontiers in Immunology,2012,3:310.
[24] SIGGERS R H,SIGGERS J,BOYE M,et al.Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs[J].The Journal of Nutrition,2008,138(8):1437-1444.
[25] HANSEN C H F,NIELSEN D S,KVERKA M,et al.Patterns of early gut colonization shape future immune responses of the host[J].PLoS One,2012,7(3):e34043.
[26] LIU H,ZHANG J,ZHANG S H,et al.Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets[J].Journal of Agricultural and Food Chemistry,2014,62(4):860-866.
[27] FUENTES S,EGERT M,JIMÉNEZ-VALERA M,et al.Administration of Lactobacillus casei and Lactobacillus plantarum affects the diversity of murine intestinal lactobacilli,but not the overall bacterial community structure[J].Research in Microbiology,2008,159(4):237-243.
[28] BEZKOROVAINY A.Probiotics:determinants of survival and growth in the gut[J].The American Journal of Clinical Nutrition,2001,73(Suppl.2):399S-405S.
[29] OHASHI Y,TOKUNAGA M,TAKETOMO N,et al.Stimulation of indigenous lactobacilli by fermented milk prepared with probiotic bacterium,Lactobacillus delbrueckii subsp bulgaricus strain 2038,in the pigs[J].Journal of Nutritional Science and Vitaminology,2007,53(1):82-86.
[30] GUILLOTEAU P,MARTIN L,EECKHAUT V,et al.From the gut to the peripheral tissues:the multiple effects of butyrate[J].Nutrition Research Reviews,2010,23(2):366-384.
[31] YU H F,WANG A N,LI X J,et al.Effect of viable Lactobacillus fermentum on the growth performance,nutrient digestibility and immunity of weaned pigs[J].Journal of Animal and Feed Sciences,2008,17(1):61-69.
[32] SUO C,YIN Y S,WANG X N,et al.Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality[J].BMC Veterinary Research,2012,8:89.
[33] WU S P,YUAN L J,ZHANG Y G,et al.Probiotic Lactobacillus rhamnosus GG mono-association suppresses human rotavirus-induced autophagy in the gnotobiotic piglet intestine[J].Gut Pathogens,2013,5(1):22.
[34] MAO Y,YU J L,LJUNGH A,et al.Intestinal immune response to oral administration of Lactobacillus reuteri R2LC,Lactobacillus plantarum DSM 9843,pectin and oatbase on methotrexate-induced enterocolitis in rats[J].Microbial Ecology in Health and Disease,1996,9(6):261-269.
[35] FORSYTH C B,FARHADIA A,JAKATE S M,et al.Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress,gut leakiness,and liver injury in a rat model of alcoholic steatohepatitis[J].Alcohol,2009,43(2):163-172.
[36] JOHNSON-HENRY K C,DONATO K A,SHEN-TU G,et al.Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157∶H7-induced changes in epithelial barrier function[J].Infection and Immunity,2008,76(4):1340-1348.
[37] ANDERSON RC,COOKSON A L,MCNABB W C,et al.Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function[J].FEMS Microbiology Letters,2010,309(2):184-192.
[38] SETH A,YAN F,POLK D B,et al.Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC-and MAP kinase-dependent mechanism[J].American Journal of Physiology:Gastrointestinal and Liver Physiology,2008,294(4):G1060-G1069.
[39] ROSELLI M,FINAMORE A,BRITTI M S,et al.The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage[J].The Journal of Nutrition,2007,137(12):2709-2716.
[40] QIN H,ZHANG Z,HANG X,et al.L.plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells[J].BMC Microbiology,2009,9:63.
[41] ZYREK A A,CICHON C,HELMSS,et al.Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζredistribution resulting in tight junction and epithelial barrier repair[J].Cellular Microbiology,2007,9(3):804-816.
[42] YEUNG C Y,CHIANG CHIAU J S,CHAN W T,et al.In vitro prevention of Salmonella lipopolysaccharide-induced damages in epithelial barrier function by various Lactobacillus strains[J].Gastroenterology Research and Practice,2013,doi:10.1155/2013/973209.
[43] WANG X Q,YANG F,LIU C,et al.Dietary supplementation with the probiotic Lactobacillus fermentum I5007 and the antibiotic aureomycin differentially affects the small intestinal proteomes of weanling piglets[J].The Journal of Nutrition,2012,142(1):7-13.
[44] MIHATSCH W A,BRAEGGER C P,DECSI T,et al.Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants[J].Clinical Nutrition,2012,31(1):6-15.
[45] WALTER J,BRITTON R A,ROOSS.Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm[J].Proceedings of National Academy of Sciences of the United States of America,2011,108:4645-4652.
[46] KO J S,YANG H R,CHANG J Y,et al.Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-α [J].World Journal of Gastroenterology,2007,13(13):1962-1965.
[47] VLASOVA A N,CHATTHA K S,KANDASAMY S,et al.Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs[J].PLoS One,2013,8(10):e76962.
[48] WEN K,LI G H,BUI T,et al.High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs[J].Vaccine,2012,30(6):1198-1207.
[49] PARVEZ S,MALIK K A,KANG S A,et al.Probiotics and their fermented food products are beneficial for health[J].Journal of Applied Microbiology,2006,100(6):1171-1185.
[50] DE KEERSMAECKER SC J,VERHOEVEN T L A,DESAIR J,et al.Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid[J].FEMS Microbiology Letters,2006,259(1):89-96.
[51] PRIDMORE R D,PITTET A C,PRAPLAN F,et al.Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity[J].FEMS Microbiology Letters,2008,283(2):210-215.
[52] OTERO M C,NADER-MACIAS M E.Inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus gasseri isolated from the vaginal tract of cattle[J].Animal Reproduction Science,2006,96(1/2):35-46.
[53] COTTER P D,HILL C,ROSS R P.Bacteriocins:developing innate immunity for food[J].Nature Reviews Microbiology,2005,3(10):777-788.
[54] BIERBAUM G,SAHL H G.Lantibiotics:mode of action,biosynthesis and bioengineering[J].Current Pharmaceutical Biotechnology,2009,10(1):2-18.
[55] CLEUSIX V,LACROIX C,VOLLENWEIDER S,et al.Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria[J].BMC Microbiology,2007,7:101.
[56] CORR S C,LI Y,RIEDEL C U,et al.Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118[J].Proceedings of the National Academy of Sciences of the United States of America,2007,104(18):7617-7621.
[57] REA M C,CLAYTON E,O’CONNOR P M,et al.Antimicrobial activity of lacticin 3,147 against clinical Clostridium difficile strains[J].Journal of Medical Microbiology,2007,56(Pt 7):940-946.
[58] BRON P A,VAN BAARLEN P,KLEEREBEZEM M.Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa[J].Nature Reviews Microbiology,2012,10(1):66-78.
[59] YAN F,CAO H W,COVER T L,et al.Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth[J].Gastroenterology,2007,132(2):562-575.
[60] BÄUERL C,PÉREZ-MARTÍNEZ G,YAN F,et al.Functional analysis of the p40 and p75 proteins from Lactobacillus casei BL23[J].Journal of Molecular Microbiology and Biotechnology,2010,19(4):231-241.
[61] YAN F,CAO H W,COVER T L,et al.Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism[J].The Journal of Clinical Investigation,2011,121(6):2242-2253.
[62] ELLI M,ZINK R,RYTZ A,et al.Iron requirement of Lactobacillus spp.in completely chemically defined growth media[J].Journal of Applied Microbiology,2000,88(4):695-703.
[63] DEEPIKA G,CHARALAMPOPOULOS D.Surface and adhesion properties of lactobacilli[J].Advances in Applied Microbiology,2010,70:127-152.
[64] LEBEER S,VANDER LEYDEN J,DE KEERSMAECKER S C J.Genes and molecules of lactobacilli supporting probiotic action[J].Microbiology and Molecular Biology Reviews,2008,72(4):728-764.
[65] TRAVASSOSL H,GIRARDIN SE,PHILPOTT D J,et al.Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition[J].EMBO Reports,2004,5(10):1000-1006.
[66] VOLZ T,NEGA M,BUSCHMANN J,et al.Natural Staphylococcus aureus-derived peptidoglycan fragments activate NOD2 and act as potent costimulators of the innate immune system exclusively in the presence of TLR signals[J].The FASEB Journal,2010,24(10):4089-4102.
[67] FERNANDEZ E M,VALENTI V,ROCKEL C,et al.Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide[J].Gut,2011,60(8):1050-1059.
[68] DELCOUR J,FERAIN T,DEGHORAIN M,et al.The biosynthesis and functionality of the cell-wall of lactic acid bacteria[J].Antonie Van Leeuwenhoek,1999,76(1/2/3/4):159-184.
[69] ZEUTHEN L H,FINK L N,FRØKIÆR H.Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gutderived lactobacilli and bifidobacteria in dendritic cells[J].Immunology,2008,124(4):489-502.
[70] GRANGETTE C,NUTTEN S,PALUMBO E,et al.Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids[J].Proceedings of the National Academy of Sciences of the United States of America,2005,102(29):10321-10326.
[71] CLAESI JJ,LEBEER S,SHEN C,et al.Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis[J].Clinical & Experimental Immunology,2010,162(2):306-314.
[72] KAJI R,KIYOSHIMA-SHIBATA J,NAGAOKA M,et al.Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages[J].The Journal of Immunology,2010,184(7):3505-3513.
[73] LEBEER S,VERHOEVEN T L A,FRANCIUS G,et al.Identification of a gene cluster for the biosynthesis of a long,galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase[J].Applied and Environmental Microbiology,2009,75(11):3554-3563.
[74] LEBEER S,CLAES I J J,VERHOEVEN T L A,et al.Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine[J].Microbial Biotechnology,2011,4(3):368-374.
[75] YASUDA E,SERATA M,SAKO T.Suppressive effect on activation of macrophages by Lactobacillus casei strain shirota genes determining the synthesis of cell wall-associated polysaccharides[J].Applied and Environmental Microbiology,2008,74(15):4746-4755.
[76] KONSTANTINOV SR,SMIDT H,DE VOSW M,et al.S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions[J].Proceedings of the National Academy of Sciences of the United States of America,2008,105(49):19474-19479.
[77] BOEKHORST J,DE BEEN M W,KLEEREBEZEM M,et al.Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs[J].Journal of Bacteriology,2005,187(14):4928-4934.
[78] REMUS D M,BONGERS R S,MEIJERINK M,et al.Impact of Lactobacillus plantarum sortase on target protein sorting,gastrointestinal persistence,and host immune response modulation[J].Journal of Bacteriology,2013,195(3):502-509.

