Intracellular adenosine-tri-phosphate levels are lower in pediatric liver transplant recipients with infection
Rao Wei,Xie Man,Sun Xiaoye,Yang Tao,Gao Wei,Zhang Jianjun,Liu Yihe,Deng Yonglin,Shen Zhongyang(.Department of Organ Transplantation,First Center Hospital,Tianjin 0092,China;2.Tianjin Key Laboratory of Organ Transplantation,Tianjin 0092,China;.Department of Digestive Diseases,First Center Hospital,Tianjin 0092,China)
【Abstract】 Objective To analyze intracellular adenosine-tri-phosphate(iATP)levels in pediatric liver transplant(PLT)recipients and to investigate its correlation with patients' clinical status.Methods Sixtythree PLT patients were recruited to this study.Patients were divided into clinically stable,and infection groups.Association of iATP levels with episodes of infection was analyzed.Results Among 63 patients,120 iATP values were obtained including serial iATP measurements from 35 patients(55.6%).The average iATP value of the 64 samples from 39 clinically stable children was(372±225)μg/L.High inter-individual iATP variability was observed in clinically stable patients and iATP levels appeared stable in individual patients with serial data.Twenty-four PLT recipients with infection had significantly lower iATP at time of infection compared with clinically stable recipients(P<0.001).Serial measurements indicated that the average iATP value decreased significantly as infection progressed(P<0.001),and increased as infection resolved(P=0.036).However,12 of the 39 clinically stable patients with low iATP values(iATP < 225 μg/L)showed no invasive infection,while 8 of the 24 infected patients showed high levels of iATP during infection(iATP > 225 μg/L).Conclusions Compared with clinically stable patients,lower iATP levels were observed in infected PLT recipients.Levels became elevated as infection resolved.The inter-individual variability observed in our study indicates that cut-off values of iATP may not be suitable for defining immune status.Serial monitoring data may be more helpful to determine the cellular immune response in PLT recipients.
【Key words】 Intracellular adenosine-tri-phosphate; Infection; Pediatrics; Liver transplantation
Introduction
Although maintenance of adequate immunosuppression is vital in preventing acute rejection following pediatric liver transplantation(PLT),overimmunosuppression may lead to undesirable complications,such as infection,cancer,lymphoproliferative disorder and drug toxicity.It is well established that infection is one of the most important causes of mortality and morbidity in all transplant recipients.1Balancing immunosuppression to prevent rejection yet minimize risk of infection or drug toxicity is a major clinical challenge.
Until recently,very few methods were applied to evaluate immune function after organ transplant.In 2002,the Food and Drug Administration(FDA)approved an in vitro assay,ImmuKnow(Cylex),to assess cell-mediated immune responses by measuring intracellular adenosine-tri-phosphate(iATP)production after CD4+cell activation.This assay was intended to indicate cellular immune function and determine the risk of infection and rejection after organ transplant.However,the potential clinical application of iATP detection remains controversial.2-4The majority of studies were carried out in adult patients,while only a few studies documented the correlation between iATP and clinical events(stable,infection and rejection)in pediatric organ recipients and iATP data in PLT recipients are very limited.Herein,we retrospectively investigate the correlation between iATP and clinical events in PLT recipients to identify whether the ImmuKnow assay is a valid tool to predict clinical events.
Methods
Patients and sample collection
Between September 2010 and January 2013,all PLT patients admitted for liver transplant at our center,First Central Hospital,Tianjin,were retrospectively recruited.Inclusion criteria were availability of iATP assay data and complete clinical records.All of the iATP tests were performed as clinical features occurred or during routine follow-up.Exclusion criteria included:>12 years of age;iATP tested before transplant;combined organ transplant;rejection and rejection-infection coincidence.
The medical records of all enrolled patients were reviewed,including the clinical status(infection,rejection,stable)and other laboratory results〔levels of tacrolimus(Tac),white cell count,lymphocyte count,and liver enzymes〕at the time of iATP testing.Demographic data included age,gender,date of birth,date of liver transplant,type of graft,and follow-up time.All clinical data were followed until March 2013.
A clinically stable status was defined as patients who had good liver functions,and general good health,in whom infection or rejection were excluded.Post-transplant infection was diagnosed based on clinical features,positive microbiologic(tests PCR and culture)and imaging.Rejection was determined by both liver function tests and biopsy-proven pathological analysis.The resolution of infection was defined as negative microbiologic tests and disappears of clinical features.
Sodium heparin anti-coagulated whole blood samples were collected and tested in our laboratory at First Central Hospital.The assay was completed within 24 hours of sample collection and all assay-related procedures were performed by specialized technicians.The immune response was measured using the FDA-approved Cylex ImmuKnow(Cylex,Columbia,MD)assay according to the manufacturer's instructions.
Statistical analysis
Data are presented as mean±standard deviation or the median and range,as appropriate.In the box and whisker diagrams,data are represented as medians(middle line in the box)with 25th and 75th percentiles(box),10th and 90th percentile(whiskers),and outliers(dots).Categorical variables were compared using the chi-square test.Differences between clinically stable patients and those with infection were analyzed using the Mann-Whitney rank sum test.The paired t- test was used to determine the difference in serial data from infected individuals before and after infection.Correlation between iATP and other clinical data were analyzed using spearman rank correlation.A P value less than 0.05 was considered statistically significant.All statistical analyses were performed using SigmaPlot software(version.12.0,Systat Software,Inc.).
Results
Demographics and baseline characteristics within clinical status groups
Between September 2010 and January 2013,109 PLT patients were admitted to our center.81 received iATP assay test but 7 patients lacked adequate clinical data,resulting in exclusion of these individuals from the study.
In the remaining 74 patients,three were older than 12 years old,three were tested for iATP levels before transplant,two received combined organ transplant,two was rejection and one was rejectioninfection coincident,leaving 63 eligible patients in total.Finally,a total of 120 immune function assays were completed in 63 patients,including 30 girls(47.6%)and 33 boys(52.4%).The median age was 1.54 years,ranging from 0.52 months to 9.84 years.Samples were obtained at a median interval time of 94 days after transplant(range of 7-2,148 days,mean time:215±320 days).
All 63 patients were of Han ethnicity and received ABO-compatible PLT with piggy-back techniques.Biliary atresia was the leading indication of PLT in 62 patients(98.4%),the final patient presenting with Caroli's disease.Fifty-two transplantations were living donor liver transplants,and eleven patients received deceased donor liver transplants.All patients received Tac-based immunosuppressive therapy after transplant.No significant difference was observed between subgroups in demographic data(Table 1).
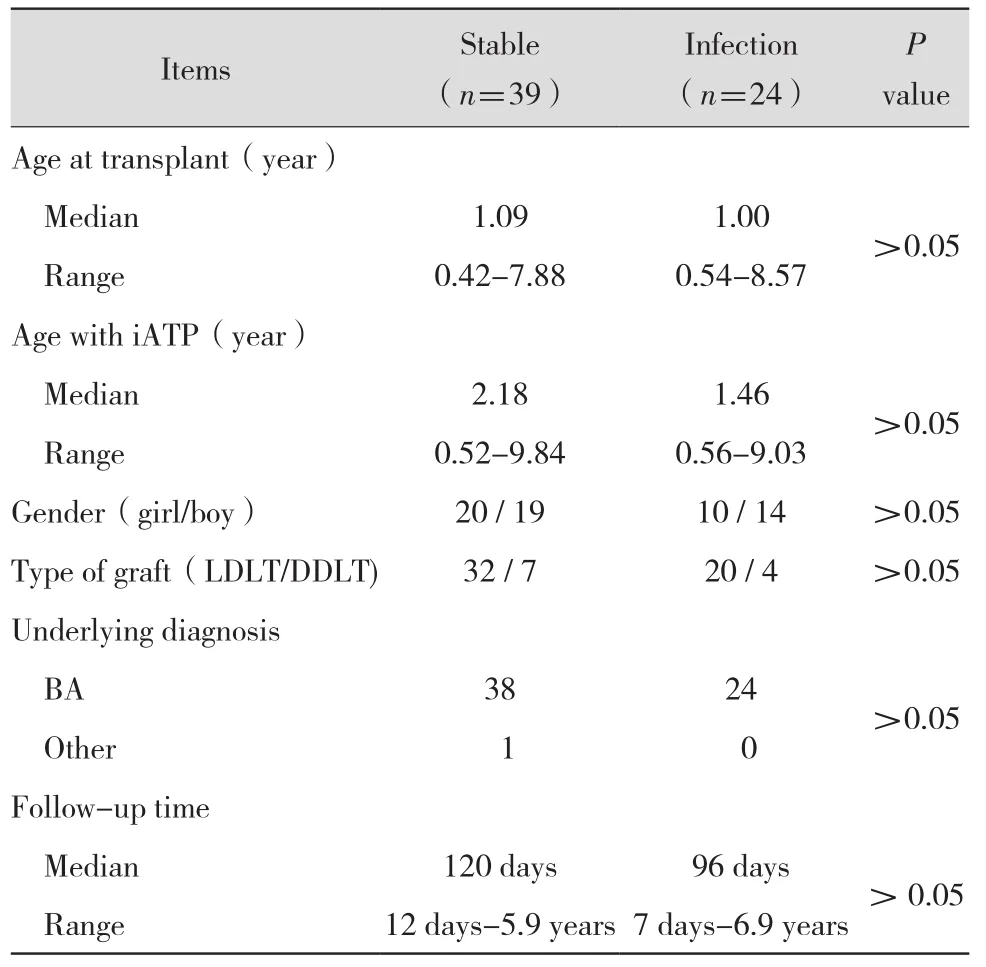
Table 1 Demographic data of pediatric liver transplant recipients
Twenty-nine episodes of infection were confirmed in 24 patients,with 21 occurrences within 6 months of transplantation.Viral infections accounted for 65.5%(19/29)of these episodes,in which cytomegalovirus(CMV)and Epstein-Barr virus(EBV)infection were most common(11/19 versus 5/19; 57.9% versus 26.3%).8 of CMV and 3 of EBV infected recipients was only viremia and others accompanied with clinical symptoms,such as fever and liver injury.Bacterial andfungal infection accounted for six and four episodes,respectively(Table 2).Acute rejection was identified in two patients by clinical manifestation and liver biopsy.Among the 120 iATP samples obtained,64 samples were collected from clinically stable patients(n=39),56 were collected from patients with infection(n=24).Serial iATP measurements were performed in 35 patients(55.6%)with a median of 2.6 samples collected(range 2-6)and a median interval of 71 days(range 14-352)between consecutive samples.

Table 2 Infections detected on surveillance
Distribution of iATP in patients with clinically stable status
The average value of iATP in clinically stable PLT recipients was 372±225 μg/L,with a median value of 325 μg/L(range 10-950 μg/L)between 7 and 2 148 days after transplantation(Figure 1).Interindividual variance of iATP was apparent in our study but a relatively stable level of iATP was observed in individual patients with serial data(Figure 2).
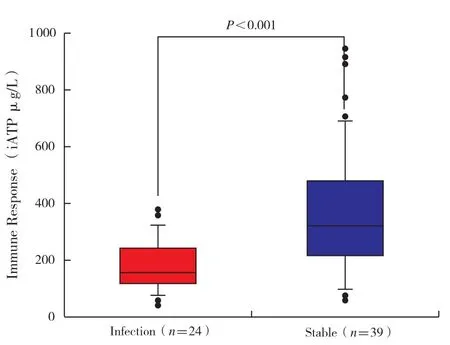
Figure 1 iATP in infection and clinically stable patient groups.Data are given as median and individual distribution; statistical analysis was calculated with the Mann-Whitney rank sum test
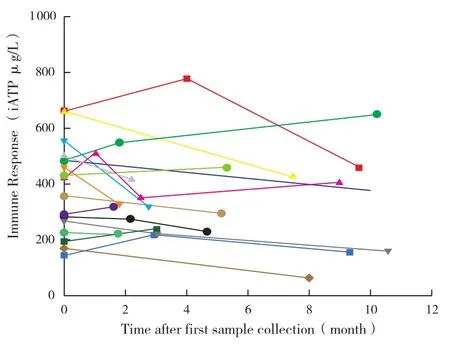
Figure 2 Distribution of serial iATP measurements in clinically stable patients
Correlation of immune function response with other clinical factors after PLT
The average trough level of Tac was 6.1±2.6 μg/L.No correlation between the trough levels of Tac and iATP was observed(Figure 3A ;r=0.202,P=0.110).Several additional clinical factors were analyzed,including age,total white cell count,lymphocyte count and time after transplantation,which may affect the immune cellular response.The level of iATP was not correlated with time after transplantation(r=-0.112,P=0.377),age(r=-0.170,P=0.179),lymphocyte count(r=0.110,P=0.438 ;data not shown),but correlated with white cell count(r=0.450,P<0.001;Figure 3B).
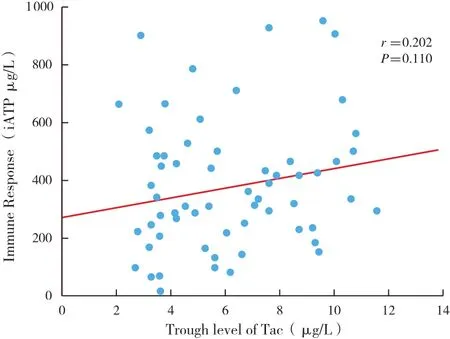
Figure 3 A No correlation was observed between iATP and trough level of Tac(r=0.202,P=0.110)

Figure 3 B The value of iATP was correlated with white blood cell count(r=0.450,P < 0.001)
Correlation of iATP with clinical status
Compared with clinically stable recipients,infected PLT recipients demonstrated significantly lower iATP levels upon infection(187±87 μg/L versus 372±225 μg/L,P < 0.001).Because of the obvious interindividual variance of iATP levels,data from infected patients undergoing serial iATP monitoring were analyzed(n=12).The average pre-infection iATP value was not significantly different from that observed in clinically stable patients(473±157 μg/L versus 372±225 μg/L,P=0.063 ; data not shown).However,the average value of iATP was significantly decreased upon infection(Figure 4 ; 189±59 μg/L versus 473±157 μg/L,P < 0.001),and became elevated as the infection resolved(Figure 4 ;341±100 μg/L versus 189±59 μg/L,P=0.036).The post-infection iATP level was similar to the value observed before infection(Figure 4; 341±100 μg/L versus 473±157 μg/L,P=0.344).Of the patients undergoing infection,eight had a relatively high level of iATP(> 225 μg/L)when infection occurred.Conversely,12 of the clinically stable patients demonstrated low iATP values(< 225 μg/L)in the absence of active infection.The sensitivity rate of iATP for infection was only 66.7%and specificity rate was 69.2% when the cut-off value was 225 μg/L.
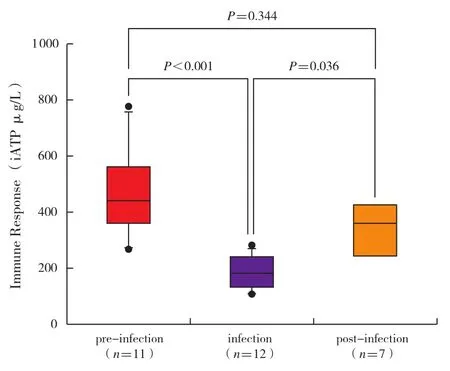
Figure 4 Serial iATP measurements in patients with infection.Statistical analysis was calculated using the paired t-test
Serial monitoring of iATP in two patients with CMV infection
Two patients with CMV infection who had serial monitoring data are presented(Figure 5).Both received liver transplant for biliary atresia within the first year of life.A close relationship between low iATP level and CMV viremia was observed,leading to regulation of immunosuppressive drugs.Viremia was accompanied by low levels of iATP,while resolution of viremia correlated with elevated iATP.No relationship was found between the trough level of Tac and CMVinfection.
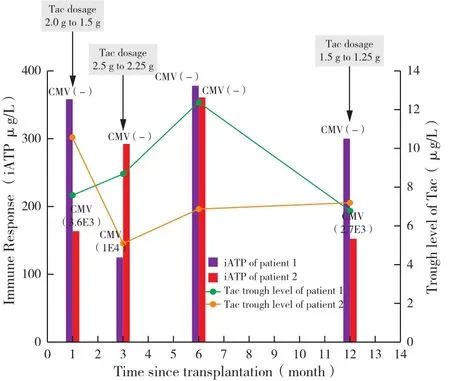
Figure 5 Clinical course of the two described patients with CMV viremia.In addition to iATP levels, trough levels of Tac and CMV PCR results are shown
Discussion
In the past decade,the Cylex ImmuKnow assay has been approved by the FDA to measure iATP levels in activated CD4+T cells after solid organ transplants,giving an evidence of the immune activation status of patients.This assay held promise as a potentially useful tool for predicting infections or rejection following transplantation.However,its actual contribution remained controversial in both adult and pediatric patients.4Rossano and colleagues5suggested that iATP values were not predictive of acute rejection or significant infections in pediatric heart transplant patients and therefore,that this assay cannot be recommended as part of the routine management of such individuals.On the contrary,a study in pediatric lung transplantation observed that the average iATP level was significantly lower in patients suffering EBV-driven post-transplant lymphoproliferative disease when compared to those without this complication.6Schulz-Juergensen and colleagues first demonstrated that iATP was a predictor for infection(n=3)and allograft rejection(n=4)in PLT recipients.7However,the low number of patients assessed limits the conclusions that may be drawn from such a study.To our knowledge,the current study is the first to assess the use of the Cylex ImmuKnow assay as a measurement of functional immunity in PLT recipients,using a relatively large infection group(n=24).We have described the distribution of iATP in PLT recipients in a single center.Importantly,although a significant association between low levels of iATP and infection was observed in our study,compatible with previous studies,high inter-individual variability of iATP values between clinically stable patients was also found.
For healthy children under 12 years,the ImmuKnow assay immune function zones of strong,moderate and low were proposed to be iATP>395,175-395 and < 175 μg/L respectively,while for healthy children over 12 years,the corresponding zones were similar to those of adults,determined as> 525 μg/L,225-525 μg/L and<225 μg/L described in the previous study8However,further research showed that children had a similar or even higher level of iATP compared with adults after liver transplant.7,9-10Israeli and colleagues10first reported the cellular immune response(measured as iATP μg/L)in PLT recipients in 2008.For children under and over the age of 12,the average value of iATP for clinically stable recipients was 322 μg/L and 286 μg/L,respectively.Recently,a study carried out in Germany found,in accordance with Israeli,that children under 12 years had a higher median iATP level than older children in both healthy patients(399 μg/L versus 342 μg/L)and stable liver transplantation recipients(280 μg/L versus 240.5 μg/L).However,no overall significant difference was observed.7In our study,the average iATP value(372±225 μg/L)was higher than the previously published results.Because biliary atresia was the most common indication for PLT in our center,and the majority of these patients received PLT withinthe first year of life(median 1.54 years),the higher iATP levels observed may be related to the younger age of patients in our study.
In comparison to the 39 clinically stable patients,the 24 patients with infection showed significantly lower iATP levels upon infection(P<0.001).After analyzing the serial sample data in infected patients,the average value of iATP before infection was similar to clinically stable patients(P=0.063),but significantly decreased during infection(P<0.001)and increasing again as infection resolved(P=0.036).This was in agreement with a previous study in adult lung transplant.11However,12 of the 39 clinically stable patients had lower iATP values(< 225 iATP μg/L)without invasive infection,while 8 of 24 infected patients had higher iATP values(> 225 μg/L)upon onset of infection.The sensitivity rate of iATP for infection was only 66.7% and specificity rate was 69.2% when the cut-off value was 225 μg/L,which was not as good as expected.
Additionally,serial sample data of clinically stable patients were analyzed,and a relatively stable value of iATP was observed for individual patients(Figure 2).We hypothesized that the previously reported discrepancies in results between researchers was due to the individual variability.Estimating the risk of clinical events based on a single iATP test appears to be arbitrary and the definition of the risk zones may not be a reliable method to predict subsequent clinical events.
No correlation between the value of iATP and trough levels of Tac was observed in our study,which was consistent with previous results in pediatric renal8,heart5liver7,9and adult lung11transplantation.Furthermore,one previous study documented that trough levels of Tac could not adequately predict the status of immunosuppression in pediatric recipients.12Our result suggested iATP might provide independent information to estimate the overall immune response,which may be useful in guiding immunosuppressive therapy in organ transplant recipients.
The retrospective observational approach of the current study is a major limitation.Further study should be focused on correlation of sequential iATP levels with patients' clinical course.A further limitation is the relatively short follow-up period,as the Cylex ImmuKnow Assay was not available in our center until 2010.However,we have given a detailed description of iATP in clinically stable,infected and rejection patients in this study,which provides preliminary data for future research.Data from larger studies relating to patient diagnosis,treatment and various immunosuppressant regimens,along with a comparison to other immune function tests following PLT may help to establish the clinical merit of this assay as a standard test for optimal monitoring of the immune function in PLT patients.
In conclusion,lower iATP levels were observed in PLT recipients undergoing infection,compared with clinically stable patients.iATP levels in these patients increased upon resolution of infection.As significant inter-individual variability of iATP was observed in our study,cut-off values of iATP may not be suitable for defining immune reactivity in PLT recipients.Serial monitoring data may be more relevant to assist the clinician in assessing the immune status of patients.Further longitudinal and larger studies are needed to evaluate the efficacy of using this assay to identify patients at risk of infection or graft rejection after PLT.

