UGT1A1*28和UGT1A1*6基因多态性与伊立替康不良反应的关系
张 勇,苏 丹,郭晓川,房 慧,白 莉
解放军总医院 肿瘤内科,北京 100853
UGT1A1*28和UGT1A1*6基因多态性与伊立替康不良反应的关系
张 勇,苏 丹,郭晓川,房 慧,白 莉
解放军总医院 肿瘤内科,北京 100853
目的探讨UGT1A1*28和UGT1A1*6基因多态性在中国人中的分布,并评价其与伊立替康不良反应之间的关系。方法收集2011年3月- 2012年3月在我科住院治疗的158例恶性肿瘤患者的外周血,检测其UGT1A1*28和UGT1A1*6基因型,其中132例使用伊立替康方案化疗,比较不同基因型患者的不良反应差异。结果158例中,UGT1A1*28野生型TA6/6者126例(79.7%),杂合突变型TA6/7者30例(19.0%),纯合突变型TA7/7者2例(1.3%);64例进行UGT1A1*6基因检测,G/G野生型40例(62.5%),G/A杂合突变型23例(35.9%),A/A纯合突变型1例(1.6%)。UGT1A1*28基因突变可增加2 ~ 4级迟发性腹泻发生率(TA6/6者15.0%、TA6/7者34.8%、TA7/7者50.0%,P=0.000);联合UGT1A1*28和UGT1A1*6基因型,野生型(TA6/6且G/G)患者发生2~4级迟发性腹泻和3~4级中性粒细胞减少的概率明显低于单点变异型和双点变异型(13.0%、22.2%、100.0%,P=0.004;8.7%、25.9%、66.7%,P=0.045)。结论UGT1A1*28和UGT1A1*6基因突变患者使用含伊立替康化疗方案时不良反应发生率较高。与单一检测一个位点相比,联合检测UGT1A1*28和UGT1A1*6基因型能更准确地预测伊立替康不良反应。
UGT1A1;基因多态性;伊立替康;不良反应
伊立替康(irinotecan,CPT-11)是半合成水溶性喜树碱衍生物,目前广泛应用于胃肠癌和小细胞肺癌的治疗,但其临床应用受到不良反应的限制,且个体差异明显。迟发性腹泻和中性粒细胞减少是伊立替康的剂量限制性毒性,两者发生率分别可达到约30%和46%,甚至可导致患者死亡[1]。药物代谢的遗传多态性是造成个体间不良反应差异的主要因素之一。伊立替康在人体组织内被羧酸酯酶(carboxylesterases,CE)水解转化为7-乙基-10-羟基喜树碱(7-ethyl-10-hydroxy-camptothecin,SN-38),SN-38为DNA拓扑异构酶Ⅰ(TopoⅠ)抑制剂,CPT-11及SN-38作用于细胞周期S期,通过抑制TopoⅠ,诱导DNA单链损伤、阻断DNA复制而产生细胞毒效应[2-3]。研究证实尿苷二磷酸葡糖苷酸转移酶1A1(UGT1A1)是SN-38体内代谢失活的关键酶,使SN-38转变为糖基化SN-38(SN-38G),UGT1A1酶的功能及其基因多态性(SNP)与伊立替康的毒性有着密切关系[4-5]。此外,亦有研究证实,不同人种的UGT1A1基因多态性分布存在明显差异,这可能导致伊立替康在不同人种的不良反应存在差异[6]。为进一步明确中国人UGT1A1基因多态性的分布情况、了解UGT1A1基因多态性与伊立替康毒性的关系、探讨检测UGT1A1基因多态性对指导个体化治疗的临床意义,本研究对158例肿瘤患者的UGT1A1基因多态性进行检测,并对采用伊立替康化疗的患者情况进行分析。
对象和方法
1 研究对象 2011年3月- 2012年3月在我科住院治疗的158例恶性肿瘤患者。肠癌105例、胃癌21例、肺癌26例、其他癌肿6例。所有患者均进行UGT1A1*28基因型检测,64例同时进行UGT1A1*6基因型检测;132例使用伊立替康为基础化疗方案。
2 UGT1A1基因检测 收集患者治疗前的外周血2 ml,抗凝处理后冻存于-20℃冰箱,根据外周血DNA提取试剂盒操作手册(北京天根公司),分批次统一提取基因组DNA。应用Primer5.0软件设计引物,PCR法扩增UGT1A1基因的相应片段,每个25 μl的PCR反应包括5 ng模板DNA、10×KOD plus buffer、1mmol/L dNTPs、0.4 mmol/L MgSO4、0.5 U KOD plus酶 (日本 TOYOBO 公司 )、0.25μmol/L正向和反向引物。PCR反应条件为:初始变性94℃2 min 30 s;变性94℃ 30 s,各个退火温度1 min,延伸68℃ 1 min,35个循环;68℃延伸7 min。UGT1A1基因片段的引物为:正向引物5'-GCCAGTTCAACTGTTGTTGC-3',反向引物5'-GTCCGTCAGCATGACATCAA-3';退火温度为57℃。应用ABI-3730测序仪双向测序分析PCR产物,Polyphred5.04对SNP进行检测分析,全部SNP经过人工校读。
3 药物不良反应评价标准 依据美国国立癌症研究所药物不良反应评价标准(NCI-CTCAE)4.0,对中性粒细胞减少、迟发性腹泻进行评价。
4 统计学方法 应用SPSS18.0软件对所有数据进行统计学分析,率的比较采用χ2检验或Fisher'S精确概率法,以P<0.05为差异有统计学意义。
结 果
1 UGT1A1*28基因多态性分布 158例患者中,126例(79.7%)UGT1A1基因启动子区TA序列呈6次重复,为TA6/6野生型;30例(19.0%)UGT1A1基因启动子区TA序列呈6次和7次重复,为TA6/7杂合突变型;2例(1.3%)呈7次重复,为TA7/7纯合突变型。UGT1A1*28基因多态性测序结果见图1。
2 UGT1A1*6基因多态性分布 158例患者中64例进行了UGT1A1*6基因检测,40例(62.5%)UGT1A1基因第1外显子211位点为G/G野生型;23例(35.9%)UGT1A1基因第1外显子211位点为G/A杂合突变型;1例(1.6%)UGT1A1基因第1外显子211位点为A/A纯合突变型。UGT1A1*6基因多态性测序结果见图2。
3 UGT1A1*28及UGT1A1*6基因多态性分布 上述64例同时进行了UGT1A1*28及UGT1A1*6基因检测,根据突变数将其分为3组:野生型(TA6/6且G/G)27例(42.2%),单点变异型(TA6/7且G/G、TA6/6且G/A)33例(51.6%),双点变异型(TA6/7且G/A、TA7/7且G/G、TA6/6且A/A)4例(6.2%)。见表1。

图 1 UGT1A1*28基因多态性代表性测序结果Fig. 1 Polymorphism sequencing of wild type TA 6/6 (A),heterozygotic genotype TA 6/7 (B), and homozygotic genotype TA 7/7 (C) in UGT1A1*28 gene
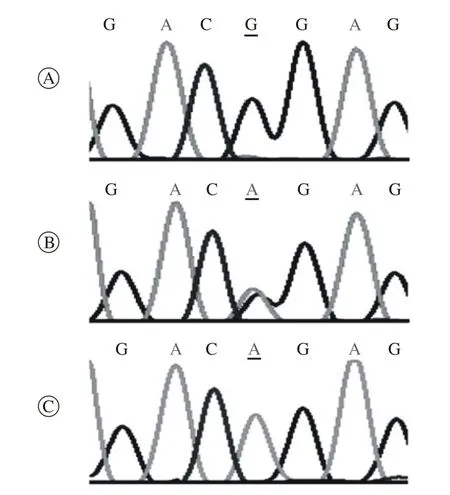
图 2 UGT1A1*6基因多态性代表性测序结果Fig. 2 Polymorphism sequencing of wild type G/G (A), heterozygotic genotype G/A (B) and homozygotic genotype A/A (C) in UGT1A1*6 gene
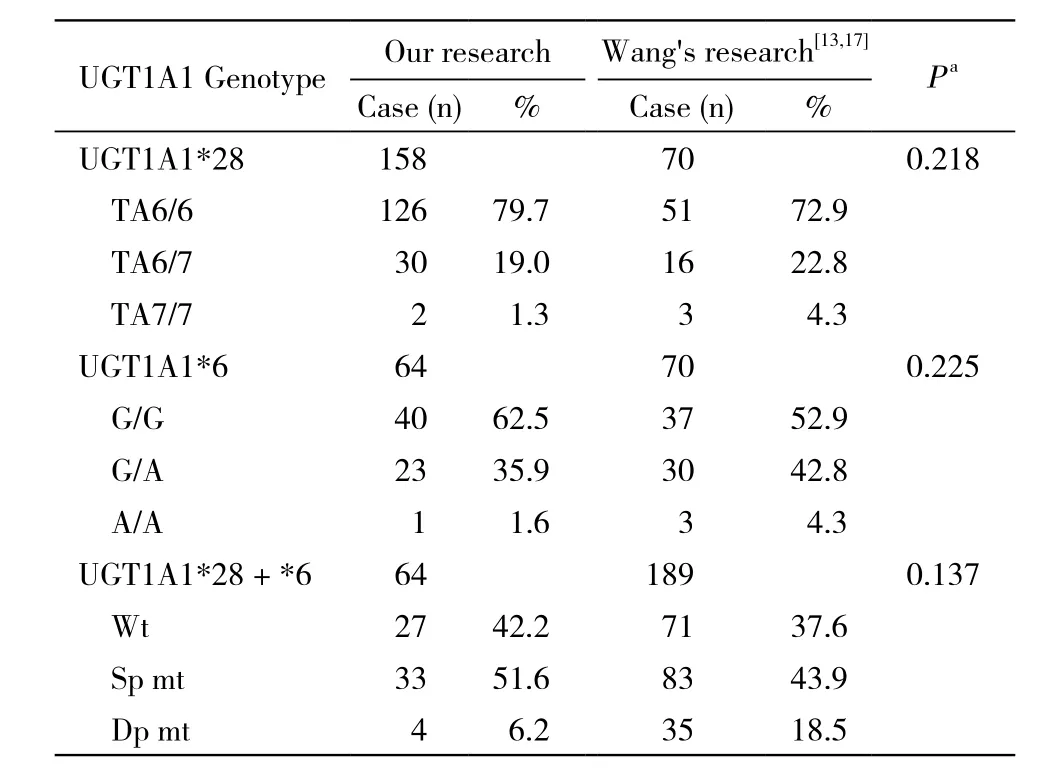
表1 本研究与其他研究中UGT1A1基因多态性分布情况的比较Tab. 1 Distribution of UGT1A1 gene polymorphism in this and other studies
4 伊立替康化疗患者的基本情况 化疗患者132例,其中男性90例,女性42例。发病年龄24 ~74岁,中位年龄43.5岁。结直肠癌90例,胃癌15例,肺癌22例,其他部位肿瘤5例。KPS评分90分116例,80分15例,70分1例。一线治疗78例,二线45例,三线及多线9例。132例均进行UGT1A1*28位点基因检测,其中53例同时检测UGT1A1*6基因位点。见表2。
5 UGT1A1基因多态性与伊立替康不良反应的相关性 132例中,UGT1A1*28野生型、杂合突变型和纯合突变型患者2 ~ 4级迟发性腹泻的发生率分别为15.0%、34.8%和50.0%,且升高趋势差异有统计学意义(P=0.000);但其3 ~ 4级迟发性腹泻和中性粒细胞减少的发生率差异无统计学意义(P>0.05)。UGT1A1*6位点各基因型患者迟发性腹泻及中性粒细胞减少的发生率均呈逐渐升高趋势,其中3 ~ 4级中性粒细胞减少的发生率分别为12.1%、31.6%和100%。联合UGT1A1*28和UGT1A1*6两位点,将53例分为UGT1A1野生型、单点变异型和双点变异型3组,迟发性腹泻和中粒细胞减少的发生率均呈逐渐升高趋势,其中2 ~4级迟发性腹泻和3 ~ 4级中性粒细胞减少发生率差异有统计学意义(P=0.004,P=0.045)。见表3。
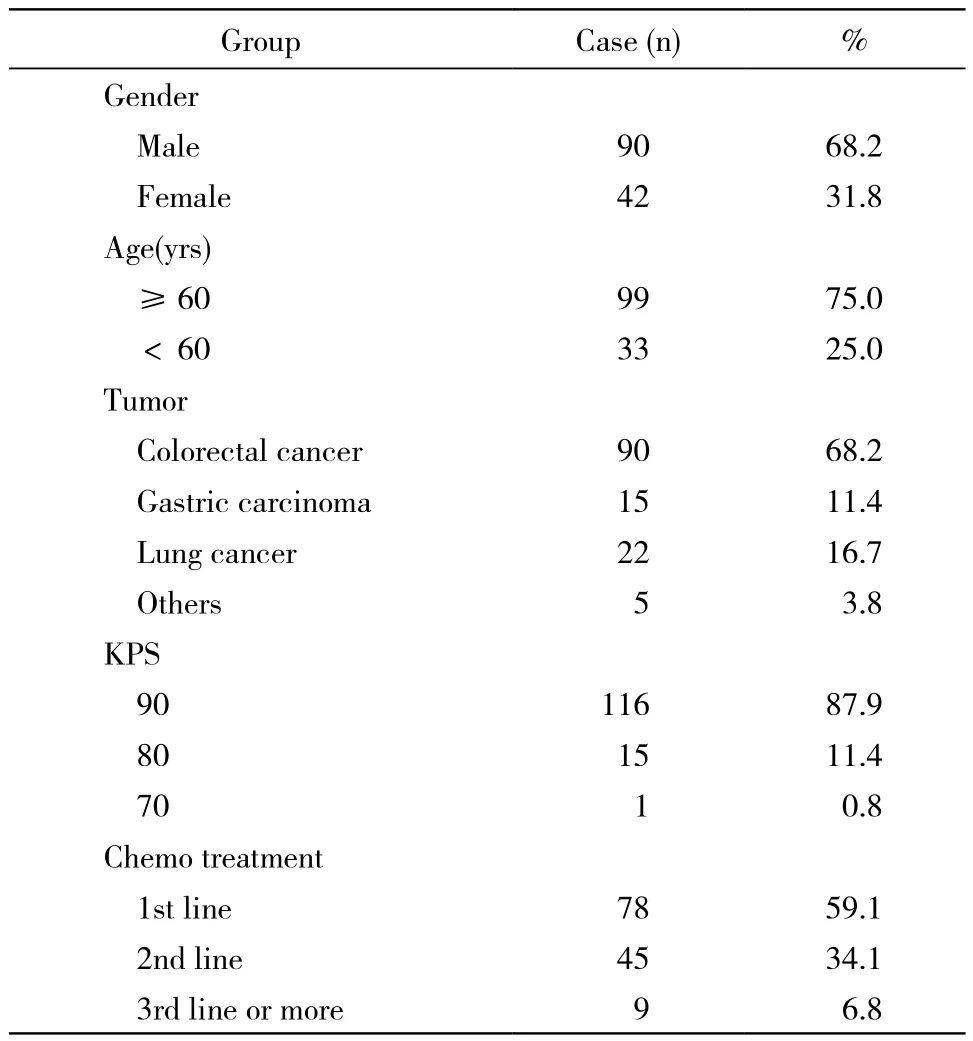
表2 132例使用伊立替康化疗方案患者的基本信息Tab. 2 Baseline parameters of 132 patients on irinotecan chemotherapy
讨 论
UGT酶是伊立替康代谢的关键酶。国内外多项研究表明UGT1A1基因多态性与该酶的功能密切相关。UGT1A1基因多态性可引起UGT1A1酶活性下降或缺失,进而影响伊立替康在人体内的代谢,造成其活性产物SN-38在体内蓄积,从而产生相关不良反应。文献报道的UGT1A1基因位点的改变多达50余种,其中UGT1A1*28和UGT1A1*6基因多态性与伊立替康不良反应的关系尤为引人关注[7-9]。
研究显示,UGT1A1*28纯合突变TA7/7在高加索人群和非洲人群中分布较高,分别占人群的10% ~ 15%和12% ~ 27%[6,10]。在亚洲人群中,该突变发生率仅为1.2%~4.7%[4,11-13]。UGT1A1*6在亚洲人群中报道较多[14-15]。韩国学者提出,由于UGT1A1*28在韩国人中非常少见,UGT1A1*6可以取代UGT1A1*28的作用[16]。本研究中158例患者检测UGT1A1*28基因型,仅有2例(1.3%)为TA7/7纯合突变,64例完成UGT1A1*6检测者中A/A纯合突变仅1例(1.6%),与王岩等[13,17]报道的比例相似(TA7/7 4.3%,A/A 4.3%),与以往汉族人群报告的结果基本相符,远低于高加索人和非洲人群。
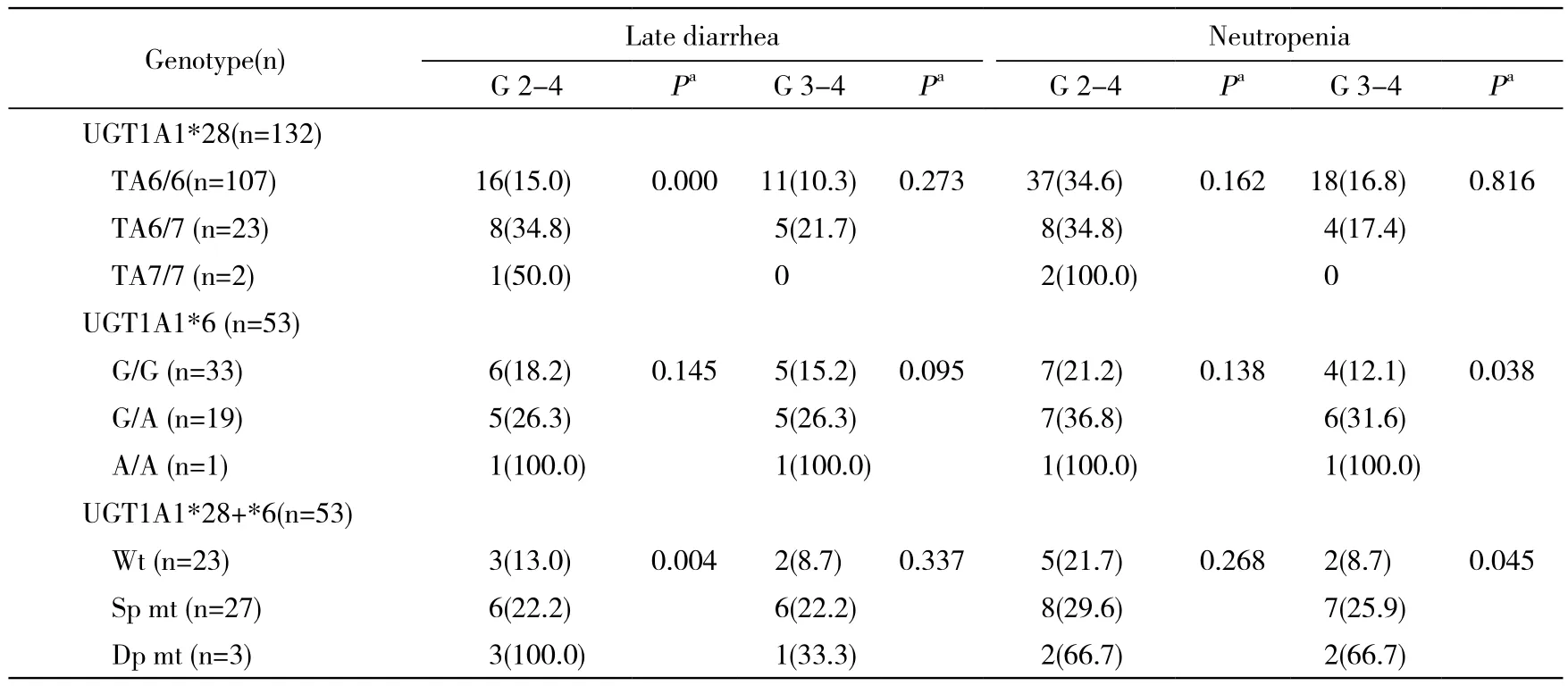
表3 UGT1A1基因多态性与迟发性腹泻、中性粒细胞减少的相关性Tab. 3 Correlation of UGT1A1 gene polymorphism with delayed diarrhea and neutropenia (n, %)
药代动力学研究显示,UGT1A1*28和UGT1A1*6突变型患者较野生型患者的SN-38葡萄糖醛酸化效率明显降低,双点变异型患者较单点变异型和野生型患者血液中SN-38浓度及SN-38的药-时曲线下面积明显升高,这表示UGT1A1基因突变型患者,尤其是纯合突变型患者发生伊立替康相关不良反应的风险可能会更高[18]。多项研究已经证实了这一推论,UGT1A1*28及UGT1A1*6突变型患者迟发性腹泻或中性粒细胞减少的发生率明显升高[5,11,13,15-17,19-20]。本研究中132例恶性肿瘤患者使用了以伊立替康为基础的化疗方案,发现UGT1A1*28基因突变可以增加患者发生2 ~ 4级迟发性腹泻的风险,野生型、杂合突变型和纯合突变的发生率逐渐升高(15.0%、34.8%、50.0%,P=0.000), 这 与 Zhou 和 Massacesi等[2,5]的 研 究结果相符。这一结果的原因可能是突变数增多导致UGT酶活性逐步下降,灭活SN-38能力逐渐降低,具体机制仍有待验证。与Han等[16]的结果类似,该位点基因多态性与中性粒细胞减少不相关,这可能与亚洲人UGT1A1*28基因突变发生率较低有关。因此,我们进一步检测了其中53例患者的UGT1A1*6基因多态性,UGT1A1*6突变可增加患者3 ~ 4级中性粒细胞减少发生比例(野生型12.1%、杂合突变型31.6%、纯合突变型100%,P=0.038)。在进一步的分析中,我们根据UGT1A1*28和UGT1A1*6位点的基因突变情况,将53例可评价不良反应的患者分为野生型、单点变异型和双点变异型3组,发现3组间2 ~ 4级迟发性腹泻和3 ~ 4级中性粒细胞减少发生率差异均有统计学意义(P=0.004,P=0.045)。与欧美人群相比,UGT1A1*28和UGT1A1*6基因突变在中国人群中较常见,随突变数增多,UGT酶活性逐渐下降,导致伊立替康代谢障碍,造成不良反应发生率升高。这提示在临床工作中联合检测UGT1A1*28和UGT1A1*6基因多态性更有助于预测伊立替康不良反应。本研究中,UGT1A1*6纯合突变型仅有1例,样本量过少,统计结果说服力不足,结论仍需大样本数据验证。
综上所述,UGT1A1基因多态性与伊立替康药物毒性存在密切联系。治疗前检测患者基因型将有助于预测不良反应,指导临床用药,规避相关风险,对实现肿瘤个体化治疗有重要意义。
1 Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal Cancer: a multicentre randomised trial[J]. Lancet, 2000, 355(9209): 1041-1047.
2 Zhou CF, Ma T, Su Y, et al. UGT1A1 gene polymorphisms and the toxicities of FOLFIRI in Chinese Han patients with gastrointestinal Cancer[J]. Anticancer Agents Med Chem, 2013, 13(2): 235-241.
3 Toffoli G, Cecchin E, Corona G, et al. Pharmacogenetics of irinotecan[J]. Curr Med Chem Anticancer Agents, 2003, 3(3):225-237.
4 Hirose K, Kozu C, Yamashita K, et al. Correlation between plasma concentration ratios of SN-38 glucuronide and SN-38 and neutropenia induction in patients with colorectal Cancer and wild-type UGT1A1 gene[J]. Oncol Lett, 2012, 3(3): 694-698.
5 Massacesi C, Terrazzino S, Marcucci F, et al. Uridine diphosphate glucuronosyl transferase 1A1 promoter polymorphism predicts the risk of gastrointestinal toxicity and fatigue induced by irinotecan-based chemotherapy[J]. Cancer, 2006, 106(5): 1007-1016.
6 Kaniwa N, Kurose K, Jinno H, et al. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C> T (P229L) found in an African-American[J]. Drug Metab Dispos, 2005, 33(3): 458-465.
7 Hazama S, Mishima H, Tsunedomi R, et al. UGT1A1*6, 1A7*3,and 1A9*22 genotypes predict severe neutropenia in FOLFIRI-treated metastatic colorectal cancer in two prospective studies in Japan[J]. Cancer Sci, 2013, 104(12):1662-1669.
8 Inoue K, Sonobe M, Kawamura Y, et al. Polymorphisms of the UDP-glucuronosyl transferase 1A genes are associated with adverse events in Cancer patients receiving irinotecan-based chemotherapy[J].Tohoku J Exp Med, 2013, 229(2): 107-114.
9 Kadakol A, Ghosh SS, Sappal BS, et al. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1)causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype[J]. Hum Mutat, 2000, 16(4): 297-306.
10 Marcuello E, Altés A, Menoyo A, et al. UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal Cancer[J]. Br J Cancer, 2004, 91(4): 678-682.
11 Premawardhena A, Fisher CA, Liu YT, et al. The global distribution of length polymorphisms of the promoters of the glucuronosyltransferase 1 gene (UGT1A1): hematologic and evolutionary implications[J]. Blood Cells Mol Dis, 2003, 31(1):98-101.
12 Yong WP, Innocenti F, Ratain MJ. The role of pharmacogenetics in cancer therapeutics[J]. Br J Clin Pharmacol, 2006, 62(1):35-46.
13 王岩,徐建明,沈琳,等.中国人尿苷二磷酸葡糖苷酸转移酶1A基因多态性与伊立替康毒性的相关性[J].中华肿瘤杂志,2007,29(12):913-916.
14 Minami H, Sai K, Saeki M, et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese:roles of UGT1A1*6 and *28[J]. Pharmacogenet Genomics, 2007,17(7): 497-504.
15 Onoue M, Terada T, Kobayashi M, et al. UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients[J]. Int J Clin Oncol, 2009, 14(2):136-142.
16 Han JY, Lim HS, Park YH, et al. Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung Cancer[J]. Lung Cancer, 2009, 63(1): 115-120.
17 王岩,葛飞娇,林莉,等.UGT1A1基因多态性与伊立替康为主方案治疗晚期结直肠癌的毒性和疗效的相关性分析[J].临床肿瘤学杂志,2012,17(11):961-966.
18 Takano M, Goto T, Hirata J, et al. UGT1A1 genotype-specific phase I and pharmacokinetic study for combination chemotherapy with irinotecan and cisplatin : a Saitama Tumor Board study[J]. Eur J Gynaecol Oncol, 2013, 34(2): 120-123.
19 Valenzuela Jiménez B, González Sales M, Escudero Ortiz V, et al. Influence of genetic polymorphisms in UGT1A1, UGT1A7 and UGT1A9 on the pharmacokynetics of irinotecan, SN-38 and SN-38G[J]. Farm Hosp, 2013, 37(2):111-127.
20 Hirasawa A, Zama T, Akahane T, et al. Polymorphisms in the UGT1A1 gene predict adverse effects of irinotecan in the treatment of gynecologic cancer in Japanese patients[J]. J Hum Genet, 2013,58(12):794-798.
Relationship between UGT1A1*28 and UGT1A1*6 gene polymorphism and adverse reactions of irinotecan-based chemotherapy
ZHANG Yong, SU Dan, GUO Xiao-chuan, FANG Hui, BAI Li
Department of Oncology, Chinese PLA General Hospital, Beijing 100853, China
Corresponding author: BAI Li. Email: baili_0795@163.com
ObjectiveTo assess the correlation of UGT1A1*28 and UGT1A1*6 gene polymorphism with adverse reactions of irinotecan-based chemotherapy by analyzing the distribution of UGT1A1 gene polymorphism in Chinese people. Methods Peripheral blood samples were taken from 158 malignant tumor patients admitted to our hospital from March 2011 to March 2012.Their UGT1A1*28 and UGT1A1*6 genotypes were detected by direct sequencing. Of the 158 patients, 132 received irinotecan chemotherapy. The adverse reactions to irinotecan chemotherapy were compared in patients with different genotypes. Results Among the 158 patients with UGT1A1*28 gene, wild genotype TA6/6, heterozygotic mutation genotype TA6/7, and homozygotic mutation genotype TA7/7 were detected in 126 (79.7%), 30 (19.0%), and 2 (1.3%) patients, respectively. Among the 64 patients with UGT1A1*6 gene, wild genotype G/G, heterozygotic mutation genotype G/A, and homozygotic mutation genotype A/A were detected in 40 (62.5%), 23 (35.9%), and 1 (1.6%) patients, respectively. The incidence of grades 2-4 delayed diarrhea was lower in patients with wild genotype TA6/6 than in those with wild genotypes TA6/7 and TA7/7 (15.0% vs 34.8% and 50.0%,P=0.000).The incidence of grades 3-4 neutropenia was signi fi cantly lower in patients with wild genotypes TA6/6 and G/G than in those with a heterozygotic or homozygotic mutation genotype or with both heterozygotic and homozygotic mutation genotypes (13.0% vs 22.2% and 100.0%, P=0.004;8.7% vs 25.9% and 66.7%, P=0.045).ConclusionThe incidence of adverse reactions to irinotecan chemotherapy is high in patients with UGT1A1*28 and UGT1A1*6 gene mutations. Detection of UGT1A1*28 and UGT1A1*6 genotypes can more accurately predict the adverse reactions to irinotecan chemotherapy than detection of UGT1A1*28 or UGT1A1*6 genotype.
UGT1A1; gene polymorphism; irinotecan; adverse reaction
R 730.53
A
2095-5227(2014)05-0489-05
10.3969/j.issn.2095-5227.2014.05.025
时间:2014-01-20 09:54
http://www.cnki.net/kcms/detail/11.3275.R.20140120.0954.001.html
2013-11-11
张勇,男,在读硕士。研究方向:消化系统肿瘤。Email:yongzhsd@163.com
白莉,女,博士,主任医师。Email: baili_0795@163.com

