BluePigment Cobalt-Aluminum Spinels Prepared by EDTA Chelating Method*
HU Mingwei,WANG Hongquan,XIA Haofu,HUANG Zhiliang,CHI Ru’an
(1.School of Material Science and Engineering,Wuhan Institute of Technology,Wuhan 430074,China;2.School of Chemical Engineering,Wuhan Institute of Technology,Wuhan 430074,China)
BluePigmentCobalt-AluminumSpinelsPreparedbyEDTAChelatingMethod*
HU Mingwei1,WANG Hongquan1,XIA Haofu1,HUANG Zhiliang1,CHI Ru’an2
(1.School of Material Science and Engineering,Wuhan Institute of Technology,Wuhan 430074,China;2.School of Chemical Engineering,Wuhan Institute of Technology,Wuhan 430074,China)
A method is proposed to prepare cobalt aluminum spinel blue pigment under a relatively low temperature.Cobalt aluminum spinels were prepared by the thermal decomposition of EDTA chelating cobalt and aluminum complex precursors which are derived from metal nitrate salts and ethylenediamine tetraacetic acid (EDTA).EDTA was used for chelating agent,cobalt nitrate hexahydrate and aluminum nitrate nonahydrate were used to synthesize the precursors.The phase and coloration of the prepared samples were characterized by the Commission International Eclairage (CIE) chromaticity diagram,X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR).The main variables which can systematically investigate the coloration and crystalline phase were cobalt-aluminum ratio,pH value of the reaction system and the calcination temperature.The optimizing process parameters to prepare cobalt aluminum spinel blue pigment:the temperature is 900 ℃ with 2 h heating preservation,the pH value is 9~10,and the cobalt-aluminum mole ratio is 1∶2.5 in the precursors.
ethylenediamine tetraacetic acid;orthogonal test;spinel;precursors;chelating ligand
1 Introduction
Cobalt blue pigments are a metal oxide miscible pigment with the spinel structure,which is also called cobalt aluminate (CoA12O4)[1].Its chemical composition is CoO and Al2O3.It has several superior performances,including thermal stability (up to 1 200 ℃),chemical stability,anti-erosion to acid and alkali,weather resistance and strong corrosion resistance.In terms of transparency,saturation,hue and refractive index,cobalt blue pigments are significantly better than other blue paint.Besides,it is non-toxic and environment friendly[2-4].With the development of the super durable coating and plastic industry,the demand for cobalt blue pigments is increasing dramatically.
Thetraditional method to prepare cobalt blue pigments is solid-phase method[5-6]which mixes solid reactant through grinding after calcination at high temperature to generate the product directly.Solid phase reaction rate is affected by the diffusion kinetics which is not easy to fulfill completely;the prepared pigment particle size distribution is not uniform,and the color,luster and chemical stability are not so good.Thus,the application of solid-phase method is limited.But it has the advantages of simple process and easy maneuverability.At present,solid-phase method is widely used in industry.In order to meet the market and take full advantage of the cobalt blue pigments,other methods have been explored:liquid-phase method[7-18]and gas-phase method[19].Through liquid-phase method,the reactants are mixed uniformly in the liquid phase.In this way,reactants can be fully contacted,and the prepared pigment particles have the advantages of small diameter,high purity,thermal stability and chemical stability.The calcination temperature of liquid-phase method is lower than that of the solid phase reaction,and the process is easy to control.Through gas phase method,the solid raw materials are evaporated at high temperature and react with each other in gas phase.The pigments prepared by gas phase reaction have the advantages of high purity and small particle size.But it also requires high energy consumption and cost[20].In this study,the cobalt blue pigments with spinel structure were prepared through the EDTA coordination chelalegal method by using the cobalt aluminum nitrate as the main raw material,and the Commission International Eclairage chromaticity diagram,X-Ray Diffraction,FTIR Investigation and Fluorescence analysis are used to characterize the samples’ phases and colorations.
2 Experiments
2.1Reagents
Co(NO3)3·6H2O (>99%,by Nanjing Chemical Reagent Co.,LTD),Al(NO3)3·9H2O (>99%,by Shanghai New Fine Chemical Plant),ethylene diamine Tetraacetic acid (EDTA,>99.99%,by Shanghai Reagent Co.,LTD),NH3·H2O (25%~28%,by Tianjin Tianli Chemical Reagent Co.,LTD) were used as reagents.
2.2Process

Fig. 1 Preparation of Cobalt Blue Pigment via EDTA Coordination Chelating Methods
A representative procedure of Co-Al-EDTA precursors is as follows:a certain amount of EDTA powder was introduced into quantificational absolute ethyl alcohol placed in a 400 mL beaker,magnetically stirred at 40 ℃;aqueous ammonia was then added dropwise into the beaker to facilitate the dissolution of EDTA,which was converted into a water-soluble ammonium salt.The resulting solution had a pH value of 8 to 10.After that,the required stoichiometric amounts of Al(NO3)3·9H2O and Co(NO3)2·6H2O were introduced into the above prepared EDTA ammonium solution at the same time,stirred at 60 ℃ for several hours until the solution was completely transformed from transparent into a purple color;then the purple solution was placed into a water bath kettle and was heated at 90 ℃ so that the excess water was removed and the polymerization was promoted.After the solution being turned into viscous atropurpureus xerogel without sediments,the sample was dried completely at 100 ℃ and grinded into powder.Then,a small amount of the resulting powder was calcined at a certain temperature in a resistance furnace for 120 min to remove the carbon impurity and cooled to room temperature.The final product samples were black or blue powder.The detailed flow diagram was shown in figure 1.Three different proportions of the molar ratio of cobalt nitrate,aluminum nitrate and EDTA were 1∶2∶3,1∶2.5∶3.5 and 1∶3∶4 respectively.
2.3OrthogonalDesign
Preparation of CoA12O4spinel cobalt blue pigments by EDTA chelating method is influenced by many factors,such as the molar ratio of cobalt nitrate and aluminum nitrate,pH value,heating temperature and calcination temperature.In order to obtain the cobalt blue pigments with intense coloration,all these factors should be studied.The factors,involving the molar ratio of cobalt nitrate and aluminum nitrate,pH value and calcination temperature,are studied to investigate the coloration and crystalline phase of the cobalt blue pigments.The orthogonal experiment is designed by using L9 (43).The various factors are shown in table 1.
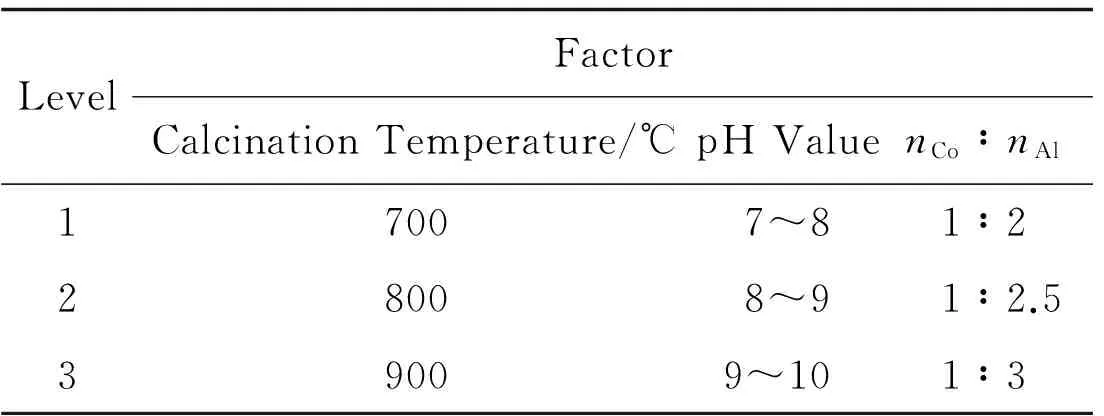
Table 1 Orthogonal Experimental Design
3 Results and Discussion
3.1ResultsofOrthogonalExperiment
The index of orthogonal experiment is the color of the samples.In the orthogonal experiment,minuscule a represents the dark aquamarine blue;minuscule b represents the sage green with slight blue;minuscule c represents atrovirens with slight blue;minuscule d represents aquamarine blue;and minuscule e represents cobalt blue.The results of orthogonal experiment are shown in table 2.
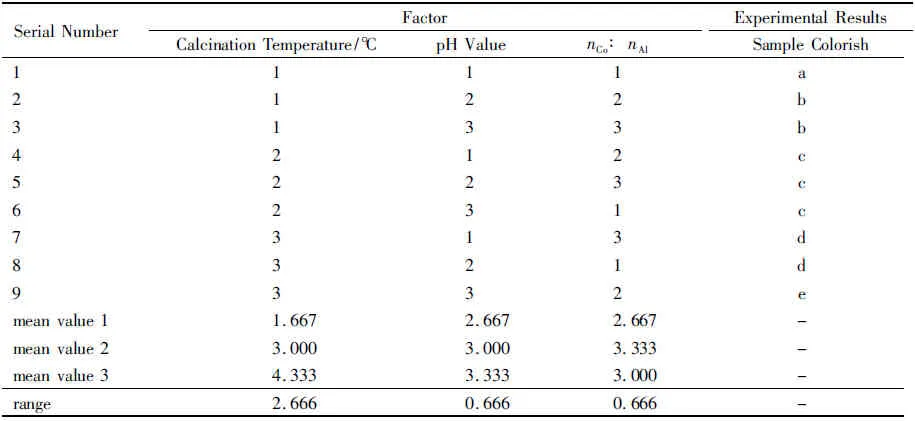
Table 2 Orthogonal Experiment Results
According to table 2,the order of the factors is as follow:calcination temperature>pH value=nCo∶nAl.The most important factor is calcination temperature,followed by pH value in the process of the reaction and the molar ratio of cobalt nitrate and aluminum nitrate.According to table 2,the optimal experimental conditions are as follows:(i) the molar ratio of cobalt nitrate and aluminum nitrate is 1∶2.5;(ii) the pH value of the reaction is 9~10;(iii) the calcination temperature is 900 ℃.
3.2EffectsofCoContentinthePrecursorsandCalcinationsTemperatureontheColoroftheSamples
The precursors were calcined at 700,800 and 900 respectively,and the coloration of the samples is shown in table 3.

Table 3 Influence of nCo/nAl and the Sintering Temperature on the Performance of the Prepared Samples
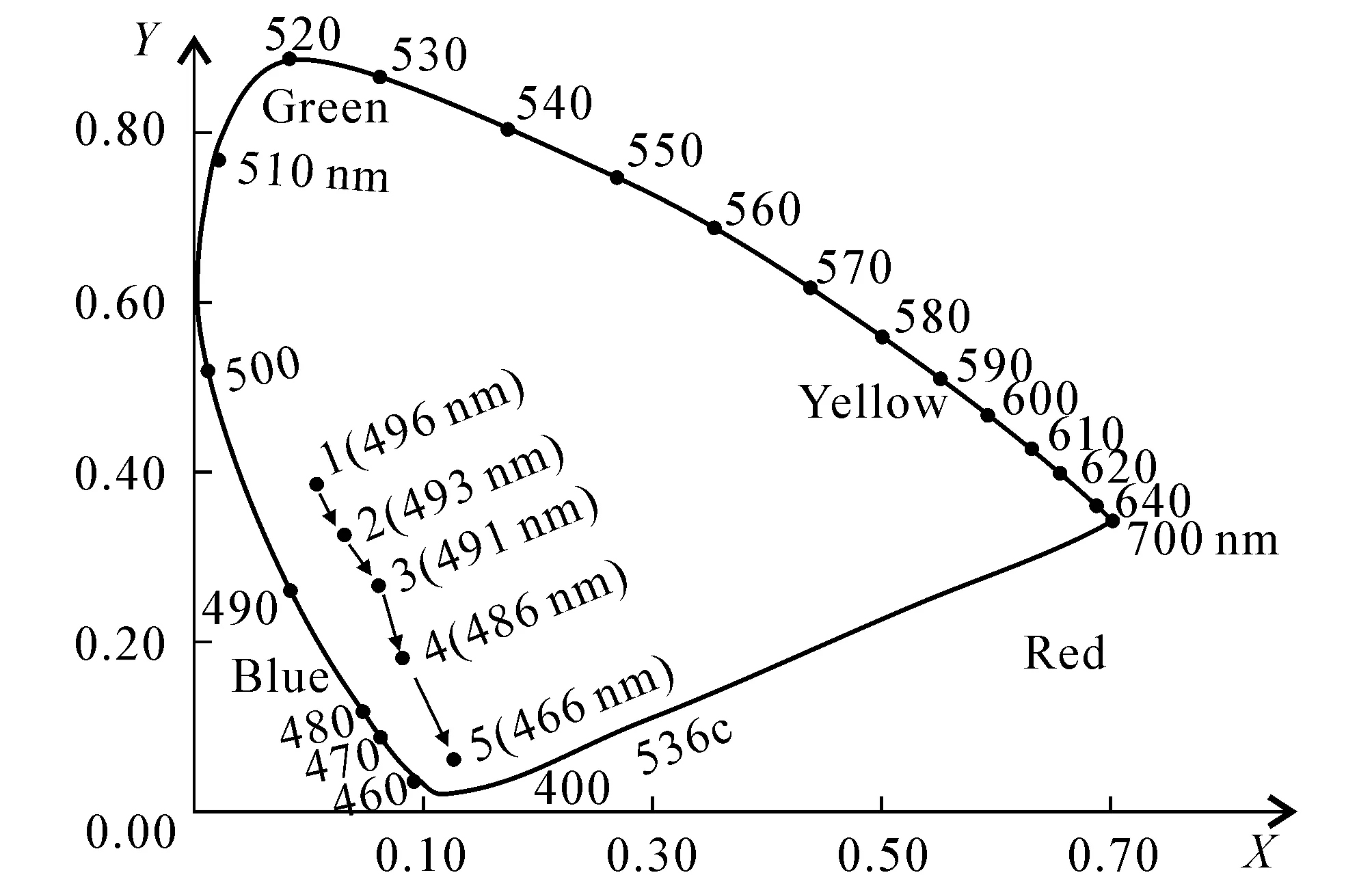
Fig. 2 Position of the Region of 5 Colors in the Chromaticity Diagram of CIE1931 Standard
The solution with standard color was prepared by potassium chloroplatinate and cobalt chloride.Compared with the standard solution visually,the color intensity of the samples can be determined.The color of the solution with 1 mg platinum and 0.5 mg cobalt is named 1 degree,which is called chrominance.The chrominance of the samples can be represented by that of the standard solution which has the similar color of the samples.The results were analyzed and calculated by CIE colorimetric[21-22].Compared with the standard chromaticity diagram of CIE 1931,the five colors were signed in the corresponding area of the coordinate paper.It is shown in figure 2.According to the figure 2,the color from 1 to 5 has become blue generally.
3.3EffectsofpHValueontheFormationofCobaltBluePrecursors
In the process of the preliminary exploration,the pH value of the chelating between cobalt and aluminum was determined in the range from 9 to 10 when the environment of the chelating of EDTA was taken into consideration.According to table 2,the pH value is suitable between 9 and 10.When the pH value of the reaction is beyond this range,pink emulsion can be observed instead of purple transparent solution.Thus,pH value between 9 and 10 is needed.
3.4XRDMeasurement
Structural phases were determined for sintered powders in a Shimadzu X-ray diffractometer using Cu Ka radiation.A continuous scan mode was used to collect 2θdata from 10°to 80° with a 0.02 sampling pitch and a 2°/min scan rate.X-ray tube voltage and current were set at 40 kV and 30 mA respectively.

①—1∶2,700 ℃;②—1∶3,700 ℃;③—1∶2,800 ℃;④—1∶3,800 ℃;⑤—1∶3,900 ℃;⑥—1∶2.5,900 ℃
(ⅰ) According to the comparison between curve ① and curve ②,crystal planes (311),(511) and (440) have CoA12O4diffraction peaks (PDF# 82-2239) of the spinel phase,which indicates that only a small amount of CoA12O4spinel is generated.In addition,the intensity and integrity of the diffraction peaks in crystal plane (220),(311),(400),(422),(511) and (440) of sample ② is better than those of sample ①.
(ⅱ) According to the comparison between curve ① and curve ③,the amount of CoA12O4spinel crystal of ③ treated at 800 ℃ is more than that of ① which treated at 700 ℃.It can be seen that with the increment of the calcination temperature,diffraction peaks of the CoA12O4spinel phase become more complete,and the intensity of the corresponding crystal plane becomes increasingly great.
(ⅲ) According to the comparison between curve ⑤ and curve ⑥,the intensity of the diffraction peaks in crystal plane (400),(422),and (511) of sample ⑤ is less than those of sample ⑥.
As can be seen from the coloration,the most complete CoA12O4spinel phase with the best coloration is at the ratio of Co and Al (nCo∶nAl=1∶2.5) in the precursors.
In summary,the results of XRD indicate that the pure CoA12O4spinel blue pigments depend on the temperature (900 ℃) of heat treatment and time (2 h),and the ratio of Co and Al (nCo∶nAl=1∶2.5) in the precursors.
3.5FTIRInvestigation
The Fourier-transform infrared (FTIR) spectra of the dried powders at different conditions were obtained on a Shimadzu FTIR-8101 by employing potassium bromide (KBr) pellet technique.The FTIR spectra of the obtained powders prepared with the different ratio of cobalt to aluminum and at calcination temperature are shown in figure 4.

①—1∶2,700 ℃;②—1∶3,700 ℃;③—1∶2,800 ℃;④—1∶3,800 ℃;⑤—1∶3,900 ℃;⑥—1∶2.5,900 ℃
We can see from figure 4,the IR spectra are similar;they all have three absorption peaks.CoAl2O4vibrations are around 505,550 and 670 cm-1,those observed lines with this spectral line are typical spinel compound and special CoAl2O4;comparing with the other curves,we know that as the temperature rises,the absorption peaks is more and more significant;at 900 ℃ and with the ratio of cobalt to aluminum 1∶2.5,the spectral line shows the most significant peaks.
3.6EffectsoftheTemperatureintheReactionProcessontheFormationofCobaltBluePrecursors
From the experiments,we find the reaction rate between EDTA and metal ions in the solution is high,so the temperature in the reaction process has little effect on the color of the cobalt blue precursors and the quality of the cobalt blue pigments.We suggest that it would be advisable to set the reactive temperature at 60 ℃.
4 Conclusions
(1) The most important factors that influence the color of the cobalt blue pigments and the formation of the crystalline phase are the molar ratio of cobalt nitrate and aluminum nitrate,pH value and calcination temperature.These factors can affect the formation of CoA12O4spinel phase and the color directly.
(2) The optimal experimental conditions are as follows:(i) the molar ratio of cobalt nitrate,aluminum nitrate and EDTA is 1∶2.5∶3.5;(ii) reactive temperature is 60 ℃;(iii) the pH value in the reaction process is between 9 and 10;(iv) the calcination temperature is 900 ℃.
[1] CHEMLAL S,LARBOTETC A.Materials Research Bulletin[J].Journal of the European Ceramic Society,2000,35(2):2 515-2 523.
[2] YANG Zongzhi.Cobalt Blue Pigments and Its Progress[J].Paint & Coatings Industry,1997(4):39-40.
[3] ZHU Jiliang,WU Shennian.Pigment Technology[M].Beijing:Chemical Industry Press,2002:293-296.
[4] ZHOU Junyi.Synthesis,Characterization and Property of Spinel Metal Oxides[D].Ji’nan:Ji’nan University,Material Science and Engineering Institute,2009.
[5] SHAO Qinghui,GU Guobang,ZHANG Lijuan,et al.Research Progress of Synthesis and Preparation of Nanophase Materials[J].Ordnance Material Science and Engineering,2005,22(4):59-63.
[6] ALEXANDRIA,VIRGINIA.DCMA Classification and Chemical Description of the Complex Inorganic Color Pigments[J].Dry Color Manufacturers’ Association,1991,21(1):54-58.
[7] HAN Yunfang,LI Xiangtang.Preparation of Cobalt-Blue Pigment by Sluggish Precipitation Method[J].Journal of Tianjin Institute of Urban Construction,2002,8(2):92-95.
[8] ZHOU Yongqiang,YU Fangli,LUO Hongjie et al.Preparation of Nanometer Cobalt Blue Pigment by Sol-Gel Method[J].Bulletin of the Chinese Ceramic Society,2006,25(5):31-33.
[9] ZHAO Ying,ZENG Yanli,ZHENG Shijun,et al.The Present Studies and Prospect on Halogen Bond[J].Journal of HeBei Normal University/Nature Science Edition,2007,31(1):85-99.
[10] MASOUD SALAVATI-NIASARI,MASOUD FARHADI-KHOUZANI,FATEMEH DAVAR.Bright Blue Pigment CoAl2O4Nanocrystals Prepared by Modified Sol-Gel Method[J].Sol-Gel Sci. Technol.,2009,52:321-327.
[11] CAO Liyun,DEN Fei,ZHANG Xinhe,et al.Preparation of Nano-CoAl2O4Blue Ceramic Pigment by Microemulsion Process[J].Glass & Enamel,2005,35(5):40-44.
[12] CHEN Jing,SHI Xiaobo,HAN Bing.The Preparation and Characteristics of Cobalt Blue Colored Mica Titania Pearlescent Pigment by Micro Emulsions[J].Dyes and Pigments,2007,75(1):766-769.
[13] DENG Xinrong,HU Guorong,PENG Zhongdong,et al.Preparation of Superfine Sized Cobalt Blue Pigment Using Precipitation-Azeotropic Distillation Method[J].Materials Review,2006,5(20):345-353.
[14] AI Jun,LU Xilong,WANG Weixing,et al.Synthesis of CoAl2O4Spinel-Type Cobalt Blue Pigments via Acid Chelating Precursor Technique[J].Journal of Ceramics,2011,32(2):178-181.
[15] CUI Yanwang,XUAN Bai,SHAO Minliu,et al.Synthesis of Cobalt-Aluminum Spinels via EDTA Chelating Precursors[J].Journal of Materials Science,2004,39:6 161-6 201.
[16] CHEN Zhizhan,SHI Erwei,ZHENG Yanqing,et al.Hydrothermal Synthesis and Optical Property of Nano-Sized CoAI2O4Pigment[J].Materials Letters,2002,55(1):281-289.
[17] HU Guorong,DENG Xinrong,CAOYanbing,et al.Synthesis of Spherical CoA12O4Pigment Particles with High Reflectivity by Polymeric-Aerosol Pyrolysis[J].Science Direct,2007,26(3):236-242.
[18] HU Guorong,CAOYanbing,DENG Xinrong,et al.Synthesis of Ultrafine Cobalt Blue Pigment Using Crystallization-Pyrolysis Process[J].Paint & Coating Industry,2006,11:15-15.
[19] CARTA G,CASARIN M,HABRA N E.MOCVD Deposition of CoAl2O4Films[J].Electrochimica Acta,2005,50(3):4 592-4 599.
[20] LIU Zhubo.Study of Spinel Cobalt Blue Pigment Preparation and Performance[D].Nanjing:Nanjing University of Science and Technology,Material Science and Engineering Institute,2008.
[21] ROBERT HUNTER.CIE Colorimetry[J].Jiangxi Building Materials,1996(4):31-42.
[22] YE Chunfang,LIU Wangling,YU Feihong.Realization of Active X Control of CIE Chromatic Spaces[J].Optical Instrument,2005(3):27-32.
(责任编辑 易必武)
乙二胺四乙酸配位螯合法制备钴铝尖晶石蓝色颜料
胡名卫1,王宏全1,夏浩孚1,黄志良1,池汝安2
(1.武汉工程大学材料科学与工程学院,湖北 武汉 430074;2.武汉工程大学化工与制药学院,湖北 武汉 430074)
提出一种可以在较低温度下焙烧制备出钴铝尖晶石蓝色颜料的方法.以六水合硝酸钴、九水合硝酸铝为主要原料,乙二胺四乙酸(EDTA)为配位螯合剂,通过EDTA配位螯合钴、铝得到有机配合物前驱体,经过焙烧制备钴蓝颜料.通过国际照明委员会的色度图谱(CIE)、X射线衍射(XRD)和傅里叶红外光谱(FTIR),分别对所制备样品的物相和呈色进行研究表征.主要考察了钴铝物质的量比、反应体系pH值、焙烧温度等工艺条件对铝酸钴尖晶石相的完整性和颜料呈色的影响.制备钴铝尖晶石蓝色颜料的最佳工艺参数是900 ℃温度下保温时间为2 h,pH值为9~10,前驱体的钴铝比为 1∶2.5.
乙二胺四乙酸;正交试验;尖晶石;前驱体;配位螯合
TQ174.5
B
1007-2985(2014)01-0072-06
date:2013-09-02
National Natural Science Foundation of China (51374155);National 973 Pre-Research Project (2011CB411901)
Biography:HU Mingwei (1990-),male,was born in Fuzhou City,Jiangxi Province,MSLA;research area is functions and application of inorganic non-metallic materials.
HUANG Zhiliang,male,was born in Wangjiang Ciy,Anhui Province,professor of School of Material Science and Engineering,Wuhan Institute of Technology.E-mail:hzl6455@126.com.
TQ174.45DocumentcodeB
10.3969/j.issn.1007-2985.2014.01.017

