新型1,3,5-三嗪衍生物的合成*
张丽慧,姜 帆,徐晓剑,周云鹏,王 洋
(1.铜仁职业技术学院,贵州 铜仁 554300;2.辽宁大学 药学院,辽宁 沈阳 110036)
·快递论文·
新型1,3,5-三嗪衍生物的合成*
张丽慧1,姜 帆2,徐晓剑2,周云鹏2,王 洋2
(1.铜仁职业技术学院,贵州 铜仁 554300;2.辽宁大学 药学院,辽宁 沈阳 110036)
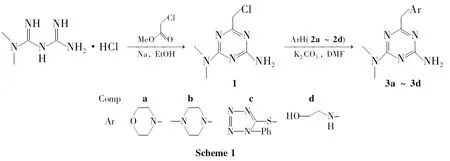
以二甲双胍盐酸盐和氯乙酸乙酯为起始原料,经环合反应制得2-二甲胺基-4-氯甲基-6-氨基-1,3,5-三嗪(1);1分别与吗啉(2a),N-甲基哌嗪(2b),1-苯基-5-巯基四氮唑(2c)和乙醇胺(2d)反应,合成了4个1,3,5-三嗪衍生物(3a~3d),其中3b~3d为新化合物,其结构经1H NMR和IR表征。
1,3,5-三嗪;衍生物;合成
1,3,5-三嗪衍生物在有机化学中占有重要的地位,其应用领域涉及医药工业、纺织工业、塑料工业、橡胶工业,可以用作杀虫剂、染料、荧光增白剂、炸药以及表面活性剂等[1]。1,3,5-三嗪衍生物具有一定的抗肿瘤活性[2-7]、抗菌活性[8-10]、抗HIV作用[11],还可以作为酶抑制剂[12]。
目前国内关于1,3,5-三嗪衍生物的合成和应用文献报道较少,进行其合成研究具有重要的现实意义。本文以二甲双胍盐酸盐和氯乙酸乙酯为起始原料,经环合反应制得2-二甲胺基-4-氯甲基-6-氨基-1,3,5-三嗪(1);1分别与吗啉(2a),N-甲基哌嗪(2b),1-苯基-5-巯基四氮唑(2c)和乙醇胺(2d)反应,合成了4个1,3,5-三嗪衍生物(3a~3d,Scheme 1),其中3b~3d为新化合物,其结构经1H NMR和IR表征。
该方法具有原料易得、步骤简单、反应条件温和、产率高、产物易分离等优点,是合成1,3,5-三嗪的好方法。
1 实验部分
1.1 仪器与试剂
X24型数字显示显微熔点仪(温度未校正);AVANCE-600型核磁共振仪(DMSO-d6为溶剂,TMS为内标);Spcetrum One型红外光谱仪(KBr压片)。
柱层析硅胶GF254,青岛海洋化工厂;二甲双胍盐酸盐和氯乙酸乙酯,国药集团化学试剂有限公司;其余所用试剂均为分析纯。
1.2 合成
(1)1的合成
在茄形瓶中加入金属钠8.31g(361.30mmol)和无水乙醇200mL,搅拌使钠溶解;加入二甲双胍盐酸盐30.00g(180.72mmol),于室温反应3h。加入氯乙酸乙酯22.05g(180.73mmol),反应5h(TLC检测)。过滤,滤饼用水(3×150mL)洗涤后经硅胶柱层析[洗脱剂:A=V(正己烷)∶V(乙酸乙酯)=5∶1]纯化得白色固体120.00g,产率59.17%,m.p.175℃~177℃;1H NMRδ:6.91(s,2H,NH2),4.24(s,2H,CH2),3.05(s,3H,CH3),3.02(s,3H,CH3);IRν:3388,3322,3179,2922,1645,1590,1560,1512,1406,1260,1219,1110,812,770cm-1。
(2)3a的合成
在反应瓶中加入12.00g(10.70mmol),2a4.65g(53.45mmol)的甲苯(20mL)溶液,于110℃(回流)反应12h(TLC检测)。冷却至室温,有黄色固体(盐酸吗啉)析出,过滤,滤液减压旋蒸至有少量白色固体析出。过滤,滤饼经硅胶柱层析(洗脱剂:A=4∶1)纯化得白色固体3a1.5g,产率60.28%,m.p.155℃~157℃;1H NMRδ:6.78(s,2H,NH2),3.55(t,J=4.8Hz,4H,OCH2),3.20(s,2H,CH2),3.05(s,3H,CH3),3.01(s,3H,CH3),2.50(t,J=4.8Hz,4H,NCH2);IRν:3365,3165,2980,2943,2848,1662,1567,1517,1476,1400,1346,1295,1218,1165,1114,1074cm-1。
(3)3b和3c的合成
在反应瓶中加入11.00g(5.35mmol),2b1.60g(16.05mmol),碳酸钾1.47g(10.70mmol)和干燥DMF 20mL,搅拌下于室温反应5h。用纯水饱和的正丁醇(3×60mL)萃取,合并萃取液,用水(3×60mL)洗涤,用无水硫酸钠干燥,经硅胶柱层析(洗脱剂:A=3∶1)纯化得白色固体3b0.70g,产率52.24%,m.p.158℃~160℃;1H NMRδ:6.76(s,2H,NH2),3.17(s,2H,CH2),3.04(s,3H,CH3),3.00(s,3H,CH3),2.50(t,J=1.8Hz,4H,CH2),2.26(t,J=1.8Hz,4H,CH2),2.11(s,3H,CH3);IRν:3475,2941,2851,2800,1637,1560,1572,1537,1456,1401,1224,1167cm-1;ESI-MSm/z:252{[M+H]+}。
用2c代替2b,用类似的方法(反应时间1h,洗脱剂:A=4∶1)合成白色固体3c1.30g,产率77.38%,m.p.226℃~228℃;1H NMRδ:7.67(m,5H,PhH),6.92(s,2H,NH2),4.37(s,2H,CH2),3.01(s,3H,CH3),2.98(s,3H,CH3);IRν:3452,3321,3120,2926,1655,1564,1536,1501,1439,1398,1385,1268,1236,1214,1110,1075cm-1;ESI-MSm/z:330{[M+H]+}。
(4)3d的合成
在反应瓶中加入15.00g(26.74mmol),2d4.90g(80.33mmol)和碳酸钾3.70g(26.81mmol),搅拌下于20℃反应3h。过滤,滤饼用甲醇100mL粗溶;过滤,除去盐,滤液加热至70℃,自然冷却至室温,析出白色固体,过滤,滤饼干燥得白色固体3d3.50g,产率66.16%,m.p.170℃~172℃,产率66.16%;1H NMRδ:6.88(s,2H,NH2),3.68(s,2H,CH2),3.64(s,1H,OH),3.58(t,J=5.4Hz,2H,OCH2),3.09(s,3H,CH3),3.03(s,3H,CH3),2.86(t,J=5.4Hz,2H,NCH2);IRν:3330,3265,3168,2907,2774,2682,1662,1570,1527,1493,1472,1451,1402,1352,1334,1264,1226,1136,1099,1066,1046,1019cm-1;ESI-MSm/z:213{[M+H]+}。
2 结果与讨论
文献[13-14]方法在合成1时,是在中性或碱性(三乙胺或甲醇钠)条件下,以THF或甲醇为溶剂,回流条件下环合。我们按该方法合成1时发现,反应时间过长,产率较低。为此,将溶剂更换成无水乙醇,甲醇钠换成金属钠,于室温就可以反应,反应时间缩短了5h,而且能够完全反应,产物微溶于乙醇,反应完全后能产物直接从溶剂中析出,直接用水洗涤过滤、烘干即可得到产物,简化了文献[13-14]方法,也提高了产率(59.17%)。
[1] Blotny G.Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis[J].Tetrahedron,2006,62:9507-9522.
[2] Brzozowski Z,Czewski F S,Gdaniec M.Synthesis,structural charactural and antitumor activity of novel 2,4-diamino-1,3,5-triazine derivatives[J].Eur J Med Chem,2000,35:1053-1064.
[3] Brzozowski Z,Czewski F S,Gdaniec M.Synthesis and antitumor activity of novel 2-amino-4-(3,5,5-trimethyl-2-pyrazolino)-1,3,5-triazine derivatives[J].Eur J Med Chem,2002,37:709-720.
[4] Sczewski F,Buakowska A,Bednarski P.Synthesis,structure and anticancer activity of novel 2,4-diamino-1,3,5-triazine derivatives[J].Eur J Med Chem,2006,41:219-225.
[5] Menieagli R,Samaritani S,Signore G,etal.In vitro ceytotoxic activities of 2-alkyl-4,6-diheteroalkyl-l,3,5-triazines:New moleeules in anticancers research[J].J Med Chem,2004,47:4649-4652.
[6] 赵维璋,倪莉云,崔智勇,等.1-取代苯基三嗪类化合物的合成和抗肿瘤活性研究[J].北京医科大学学报,1995,27(03):233-235.
[7] Nie Z,Perretta C,Erickson P,etal.Structure-based design,synthesis and study of pyrazolo[l,5-a][1,3,5]triazine derivatives as potent inhibitors of protein kinase CK2[J].Bioorg Med Chem Lett,2007,17:4191-4195.
[8] Lebreton S,Newcombe N,Bradley M.Antibacterial single-bead screening[J].Tetrahedron,2003,59:10213-10222.
[9] Lubbers T,Angehrn P,Gmunder H,etal.Design,synthesis and structure-activity relationship studies of ATP analogues as DNA gyrase inhibitors[J].Bioorg Med Chem Lett,2000,10:821-826.
[10] Tsital P,Antoniadou-Vyzal E,Hamodrakas S J,etal.Synthesis,crystal structure and biological properties of a new series of lipophilics-triazines,dihydrofolate reduetase inhibitors[J].Eur J Med Chem,1993,28:149-158.
[11] Naicker K P,Jiang S B,Lu H.Synthesis and anti-HIV-1activity of 4-[4-(4,6-bisphenylamino-1,3and 5triazin-2-ylamino)-5-methoxy-2-methylphenylazo]-5-hydroxynaphthalene-2,7-disulfonic acid and its derivatives[J].Bioorg Med Chem Lett,2004,12:1215-1220.
[12] Garaj V,Puccetti L,Fasolis G.Carbonic anhydrase inhibitors:Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I,II and IX[J].Bioorg Med Chem Lett,2005,15:3102-3108.
[13] Zheng M F,Xu C H,Ma J W.Synthesis and antitumor evaluation of a novel seriesof triaminotriazine derivatives[J].Bioorg Med Chem,2007,15:1815-1827.
[14] Brzozowski Z,Saezewski F,Gdaniec M.Synthesis,structural characterization and antitumor activity of novel 2,4-diamino-1,3,5-triazine derivatives[J].Eur J Med Chem,2000,35:1053-1064.
Synthesisof1,3,5-TriazineDerivatives
ZHANG Li-hui1, JIANG Fang2,XU Xiao-jian2, ZHOU Yun-peng2, WANG Yang2
(1.Tongren Polytechnic College,Tongren 554300,China;2.College of Pharmacy,Liaoning University,Shenyang 110036,China)
2-Dimethylamino-4-chloromethyl-6-amino-1,3,5-triazine(1)was prepared by cyclization of dimethybiguanide hydrochlorid with ethyl chloroacetate.Four 1,3,5-triazine derivatives(3a~3d)were synthesized by 1with morpholine(2a),1-methylpiperazine(2b),1-phenyl-1H-tetrazole-5-thiol(2c)and 2-aminoethanol(2d),respectively.3b~3dwere new compounds and the structures were characterized by1H NMR and IR.
1,3,5-triazine;derivative;synthesis
2014-03-30;
2014-04-28
张丽慧(1986-),女,土家族,贵州铜仁人,硕士研究生,主要从事药物合成的研究与教学。E-mail:lihui8852032@163.com
周云鹏,实验师,E-mail:ZYP@lnu.edu.cn;王洋,助理研究员,E-mail:13940355775@163.com
O626.23
A
1005-1511(2014)04-0507-03
——非均布滤饼的局部比阻与平均比阻的测定与计算方法

