1-取代-4-[5-(4-取代苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪类衍生物的合成及其抑菌活性*
吴 琴,王贞超,魏 学,薛 伟
(1.贵州大学 精细化工研究开发中心 教育部绿色农药和生物工程重点实验室,贵州 贵阳 550025;2.贵州理工学院 制药工程学院,贵州 贵阳 550003)
·研究论文·
1-取代-4-[5-(4-取代苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪类衍生物的合成及其抑菌活性*
吴 琴1,2,王贞超1,魏 学1,薛 伟1
(1.贵州大学 精细化工研究开发中心 教育部绿色农药和生物工程重点实验室,贵州 贵阳 550025;2.贵州理工学院 制药工程学院,贵州 贵阳 550003)
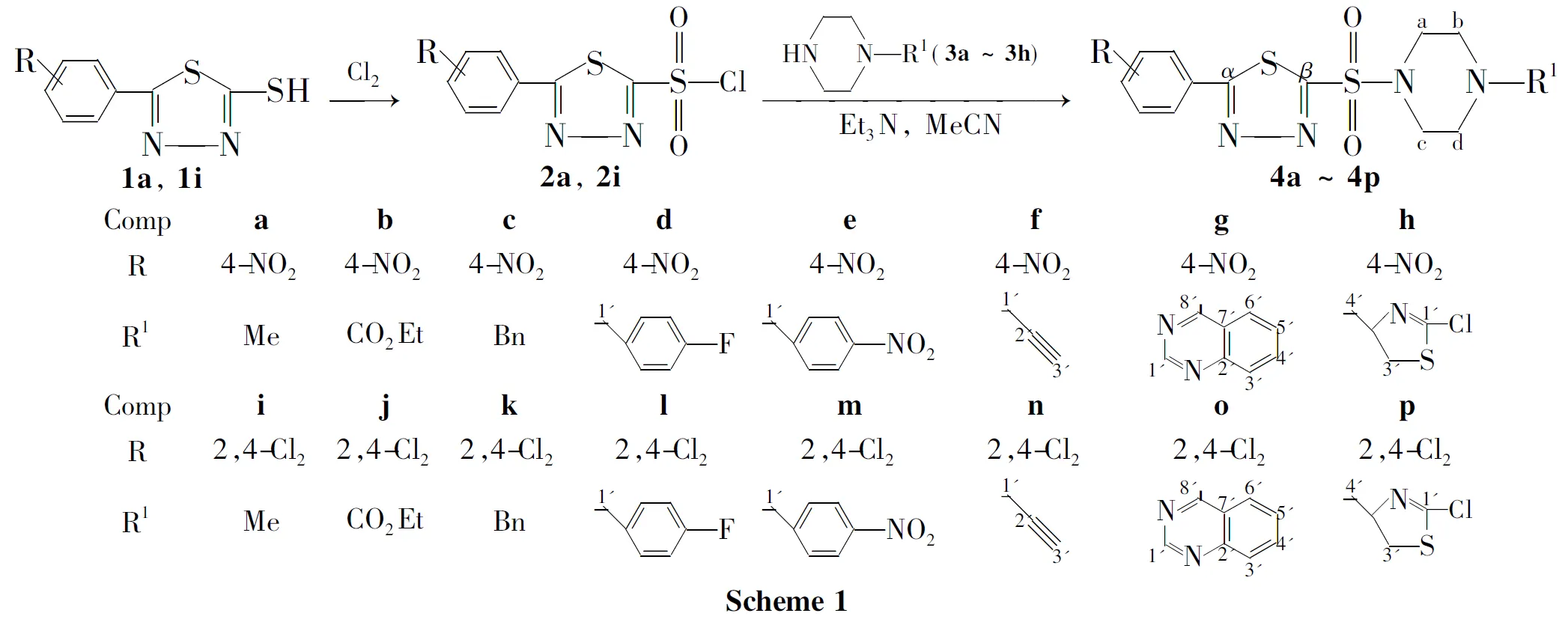
根据活性亚结构拼接原理,将1,3,4-噻二唑环和哌嗪环通过磺酰基桥接,设计并合成了16个新型的1-取代-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪类衍生物(4a~4p),其结构经1H NMR,13C NMR,IR和元素分析表征。考察了溶剂、缚酸剂和反应温度对1-甲基-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4a)产率的影响。合成4a的最佳反应条件为:乙腈为溶剂,三乙胺为缚酸剂,于25℃反应5h,产率75%。采用生长速率法研究了4a~4p对小麦赤霉菌、苹果腐烂病菌和辣椒枯萎病菌的抑制活性。结果表明:在用药量为50μg·mL-1时,1-(4-氟苄基)-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4d)和1-丙炔基-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4n)对三种病菌均具有较好的抑制活性。
1,3,4-噻二唑;哌嗪;磺胺;合成;抑菌活性
磺酰胺衍生物具有抗菌、杀虫、抗真菌和抗病毒等多种生物活性[1-5]。如日本石原公司开发的杀菌剂氰霜唑,对卵菌纲类病害具有良好的抑制活性;美国Dow Agrosciences公司开发的除草剂双氟磺草胺,主要用于苗后防除冬小麦和玉米田阔叶杂草。寻找新型结构的磺酰胺类化合物成为药物开发的一个重要方向。
哌嗪类化合物也显示了广谱的生物活性,在抗真菌、抗病毒、抗肿瘤、杀虫和除草等领域中发挥着重要作用[6-8]。胡国强等[9]在合成系列稠杂环的新化合物时发现,和母体结构相比,极性碱性哌嗪基的引入有利于提高化合物的活性。1,3,4-噻二唑类化合物具有独特的抗真菌、病毒、杀虫、除草和抗氧化等活性[10-13],如叶青双能有效防治水稻白叶枯病;诺华公司开发的除草剂Fluthiacetmethy,能完全控制玉米和马铃薯中的阔叶杂草。
本课题组在前期工作[14-18]中合成了含1,3,4-噻二唑杂环的硫醚、亚砜和砜类化合物,发现其中部分化合物表现出较好的生物活性。在此基础上,通过引入磺酰胺基团,进一步进行结构改造,有望得到较高抑菌活性的新化合物。为此,本文根据活性亚结构拼接原理,将1,3,4-噻二唑环和哌嗪环通过磺酰基桥接,设计并合成了16个新型的1-取代-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪类衍生物(4a~4p,Scheme 1),其结构经1H NMR,13C NMR,IR和元素分析表征。并采用生长速率法研究了4a~4p对小麦赤霉菌,苹果腐烂病菌和辣椒枯萎病菌的抑制活性。
1 实验部分
1.1 仪器与试剂
XT-5型显微熔点仪(温度未校正);JEOL-ECX 500MHz型核磁共振仪(DMSO-d6为溶剂,TMS为内标);IR Prestige-21型红外光谱仪(KBr压片);Elementar Vario-III型元素分析仪。
5-(4-硝基苯基)-2-巯基-1,3,4-噻二唑(1a)和5-(2,4-二氯苯基)-2-巯基-1,3,4-噻二唑(1i)按文献[17]方法合成;其余所用试剂均为分析纯或化学纯。
1.2 合成
(1)2a和2i的合成
在反应瓶中依次加入1a2.0mmol,1,2-二氯乙烷12mL和水6mL,冰盐浴冷却,搅拌下加入浓盐酸3mL,于0℃通入氯气15min(反应体系呈黄绿色)。快速分液后脱溶,有机相用无水硫酸镁干燥得淡黄色固体5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰氯(2a)0.38g,收率63%,m.p.123℃~125℃。
用类似的方法合成白色固体5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰氯(2i)0.32g,收率50%,m.p.92℃~95℃。
(2)4a~4p的合成通法
在反应瓶中依次加入单取代哌嗪3a~3h1.0mmol和无水乙腈5mL,搅拌使其溶解;缓慢(15min)滴加2a(1.0mmol)的无水乙腈(10mL)溶液;滴加三乙胺0.2mL,滴毕,于25℃反应5h。抽滤,滤饼用无水乙腈洗涤,干燥后用混合溶剂[V(DMF)∶V(EtOH)=1∶2]重结晶得4a~4h。
用2i替代2a,用类似的方法合成4i~4p。
1-甲基-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4a):黄色晶体,收率75%,m.p.203℃~205℃;1H NMRδ:8.24(d,J=10.00Hz,2H,ArH),8.36(d,J=10.00Hz,2H,ArH),2.49~2.51(m,8H,a,b,c,d-H),2.23(s,3H,CH3);13C NMR(125MHz,下同)δ:170.7,166.2,149.9,134.5,130.3,125.2,53.9,46.3,45.6;IRν:3111,2941,2910,2796,1519,1340,1319,1172,1087,983,604,592,518cm-1;Anal.calcd for C13H15N5O4S2:C 42.27,H 4.09,N 18.96;found C 42.10,H 3.79,N 19.31。
1-乙氧羰基-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4b):白色晶体,收率71%,m.p.212℃~214℃;1H NMRδ:8.24~8.43(m,4H,ArH),4.01(q,J=7.45Hz,2H,CH2in Et),3.34~3.35(m,8H,a,b,c,d-H),1.15(t,J=6.85Hz,3H,CH3in Et);13C NMRδ:170.9,169.1,165.8,155.5,154.8,149.9,149.6,134.9,134.5,61.6,46.2,43.0,15.0;IRν:3107,3076,1683,1597,1512,1489,1373,1323,1166cm-1;Anal.calcd for C15H17N5O6S2:C 42.15,H 4.01,N 16.38;found C 42.45,H 4.40,N 16.08。
1-苄基-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4c):淡黄色晶体,收率68%,m.p.223℃~225℃;1H NMRδ:8.42(d,J=10.00Hz,2H,ArH),8.36(d,J=10.00Hz,2H,ArH),7.22~7.32(m,5H,ArH),3.51(s,2H,CH2in Bn),3.32~3.35(m,8H,a,b,c,d-H);13C NMRδ:170.8,166.1,149.9,138.1,134.5,130.3,129.3,128.8,127.6,125.1,61.8,51.9,46.7;IRν:3109,2916,1699,1520,1361,1338,1172,1116,983,854,721,592cm-1;Anal.calcd for C19H19N5O4S:C 51.22,H 4.30,N 15.72;found C 51.62,H 4.26,N 15.42。
1-(4-氟苄基)-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4d):白色晶体,收率49%,m.p.203℃~205℃;1H NMRδ:8.39(d,J=10.00Hz,ArH),8.18(d,J=10.00Hz,2H,ArH),6.97~7.26(m,4H,ArH),3.52(s,2H,1′-Η),2.69~3.51(m,8H,a,b,c,d-H);13C NMRδ:169.7,166.8,149.9,134.4,130.7,129.3,124.8,115.5,115.3,61.7,51.9,46.7;IRν:3113,2868,2837,2777,1598,1508,1373,1340,1319,1178,1126,948,852,727,592,549cm-1;Anal.calcd for C19H18N5O4S2F:C 51.22,H 4.30,N 15.72;found C 51.62,H 4.26,N 15.42。
1-(4-硝基苄基)-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4e):白色晶体,收率65%,m.p.232℃~234℃;1H NMRδ:8.40(d,J=10.00Hz,2H,ArH),8.17~8.19(m,4H,ArH),7.49(d,J=10.00Hz,2H,ArH),2.63~3.66(m,10H,a,b,c,d,1′-H);13C NMRδ:170.8,169.9,166.1,150.0,134.4,130.3,125.1,124.0,114.9;IRν:3109,2927,1598,1520,1456,1367,1346,1174,954,852,723,590cm-1;Anal.calcd for C19H18N6O6S2:C 46.52,H 3.70,N 16.13;found C 46.76,H 3.64,N 16.51。
1-丙炔基-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4f):淡黄色针状晶体,收率58%,m.p.208℃~211℃;1H NMRδ:8.39(d,J=10.00Hz,2H,ArH),8.18(d,J=10.00Hz,2H,ArH),3.56(t,J=10.00Hz,4H,a,b,c,d-H),3.36(s,2H,1′-H),2.72(t,J=10.00Hz,4H,a,b,c,d-H),2.29(s,1H,3′-H);13C NMRδ:170.8,166.2,149.9,134.4,130.3,125.1,50.5,46.2,46.0;IRν:3107,2931,2823,1640,1584,1348,1315,1126,956,854,729,590cm-1;Anal.calcd for C15H15N5O4S2:C 45.79,H 3.84,N 17.80;found C 45.98,H 3.74,N 17.56。
1-(4-喹唑啉基)-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4g):黄色晶体,收率67%,m.p.234℃~236℃;1H NMRδ:8.78(s,1H,1-H),8.40(d,J=10.00Hz,2H,ArH),8.19(d,J=10.00Hz,2H,ArH),7.51~7.96(m,4H,3′,4′,5′,6′-H),3.94~3.76(m,8H,a,b,c,d-H);13C NMRδ:170.9,166.0,164.2,153.9,151.6,149.9,134.4,133.5,130.2,128.5,126.5,125.7,125.1,116.3,48.9,46.3;IRν:3109,2916,2891,1568,1429,1377,1328,1174,945,854,773,688,592,528cm-1;Anal.calcd for C20H17N7O4S2:C 49.68,H 3.54,N 20.28;found C 49.74,H 3.86,N 19.98。
1-[(2-氯噻唑-4-基)甲基]-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4h):白色晶体,收率68%,m.p.229℃~231℃;1H NMRδ:8.42(d,J=10.00Hz,2H,ArH),8.19(d,J=10.00Hz,2H,ArH),7.26(s,1H,3′-H),3.71(s,2H,4′-H),3.54~2.66(m,8H,a,b,c,d-H);13C NMRδ:170.9,166.1,150.0,134.4,130.3,125.1;IRν:3107,2914,2368,1597,1520,1450,1353,1332,1176,956,852,725,686,509cm-1;Anal.calcd for C16H15N6O4S2Cl:C 39.46,H 3.10,N 17.26;found C 39.25,H 3.02,N 17.01。
1-甲基-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4i):白色晶体,收率54%,m.p.152℃~154℃;1H NMRδ:7.45~8.40(m,3H,ArH),2.49~2.51(m,8H,a,b,c,d-H),2.23(s,3H,CH3);13C NMRδ:166.4,166.3,138.6,133.6,132.1,130.7,128.3,126.5,54.1,46.5,45.9;IRν:3089,2935,2800,1640,1456,1373,1352,1176,1155,958,729,619,595cm-1;Anal.calcd for C13H14N5O2S2Cl2:C 39.70,H 3.59,N 14.24;found C 39.30,H 3.79,N 14.64。
1-乙氧羰基-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4j):白色晶体,收率63%,m.p.139℃~141℃;1H NMRδ:8.38(d,J=8.55Hz,ArH),7.60(s,1H,ArH),7.45(d,J=8.6Hz,1H,ArH),4.11(q,J=5.92Hz,2H,CH2in Et),3,49~3,65(m,8H,a,b,c,d-H),1.25(t,J=6.85Hz,3H,CH3in Et);13C NMRδ:166.6,155.1,138.7,133.6,132.0,130.7,128.4,126.3,62.0,46.5,14.6;IRν:3080,2987,2870,1690,1471,1382,1365,1163,952,862,727,596cm-1;Anal.calcd for C15H16N4O4S2Cl2:C 39.92,H 3.57,N 12.41;found C 39.67,H 3.49,N 12.18。
1-苄基-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4k):白色晶体,收率56%,m.p.125℃~127℃;1H NMRδ:8.13(d,J=10.00Hz,1H,ArH),7.47(d,J=10.00Hz,1H,ArH),7.32~7.34(m,5H,ArH),7.26~7.30(m,1H,ArH),3.62(t,J=10.00Hz,4H,a,b,c,d-H),3.58(s,2H,CH2in Bn),2.59(t,J=10.00Hz,4H,a,b,c,d-H);13C NMRδ:173.5,152.6,131.9,131.2,130.2,129.2,128.6,128.5,127.8,127.5,63.0,52.1,49.8;IRν:3089,2935,2800,1640,1456,1373,1352,1176,1155,958,729,619,595cm-1;Anal.calcd for C19H18N4O2S2Cl2:C 48.62,H 3.87,N 11.94;found C 48.42,H 3.69,N 11.58。
1-(4-氟苄基)-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4l):白色晶体,收率72%,m.p.140℃~142℃;1H NMRδ:8.13(d,J=10.00Hz,1H,ArH),7.47(d,J=10.00Hz,1H,ArH)),7.32~7.33(m,1H,ArH),7.03~7.26(m,4H,ArH),3.61(t,J=10.00Hz,4H,a,b,c,d-H),3.53(s,2H,1′-H),2.58(t,J=10.00Hz,4H,a,b,c,d-H);13C NMRδ:168.9,135.9,131.9,131.2,130.7,130.1,127.7,115.4,115.2,62.1,51.8,49.5;IRν:3111,2956,2850,1640,1500,1444,1375,1317,1240,1103,906,806,596,503cm-1;Anal.calcd for C19H17N4O2S2Cl2:C 46.82,H 3.52,N 11.50;found C 46.55,H 3.75,N 11.39。
1-(4-硝基苄基)-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4m):白色晶体,收率79%,m.p.163℃~165℃;1H NMRδ:8.29~8.34(m,3H,ArH),7.72~8.03(m,4H,ArH),3.25~3.97(m,10H,a,b,c,d,1′-H);13C NMRδ:166.6,166.5,147.4,145.4,133.6,132.0,130.7,129.4,128.4,126.4,123.7,61.6,52.2,46.6;IRν:3074,2937,2912,2819,1606,1587,1501,1361,1328,1168,1109,954,729,707cm-1;Anal.calcd for C19H17N5O4S2Cl2:C 44.36,H 3.33,N 13.61;found C 44.58,H 3.03,N 13.84。
1-丙炔基-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4n):黄色针状晶体,收率71%,m.p.137℃~139℃;1H NMRδ:8.39(d,J=10.00Hz,1H,ArH),7.61(d,J=10.00Hz,1H,ArH),7.46~7.48(m,1H,ArH),3.53(t,J=10.00Hz,4H,a,b,c,d-H),3.35(s,2H,1′-H),2.71(t,J=10.00Hz,4H,a,b,c,d-H),2.28(s,1H,3′-H);13C NMRδ:166.4,166.3,138.6,133.5,132.0,130.7,128.3,126.4,77.8,73.9,50.9,46.7,46.5;IRν:3084,2947,2819,1585,1456,1357,1317,1118,950,831,727,634,590,466cm-1;Anal.calcd for C15H14N4O2S2Cl2:C 43.17,H 3.38,N 13.42;found C 43.34,H 3.45,N 13.27。
1-(4-喹唑啉基)-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4o):白色晶体,收率64%,m.p.104℃~107℃;1H NMRδ:8.77(s,1H,1-H),7.62~8.41(m,4H,3′,4′,5′,6′-H),7.46~7.50(m,3H,ArH),3.73~3.92(m,8H,a,b,c,d-H);13C NMRδ:153.9,151.7,137.9,133.5,132.9,130.9,129.0,128.6,126.5,125.7,116.3,48.9,46.3;IRν:3113,2969,2843,1610,1570,1498,1370,1340,1263,1140,964,827,775cm-1;Anal.calcd for C20H16N6O2S2Cl2:C 47.34,H 3.18,N 16.56;found C 46.99,H 3.46,N 16.35。
1-[(2-氯噻唑-4-基)甲基]-4-[5-(2,4-二氯苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪(4p):白色晶体,收率72%,m.p.139℃~141℃;1H NMRδ:7.71~8.32(m,3H,ArH),7.62(s,1H,3′-H),3.57(s,2H,4′-H),2.47~3.34(m,8H,a,b,c,d-H);13C NMRδ:167.1,167.0,166.0,137.9,133.6,133.5,132.9,130.9,129.0,126.7,51.2;IRν:3078,2937,2829,1585,1456,1363,1334,1172,956,798,725,623,590,553cm-1;Anal.calcd for C16H14N5O2S3Cl3:C 37.62,H 2.76,N 13.71;found C 37.52,H 2.81,N 13.54。
1.3 抑菌活性测定
以多菌灵为阳性对照组,采用生长速率法[19],在用药量为50μg·mL-1下测试4a~4p对小麦赤霉菌(G.zeae),苹果腐烂病菌(C.mandshurica)和辣椒枯萎病菌(F.oxysporum)的抑制活性。
2 结果与讨论
2.1 合成
(1)2的合成
在2的合成中发现溶剂对氯氧化反应有较大影响。为此,考察了溶剂对2i收率的影响,结果见表 1。由表1可见,以A和B为溶剂时,收率较低;以C为溶剂时,当体积比为4∶2∶1时,收率较高(52%)。合成2i的最佳溶剂为C,即V(ClCH2CH2Cl)∶V(H2O)∶V(HCl)=4∶2∶1。
(2)4的合成
以合成4a为模板,考察溶剂、缚酸剂和反应温度对4a收率的影响,结果见表2。由表2可见,当温度、反应时间和缚酸剂不变,选用乙腈为溶剂时收率最高;当温度、反应时间和溶剂不变时,以三乙胺为缚酸剂时收率最高,以吡啶为缚酸剂时,收率仅46%。其可能原因是吡啶盐对4a有一定的溶解作用;当以乙腈为溶剂,三乙胺为缚酸剂,反应时间为5h时,随着温度的升高,收率有所提高;温度升至35℃时,收率反而降低。由此可见,合成4a的最佳条件为:乙腈为溶剂,三乙胺为缚酸剂,于25℃反应5h,收率75%。
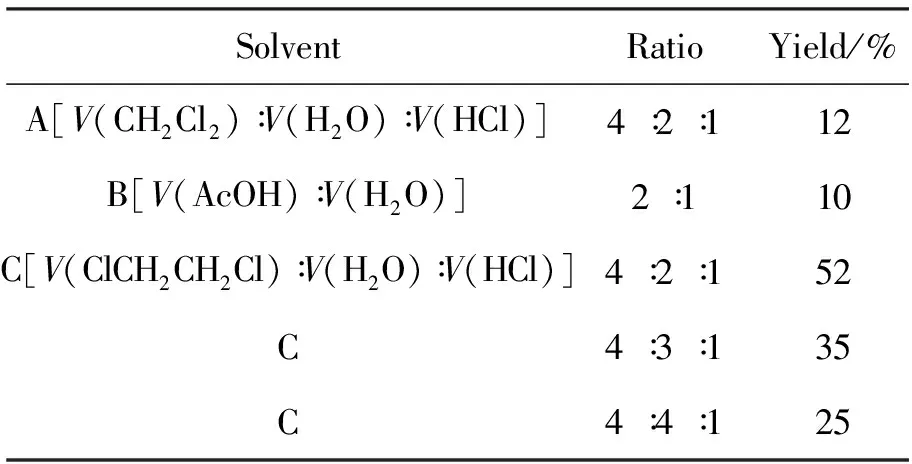
表1 溶剂对合成2i的影响*Table1 Effect of solvent on synthesizing 2i
*solvent volume 20mL,other reaction conditions as same as 1.2(1)

表2 反应条件对合成4a的影响*Table2 Effect of reaction conditions on synthesizing 4a
*other reaction conditions as same as 1.2(2)
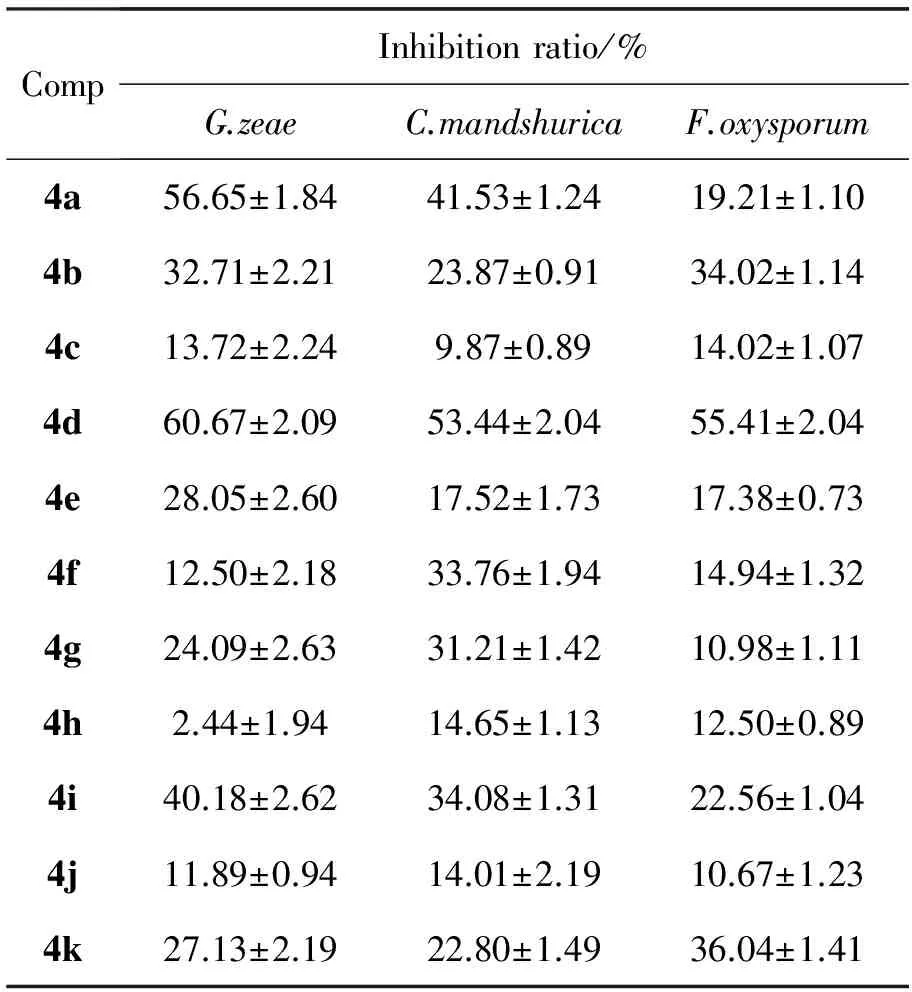
表3 4a~4p的抑菌活性Table3 Antibacterial activities of 4a~4p
续表3
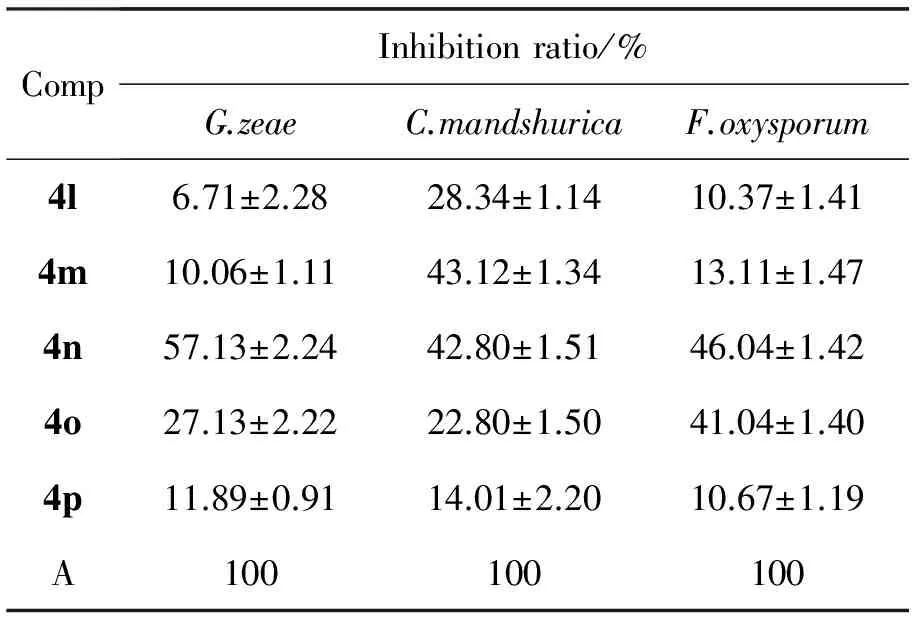
CompInhibitionratio/%G.zeaeC.mandshuricaF.oxysporum4l6.71±2.2828.34±1.1410.37±1.414m10.06±1.1143.12±1.3413.11±1.474n57.13±2.2442.80±1.5146.04±1.424o27.13±2.2222.80±1.5041.04±1.404p11.89±0.9114.01±2.2010.67±1.19A100100100
A=Carbendazim
2.2 抑菌活性
4a~4p的抑菌活性见表3。由表3可见,4a~4p对三种菌均有一定抑菌活性,其中4d和4n对三种病菌的抑制活性较好,但均次于阳性对照药多菌录灵。
3 结论
设计并合成了16个新型的1-取代-4-[5-(4-硝基苯基)-1,3,4-噻二唑-2-磺酰基]哌嗪类衍生物(4a~4p)。合成4a的最佳反应条件为:乙腈为溶剂,三乙胺为缚酸剂,于25℃反应5h,收率75%。
抑菌活性测试结果表明:在用药量为50μg·mL-1时,4d和 4n对小麦赤霉菌,苹果腐烂病菌和辣椒枯萎病菌均有较好的抑制活性。
[1] Andrighetti Fröhner C R,Oliveira K N,Gaspar Silva D,etal.Synthesis,biological evaluation and SAR of sulfonamide 4-methoxychalcone derivatives with potential antileishmanial activity[J].Eur J Med Chem,2009,44(2):755-763.
[2] Schobert R,Stehle R,Walter H.Tipranavir analogous 3-sulfonylanilidotetronic acids:New synthesis and structure-dependent anti-HIV activity[J].Tetrahedron,2008,64(40):9401-9407.
[3] Keche A P,Hatnapure G D,Tale R H,etal. A novel pyrimidine derivatives with aryl urea,thiourea and sulfonamide moieties:Synthesis,anti-inflammatory and antimicrobial evaluation[J].Bioorg Med Chem Lett,2012,22:3445-3448.
[4] Ozawa Y,Kusano K,Owa T,etal.Therapeutic potential and molecular mechanism of a novel sulfonamide anticancer drug,indisulam(E7070)in combination with CPT-11for cancer treatment[J].Cancer Chemother Pharmacol,2012,69(5):1353-1362.
[5] Zoumpoulakis P,Camoutsis C,Pairas G,etal.Synthesis of novel sulfonamide-1,2,4-triazoles,1,3,4-thiadiazoles and 1,3,4-oxadiazoles,as potential antibacterial and antifungal agents[J].Bioorg Med Chem,2012,20(4):1569-1583.
[6] Dong M X,Lu L,Li H T,etal.Design,synthesis and biological activity of novel 1,4-disubstituted piperidine/piperazine derivatives as CCR5antagonist-based HIV-1entry inhibitors[J].Bioorg Med Chem Lett,2012,22(9):3284-3286.
[7] Hatnapure G D,Keche A P,Rodge A H,etal.Synthesis and biological evaluation of novel piperazine derivatives of flavone as potent anti-inflammatory and antimicrobial agent[J].Bioorg Med Chem Lett,2012,22(20):6385-6390.
[8] Waszkielewicz A M,Gunia A,Szkaradek N,etal.Synthesis and evaluation of pharmacological properties of some new xanthone derivatives with piperazine moiety[J].Bioorg Med Chem Lett,2013,23(15):4419-4423.
[9] 胡国强,侯莉莉,谢松强,等.非对称双杂环取代均三唑并噻二唑哌嗪衍生物的合成及抗菌活性[J].应用化学,2009,26(1):63-66.
[10] Yang X H,Xiang L,Li X,etal.Synthesis,biological evaluation and molecular docking studies of 1,3,4-thiadiazol-2-amide derivatives as novel anticancer agents[J].Bioorg Med Chem,2012,20(9):2789-2795.
[11] Aggarwal N,Kumar R,Dureja P,etal.Synthesis of novel nalidixic acid-based 1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives as potent antibacterial agents[J].Chem Biol Drug Des,2012,79(4):384-397.
[12] Guan P,Sun F E,Hou X B,etal.Design,synthesis and preliminary bioactivity studies of 1,3,4-thiadiazole hydroxamic acid derivatives as novel histone deacetylase inhibitors[J].Bioorg Med Chem 2012,20(12):3865-3872.
[13] El Sayed W A,Metwally M,Nada D S,etal.Synthesis and antimicrobial activity of new substituted 5-(pyridine-3-yl)-1,3,4-thiadiazoles and their sugar derivatives[J].J Heterocycl Chem,2013,50(2):194-201.
[14] Chen C J,Song B A,Yang S,etal.Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives[J].Bioorg Med Chem,2007,15(12):3981-3989.
[15] Liu F,Luo X Q,Song B A,etal.Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety[J].Bioorg Med Chem,2008,16(7):3632-3640.
[16] Yang S,Li Z,Jin L H,etal.Synthesis and bioactivity of 4-alkyl(aryl)thioquinazoline derivatives[J].Bioorg Med Chem Lett,2007,17(8):2193-2196.
[17] 杨超,杨松,宋宝安,等.2-取代硫醚(砜)-5-(4-硝基或4-甲氧苯基)-1,3,4-噻二唑类化合物的合成及抑菌活性[J].有机化学,2010,30(9):1327-1334.
[18] 宋宝安,陈才俊,杨松,等.2-取代硫醚-5-(3,4,5-三甲氧基苯基)-1,3,4-噻二唑类化合物的合成、结构与体外抗癌活性[J].化学学报,2005,63(18):1720-1726.
[19] 孔凡彬,高扬帆,陈锡岭,等.9种药剂对玉米小斑病菌的室内抑菌试验[J].广西农业科学,2006,37:148-149.
SynthesisandAntibacterialActivitiesof1-Substituted-4-[5-(4-sub-stitutedphenyl)-1,3,4-thiadiazol-2-sulfonyl]piperazineDerivatives
WU Qin1,2, WANG Zhen-chao1, WEI Xue1, XUE Wei1
(1.Key Laboratory of Green Pesticide and Bioengineering,Ministry of Education,Research and Development Center for Fine Chemicals,Guizhou University,Guiyang 550025,China;2.School of Pharmaceutical Engineering,Guizhou Institute of Technology,Guiyang 550003,China)
Sixteen novel 1-substituted-4-[5-(4-substituted phenyl)-1,3,4-thiadiazol-2-sulfonyl]piperazine derivatives(4a~4p)were designed and synthesized by connection reaction of 1,3,4-thiadiazol withN-substitued piperazine.The structures were characterized by1H NMR,13C NMR,IR and elemental analysis.Effects of the solvent,acid acceptor and reaction temperature on the yield of 1-methyl-4-[5-(4-nitrophenyl)-1,3,4-thiadiazol-2-sulfonyl]piperazine(4a)were investigated.The optimum reaction conditions for synthesizing4awith the yield of 75% at 25℃ for 5h were as follows:acetonitrile was solvent and triethylamine was acid acceptor.The antibacterial activities of4a~4pagainstG.zeae,C.mandshuricaandF.oxysporumwere investigated by growth rate method.The results indicated that 1-methyl-4-[5-(4-fluorobenzyl)-1,3,4-thiadiazol-2-sulfonyl]piperazine(4d)and 1-methyl-4-[5-(4-propinyl)-1,3,4-thiadiazol-2-sulfonyl]piperazine(4n)exhibited better antibacterial activities at 50μg·mL-1.
1,3,4-thiadiazol;piperazine;sulfonamide;synthesis;antibacterial activity
2014-01-24;
2014-03-12
国家自然科学基金资助项目(20762002);国家十二五科技支撑计划资助项目(2011BAE06B04-09)
吴琴(1974-),女,汉族,贵州贵阳人,副教授,主要从事新农药设计与合成的研究。E-mail:wuqin1974@163.com
O626.25;O626.32
A
1005-1511(2014)04-0429-06

