脂肪族聚酯功能化研究进展*
沈 慧,李 阳,刘 钰
(电子科技大学 微电子与固体电子学院 生物材料教研室,四川 成都 610054)
·综合评述·
脂肪族聚酯功能化研究进展*
沈 慧,李 阳,刘 钰
(电子科技大学 微电子与固体电子学院 生物材料教研室,四川 成都 610054)
从六方面(卤化、羟基化、羧基化、氨基化、不饱和键功能化和其他功能化)综述了生物可降解线性脂肪族聚酯的功能化研究进展。参考文献60篇。
线性脂肪族聚酯;功能化;合成;综述
生物可降解线性脂肪族聚酯及共聚物由于具有生物可降解性、良好的生物相容性成为应用较多的可吸收植入材料和良好的组织工程材料,同时在药物缓控释材料中也有非常重要的应用前景[1]。但其主链的疏水性和骨架上缺少活性官能团,使其应用受限[2]。近年来,由于生物医学的发展,使功能化可生物降解线性聚酯成为更有吸引力的生物材料。聚酯多为半结晶性,疏水性较强,缺乏活性官能团。改善的方法之一就是引入官能团,如引入羟基和羧基可提高亲水性和可降解性,调节力学、热学、化学和生物学性质。功能化后的线性脂肪族聚酯仍属生物可降解聚合物,被广泛应用于生物医学领域。
线性脂肪族聚酯的聚合方法主要有三种:(1)羟基酸分子与二醇或二酸化合物缩聚[2-4];(2)内酯或其他环二酯的开环聚合[5-6];(3)酶催化聚合[7-8]。前两种方法可统称为化学合成功能化。本文仅对该法进行综述。
化学功能化中常采用两种策略:(1)在单体中直接引入官能团,保护以免副反应发生,聚合后再脱保护。该法需复杂的单体制备过程,冗长的官能团保护与脱保护,但可得到结构可控性较强的功能化聚酯。(2)先合成传统的线性聚酯,再以化学修饰于聚合物链上引入官能团。该法条件苛刻,结构可控性较差[9-10]。
本文主要从六方面(卤化、羟基化、羧基化、氨基化、不饱和键功能化和其他功能化)综述生物可降解线性脂肪族聚酯功能化的研究进展。
1 卤化
卤原子性质活泼,是很好的离去基团,因此可先卤化再进行其它功能化反应。如,将6-氯环己酮氧化成6-氯-己内酯,再开环均聚或共聚得到含氯原子侧基的聚酯[11]。通过氯原子同叠氮化钠的取代反应,聚酯主链上能引入叠氮基团。叠氮基可同众多具有三键官能团的化合物进行点击反应,从而在聚酯中引入其它侧链[12]。
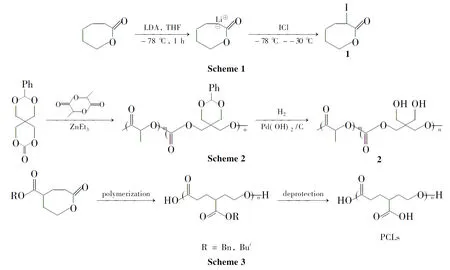
Habnouni等[13]合成了一个新的功能化内酯α-碘-ε-己内酯 (1,Scheme 1);1聚合后就得到分子量分布窄的含碘侧基的聚酯。
Lenoir等[14]通过α-氯-ε-己内酯单体和ε-己内酯共聚,再通过原子自由基转移反应(ATRP)接枝聚甲基丙烯酸甲酯或聚丁烯基苯甲酸。该反应目前扩展到了其它官能团,如醇、羧酸、环氧化物和胺。Hedrick等[15]通过γ-溴-ε-己内酯的均聚,在0℃下甲苯溶液中通过Al(OPri)3引发,得到的聚酯具有可预测的分子量和窄的分子量分布。这个含溴的聚酯开辟了通过直接转化得到新的功能化聚酯的道路。
2 羟基化
引入羟基可影响聚酯的亲水性、降解率、结晶度和极性,且羟基可进一步功能化[16]。线性聚酯的首次羟基化是通过环氧十一烯酸的直接缩聚[17],但所得产物纯度不高。羟基化也可通过三步法实现:(1)合成含有被保护羟基的内酯;(2)内酯再开环聚合;(3)去保护[18]。
Wang等[19-22]以双端羟基的聚己内酯同富马酸氯反应合成线型脂肪族不饱和聚酯。其中双键在光引发剂作用下发生交联反应,形成聚己内酯的交联网络结构。Takasu等[23]报道了含悬挂羟基的二醇和二羧酸的化学选择性缩聚合成悬挂羟基聚酯的一步合成法,包括酒石酸和苹果酸,在温和条件下,用稀土三氟[如Sc(OTf)3,Y(OTf)3,Sm(OTf)3,Yb(OTf)3和Sc(NTf2)3]催化,得到聚合物的分子量为Mn>1.0×104g·mol-1。Xie[24]等合成了含伯羟基侧基的聚酯。由于亲水性的改变,脱保护后聚酯的降解速率比脱保护前明显加快。随后,又以mPEG为引发剂引发L-LA和PTO开环共聚,合成了两亲嵌段共聚物(2,Scheme 2)[25]。
Wang等[26]通过甘油和癸二酸缩聚,甘油/癸二酸的摩尔比是1∶1,得到的聚合物只有少量的交联且羟基是直接连在网状物骨架上。You[27]等报道了一种简单的路线制备带有羟基化的功能化聚酯,聚(癸二甘油二酯)。其关键步骤是环氧化物开环聚合反应,而不是产生聚(癸二酸甘油)(PGS)的传统缩聚。相比于PGS,PSeD的线性主链上有更多的羟基官能团,更高分子量和窄的分布,还具有良好的细胞相容性。Zhang等[28]合成了一种新的生物可降解的功能化聚酯聚(丁二酸丁二醇酯-共-丁烯酸盐)[P(BS-co-BM)],脱保护得到悬挂羟基官团的P(BS-co-BM)。脱保护后的产物P(BS-co-55mol% BM)显示比预期对应的P(BS-co-55mol% BBM)有较高的熔点,结晶性和较低玻璃化转变温度。Miao[29]等将油酸经酶促环氧化得到环氧化油酸,这种单体在较高温下可自开环生成含侧链经基及长链烷基的高分子量聚酯。
Hedrick等[30]报道了γ-苄氧基-ε-己内酯和γ-2,2-双(苯双氧甲基)丙酸酯-ε-己内酯的合成。1,4-环己二醇的单保护是通过与苄基溴或者2,2-双(苯双氧甲基)丙酸氯以中等产率反应来完成。两种产物都可被氯铬酸吡啶嗡氧化,分别得到被保护的羟基和双羟基功能化的环己酮。最后,通过Pd/C的催化氢解得到功能化聚合物。
Saulnier等[31]以辛酸亚锡为引发剂,通过L-丙交酯和3-(1,2,3,4-四氧代丁基-二异亚丙基)-1,4-二恶烷-2,5-二酮的开环共聚制备了羟基被保护的聚(乙醇酸-共-葡糖酸)和聚(L-乳酸-co-羟基乙酸-co-葡糖酸)。Wolf等[32]以异辛酸亚锡或者有机碱为催化剂,分别在本体和溶液中进行共聚合成了带有羟基端官能团的聚(L-丙交酯)共聚物,两者的Mn在(1200~34000)g·mol-1,且支化度可通过改变单体/引发剂的比例来调整。
Dhamaniya等[33]报道了2,3-异亚丙基-L-酒石酸盐与丁二酸二甲酯或己二酸二甲酯与市售烷二醇(如丁二酸二甲酯与1,6-己二醇)在四异丙氧基钛为催化剂下本体缩聚,接着异亚丙基的选择性脱保护后就得到了侧链是羟基的功能化聚酯。
Hao等[34]先将微溶于氯仿的聚(丁烯酒石酸盐)(PBF)乙酰基封端,接着OsO4和NMO为催化剂,氯仿和甲醇的混合溶剂中成功合成新型水溶性聚(丁烯酒石酸)(PBT),其具有较高的玻璃化转变温度和低于PBF合成前体的热稳定性。Leemhuis[35-37]等先后报道了带有悬挂羟基的功能化聚酯——聚(乳酸-无规-羟甲基乙醇酸)[poly(HMMG-L)]和聚(乙醇-交替-羟甲基乙醇酸)[poly(HMG-CL)]的合成。
3 羧基化
Mahmud等[38]合成了在PCL嵌段上带有羧基侧链的聚(环氧乙烷)-b-聚(ε-己内酯)(PEO-b-PCL)嵌段共聚物。如,α-苄基羧酸酯-ε-己内酯。用甲氧基PEO(5000g·mol-1)为引发剂,辛酸亚锡作为催化剂,α-苄基羧酸酯-ε-己内酯的ROP制备PEO-b-聚(α-苄基羧酸酯的ε-己内酯)(PEO-b-PBCL)。进一步催化脱苄制备PEO-b-PCCL。PEO-b-PBCL和PEO-b-PCCL共聚物的分子量分布分别是1.74和1.52,球形胶束的平均直径分别为62nm和20nm。Kulshrestha等[39]通过己二酸,1,8-辛二醇和双(羟甲基)丁酸,成功制备了具有侧挂羧酸基团的直链脂族族聚酯。
Ouchi等[40]通过二苄基苹果酸酯的开环聚合合成可生物降解聚(α-苹果酸)。为了获得较高分子量且侧链有可修饰基团的可生物降解乳酸类聚酯 ,二苄基苹果酸酯与L-双丙交酯开环共聚得到聚(α-苹果酸-共-乳酸)。聚(α-苹果酸)水解的结果表明该主链被无规裂解并缓慢排出体外。Zhang等[41]根据功能化的ε-己内酯合成了pH敏感的带有羧基的甲氧基聚(乙二醇)-嵌段-聚(ε-己内酯)[mPEG-b-P(2-CCL-co-6-CCL)]。带有侧链羧基的PCLs 是由Hedrick和他的同事通过苄基γ-(ε-己内酯)羧酸或叔丁基-γ-(ε-己内酯)羧酸开环聚合,然后酸脱保护来制备的[30](Scheme 3)。
He等[42]用ROP的方法合成了具有高分子量的含聚β-苄基马来酮酯的PLMA 共聚物。细胞无论在d-PLMA表面和支架内都生长良好,d-PLMA的细胞亲和性比PLLA更好。
4 氨基化
含有保护氨基的ε-己内酯单体——γ-(氨基甲酸苄基酯)-ε-己内酯(γ-CAB-ε-CL)成功被Yan等[43]合成。以辛酸亚锡为催化剂,于130℃其与己内酯开环聚合共聚来合成共聚物。将γCABεC结构单元连接到聚己内酯上,导致了形态从半结晶至无定形的改变。保护基团经Pd/C催化氢解除去,获得了侧链上有一级氨基的聚己内酯。Elisseeff等[44]合成了含有赖氨酸的ε-胺侧链聚合物,如,聚(乳酸-co-赖氨酸)。聚天冬氨酸的侧链接枝到聚合物氨基上得到PLAL-ASP。该聚合物与过量的甲基丙烯酸酐反应,连接到PLAL-ASP甲基丙烯酸酯基的含量为5%~22%(取决于反应时间和温度)。该聚合物有弹性,并具有组织工程应用的机械完整性。Caponetti等[45]合成了含一个聚(L-乳酸-共-L-赖氨酸)骨架和聚(L-赖氨酸),聚(D,L-丙氨酸)或 聚(L-天冬氨酸)侧链的共聚物。Alice等[46]在控制纳米级尺寸下通过分子链交联方法制备了多功能聚酯。首先2,2′-(亚乙二氧基)双(乙胺)耦合,再与δ-戊内酯和α-烯丙基-δ-戊内酯,α-炔丙基-δ-戊内酯和2-氧杂环庚烷-1,5-二酮开环聚合得到线性聚酯(带有缩酮、氨基和羟基)。
5 不饱和碳碳键
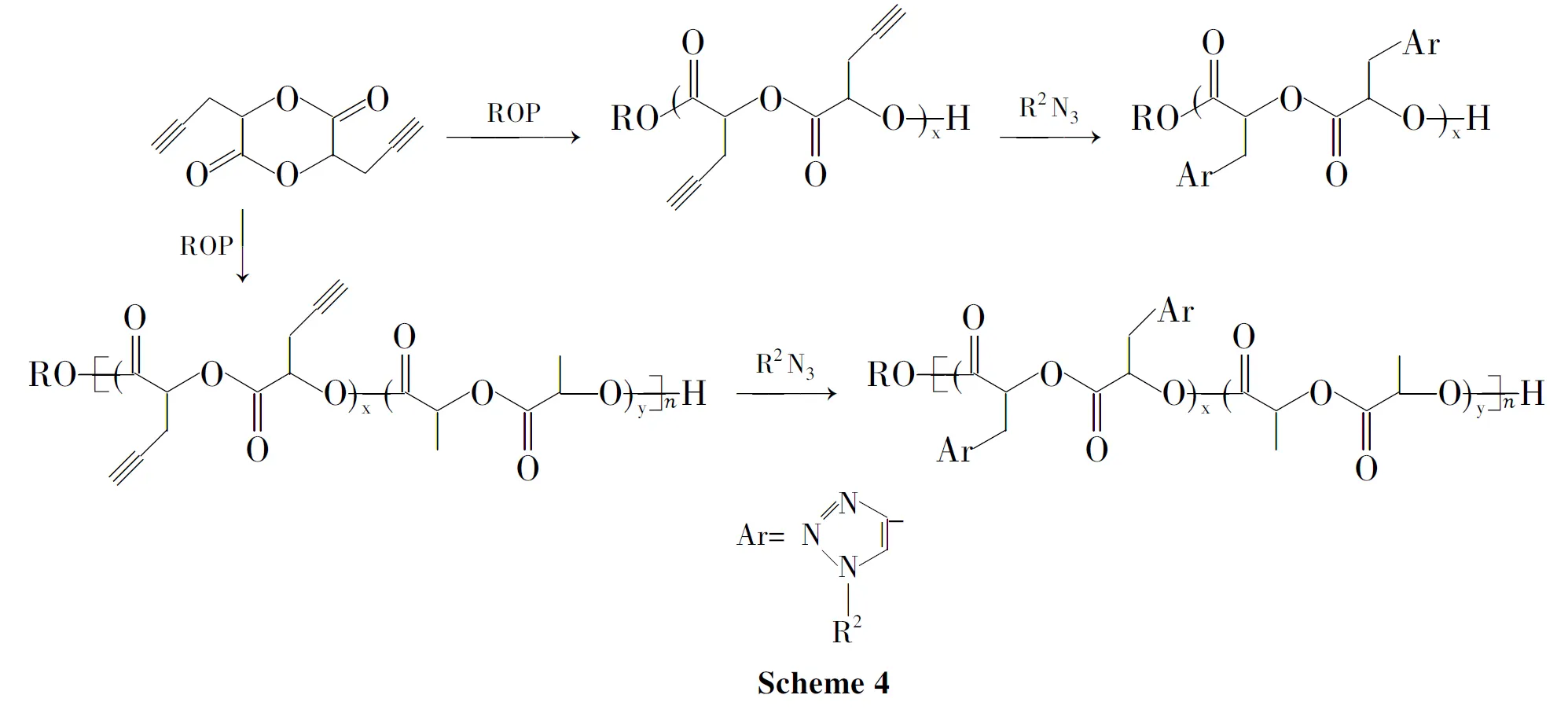
Jiang[47]等制备了双三键取代的乙交酯单体3,6-二炔丙基-1,4-二氧-2,5-二酮(PGL),并合成其均聚物、同丙交酯的无规及嵌段共聚物。这种聚合物可同含叠氮基团的化合物或聚合物反应来修饰改性(Scheme 4)。
Biiliet[48]合成了2-甲基-2-炔丙基-1,3-丙二醇,将其同二元酸、二元醇在阻聚剂对苯二酚存在下进行常规高温缩合,得到主链含炔键侧基的功能化线性聚酯。通过炔键和叠氮基团的Huisgen 1,3-环加成反应,对功能化聚酯进行修饰,得到高产率的刷状聚合物。
Riva等[49]用α-氯-ε-己内酯(α-Cl-ε-CL)在引发剂作用下开环聚合,然后通过聚(α-叠氮-ε-己内酯-共-ε-己内酯)无规共聚物的叠氮基替代悬挂的氯,再进行 Huisgen 1,3-偶极炔烃的环加成反应,这是比较有效的聚己内酯接枝取代方法。据这一方法,悬挂羟基、酯基和原子转移自由基聚合的引发剂被成功连接到PCL。5-炔丙基-戊内酯也是一种不饱和功能基的环状单体,可进行开环均聚或与己内脂、丙交酯等单体共聚,合成了含炔丙基侧基的功能化聚酯[50]。
Su等[51]报道了两亲性接枝嵌段功能化聚酯(PαN3CL-g-alkyne)-b-PCL的合成及功能化。将PMEs或PMPEGs接枝到PaN3CL-b-PCL上,这种两亲性聚合物在水溶液中自组装成胶束。在25℃时临界胶束浓度为(8.2~39.8)mg·mol-1。Parrish等[12]通过α-炔丙基-δ-戊内酯和ε-己内酯的可控开环聚合了带有悬挂乙炔官能团的脂肪族聚酯。随后,用点击化学在聚酯上接枝聚(乙二醇)和低聚物基团;该两亲性接枝聚酯具有生物相容性,可在一定范围内作为生物材料使用。Wurth等[52]提出了通过ε-己内酯和单体乙基2-甲基-4-戊烯酸氧化物的共聚得到聚(ε-己内酯-共-乙基-2-甲基-4-戊烯酸氧化物)。这个方法开拓了生物医学领域PCL应用的新途径。Målberg等[53]通过缩聚得到了不饱和脂肪族聚酯聚(丁-2-烯-1,4-二基丙二酸二甲酯)(PBM)。PCL-PBM和PLLA-PBM线性聚合物易交联而得到更高强度和机械性能,所以该物质可以用于用于弹性体网络和大分子引发剂。Parrish[54]等合成了一种双键取代的戊内酯,将其同己内酯在Sn(Otf)2催化下合成了双键功能化的共聚物。双键可改性为羟基,再同羧基封端的mPEG反应得到mPEG接枝的两亲性共聚物。
6 其他功能化
1,4-环己二酮经Bayer-Villiger氧化反应可高产率合成羰基功能化己内酯(OPD);OPD由锡类化合物催化开环均聚合或同其它单体如CL,LA等共聚,能获得含不同功能基团的聚酯[55-57]。聚酯上的羰基碳原子上易发生亲核加成反应,从而对聚酯进行修饰。Mayer[58]等以氨基封端的PEG为亲核试剂,同P(CL-co-OPD)进行加成反应合成了亲水链PEG修饰的聚酯,反应条件温和。聚酯中接枝的PEG较多时,可溶于水相。Horn[59]等也合成了P(CL-co-OPD),以含氨氧基的小分子荧光染料和药物同时对这种聚酯进行修饰,室温下即可在聚酯上引入不同比例的小分子,使聚酯含有两种不同功能性侧基。以双端官能团1,6-二(氨氧基)己烷同聚合物反应,得到了一种交联的聚酯凝胶。Yang等[60]合成了悬挂缩酮和活性丙烯酸酯与己内酯的共聚物,体外实验显示,该聚合物毒性低,可应用于组织工程支架。Zheng等[61]合成了多官能化的脂肪族聚酯,包含聚(富马酸丁二醇酯)和聚(1,2-环氧琥珀酸)两部分,并且当与后者结合时,其Tm和Tg稳定,适用于低廉的生物药品。
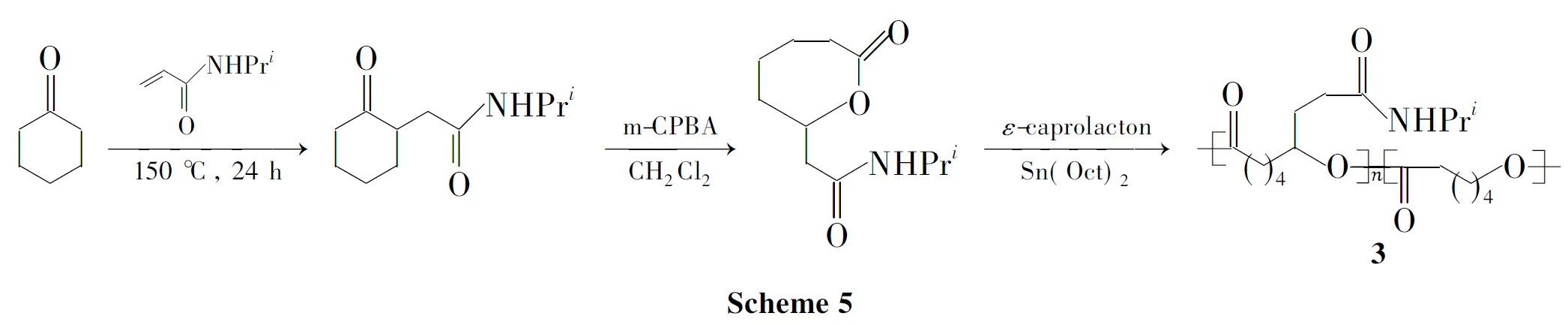
Dai等[62]首次合成了带有N-异丙基官能团的脂肪族聚酯(3,Scheme 5)。与PCL相比,poly(CCL-co-CL)具有较低结晶度和较高降解率。
Tian等[57]合成了被酮基修饰的聚己内酯。1,4,8-三氧杂[4,6]-9-十一烷酮以铝醇盐作为催化剂在甲苯中先均聚,接着用三苯基四氟硼酸盐脱去缩醛的反应合成新的脂肪族聚酯聚(γ-酮-ε-己内酯)。与PCL相比,该聚合物有较高结晶度,较高熔融温度,还可以光降解,减少白色污染。
7 总结与展望
生物可降解脂肪族聚酯的功能化不仅扩大了生物材料的应用范围,而且也促进了生物医学领域的发展。化学修饰的聚合物很有可能会引起生物可降解聚合物的性质改变,但可以通过适当的设计手段使合成的聚合物不仅保持其本身优点,还可得到多种性质共存并进一步修饰的可能性。如:组织工程支架需要良好的生物相容性、细胞粘附性和生物可降解性,药物运输系统需要智能可控的刺激相应性能等。生物可降解聚合物功能化的发展趋势如下:(1)功能化过程应该更简单且有效;(2)合成的聚酯不只是连接不同的功能基,而要各种基团间协同作用;(3)应扩大应用范围,包括潜在的体内应用。线性脂肪族聚酯的功能化将会对促进生物医学的发展做出巨大贡献。
[1] Jazkewitsch O,Mondrzyk A,Staffel R,etal.Cyclodextrin-modified polyesters from lactones and from bacteria:An approach to new drug carrier systems[J].Macromolecules,2011,44:1365-1371.
[2] Yildirim E D,Ayan H,Vasilets V N,etal.Effect of dielectric barrier discharge plasma on the attachment and proliferation of osteoblasts cultured over poly(ε-caprolactone)scaffolds[J].Plasma Processes and Polymers,2008,5:58-66.
[3] Albertsson A C,Varma I K.Aliphatic polyesters:Synthesis,properties and applications[J].Adv Polym Sci,2002,157:1-40.
[4] Hyon S H,Jamshidi K,Ikada Y.Synthesis of polylactides with different molecular weights[J].Biomaterials,1997,18:1503-1508.
[5] Pounder R J,Dove A P.Towards poly(ester)nanoparticles:Recent advances in the synthesis of functional poly(ester)s by ring-opening polymerization[J].Polymer Chemistry,2010,1:260.
[6] Velthoen I W,Dijkstra P J,Feijen J.AB2functional polyesters via ring opening polymerization:Synthesis and characterization[J].Macromolecular Chemistry and Physics,2009,210:689-697.
[7] Kobayashi S,Uyama H,Kimu S.Enzymatic polymerization[J].Chem Rev,2001,101:3793-3818.
[8] Loos K,Stadler R.Synthesis of amylose-block-polystyrene rod-coil block copolymers[J].Macromolecules,1997,30:7641-7643.
[9] Saulnier B,Coudane J,Garreau H,etal.Hydroxyl-bearing poly(α-hydroxy acid)-type aliphatic degradable polyesters prepared by ring opening(co)polymerization of dilactones based on glycolic,gluconic and l-lactic acids[J].Polymer,2006,47:1921-1929.
[10] Ponsart S,Coudane J,Vert M.A novel route to poly(ε-caprolactone)-based copolymers via anionic derivatization[J].Biomacromolecules,2000,1:275-81.
[11] Riva R,Schmeits S,Stoffelbach F,etal.Combination of ring-opening polymerization and “click” chemistry towards functionalization of aliphatic polyesters[J].Chemical Communications,2005:5334.
[12] Parrish B,Breitenkamp R B,Emrick T.PEG-and peptide-grafted aliphatic polyesters by click chemistry[J].J Am Chem Soc,2005,127:7404-7410.
[13] Habnouni S E,Darcos V,Coudane J.Synthesis and ping opening polymerization of a new functional lactone,alpha-Iodo-epsilon-caprolactone:A novel route to functionalized aliphatic polyesters[J].Macromolecular Rapid Communications,2009,30:165-169.
[14] Lenoir S,Riva R,Lou X,etal.Ring-opening polymerization ofα-chloro-ε-caprolactone and chemical modification of poly (α-chloro-ε-caprolactone)by atom transfer radical processes[J].Macromolecules,2004,37:4055-4061.
[15] Detrembleur C,Mazza M,Hedrick J,etal.Ring-opening polymerization ofγ-bromo-ε-caprolactone:A novel route to functionalized aliphatic polyesters[J].Macromolecules,2000,33:14-18.
[16] Hans M,Keul H,Moeller M.Poly(ether-ester)conjugates with enhanced degradation[J].Biomacromolecules,2008,9:2954-62.
[17] White J E,Earls J D,Sherman J W,etal.Step-growth polymerization of 10,11-epoxyundecanoic acid:Synthesis and properties of a new hydroxy-functionalized thermoplastic polyester[J].Polymer,2007,48:3990-3998.
[18] Lecomte P,Riva R,Schmeits S,etal.New prospects for the grafting of functional groups onto aliphatic polyesters.Ring-opening polymerization ofα- orγ-substituted-caprolactone followed by chemical derivatization of the substituents[J].Macromolecular Symposia,2006,240:157-165.
[19] Wang S,Lu L,Gruetzmacher J A,etal.A biodegradable and cross-linkable multiblock copolymer consisting of poly(propylene fumarate)and poly(e-caprolactone):Synthesis,characterization,and physical properties[J].Macromolecules,2005:7358-7370.
[20] Jabbari E,Wang S,Lu L,etal.Synthesis,material properties,and biocompatibility of a novel self-cross-linkable poly(caprolactone fumarate)as an Injectable tissue engineering scaffold[J].Biomacromolecules,2005,6:2503-2511.
[21] Wang S,Lu L,Gruetzmacher J,etal.Synthesis and characterizations of biodegradable and crosslinkable poly(ε-caprolactone fumarate),poly(ethylene glycol fumarate),and their amphiphilic copolymer[J].Biomaterials,2006,27:832-841.
[22] Wang S,Yaszemsk M J,Gruetzmacher J A,etal.Photo-crosslinked poly(3-caprolactone fumarate)networks:Roles of crystallinity and crosslinking density in determining mechanical properties[J].Polymer,2008,49:5692-5699.
[23] Takasu A,Shibata Y,Narukawa Y,etal.Chemoselective dehydration polycondensations of dicarboxylic acids and diols having pendant hydroxyl groups using the room temperature polycondensation technique[J].Macromolecules,2007,40:151-153.
[24] Xie Z,Lu C,Chen X,etal.Synthesis and characterization of novel poly(ester carbonate)s based on pentaerythritol[J].Journal of Polymer Science Part A:Polymer Chemistry,2007,45:1737-1745.
[25] Hu X,Liu S,Chen X,etal.Biodegrad able amphiphilic block copolymers bearing protected hydroxyl groups:Synthesis and characterization[J].Biomacromolecules,2008,9:553-560.
[26] Wang Y,Ameer G A,Sheppard B J,etal.A tough biodegradable elastomer[J].Nature Biotechnology,2002,20:602-606.
[27] You Z,Cao H,Gao J,etal.A functionalizable polyester with free hydroxyl groups and tunable physiochemical and biological properties[J].Biomaterials,2010,31:3129-3138.
[28] Zhang S,Yang J,Liu X,etal.Synthesis and characterization of poly(butylene succinate-437-butylene malate):A new biodegradable copolyester bearing hydroxyl pendant Groups[J].Biomacromolecules,2003,4:437-445.
[29] Miao S,Zhang S,Su Z,etal.Chemoenzymatic synthesis of oleic acid-based polyesters for use as highly stable biomaterials[J].Journal of Polymer Science Part A:Polymer Chemistry,2008,46:4243-4248.
[30] Trollsås M,Lee V Y,Hedrick J,etal.Hydrophilic aliphatic polyesters:Design,synthesis,and ring-opening polymerization of functional cyclic esters[J].Macromolecules,2000,33:4619-4627.
[31] Saulnier B,Coudane J,Garreau H,etal.Hydroxyl-bearing poly(α-hydroxy acid)-type aliphatic degradable polyesters prepared by ring opening (co)polymerization of dilactones based on glycolic,gluconic and l-lactic acids[J].Polymer,2006,47:1921-1929.
[32] Wolf F K,Frey H.Inimer-promoted synthesis of bran ched and hyperbranched polylactide copolymers[J].Macromolecules,2009,42:9443-9456.
[33] Dhamaniya S,Jacob J.Synthesis and characterization of copolyesters based on tartaric acid derivatives[J].Polymer Bulletin,2011,68:1287-1304.
[34] Hao Q,Yang J,Li Q,etal.New facile approach to novel water-soluble aliphatic poly(butylene tartarate)s bearing reactive hydroxyl pendant groups[J].Biomacromolecules,2005,6:3474.
[35] Leemhuis M,van Steenis Jan H,van Uxem Michelle J,etal.A versatile route to functionalized dilactones as monomers for the synthesis of poly(α-hydroxy)acids[J].European Journal of Organic Chemistry,2003,2003:3344-3349.
[36] Leemhuis M,Nostrum C F V,Kruijtze J A W,etal.Functionalized poly(R-hydroxy acid)s via ring-opening polymerization:Toward hydrophilic polyesters with pendant hydroxyl groups[J].Macromolecules,2006,39:3500-3508.
[37] Leemhuis M,Kruijtzer J A W,Nostrum C F C,etal.In vitro hydrolytic degradation of hydroxyl-functionalized poly(α-hydroxy acid)s[J].Biomacromolecules,2007,8:2943-2949.
[38] Mahmud A,Xiong X B,Anifar A L.Novel self-associating poly(ethyle ne-oxide)-block-poly(ε-caprolactone)block copolymers with functional side groups on the polyester block for drug delivery[J].Macromolecules,2006,39:9419-9428.
[39] Kulshrestha A S,Sahoo B,Gao W,etal.Lipase catalysis.A direct route to linear aliphatic copolyesters of bis(hydroxymethyl)butyric acid with pendant carboxylic acid groups[J].Macromolecules,2005,38:3205-3213.
[40] Ouchi T,Fujino A.Synthesis of poly(a-malic acid)and its hydrolysis behavior in vitro[J].Die Makro Chemie,2003,190:1523-1530.
[41] Zhang Y,Li J H,Du Z Z.Synthesis and pH-responsive assembly of methoxy poly(ethylene glycol)-b-poly(ε-caprolactone)with pendant carboxyl groups[J].Poly Sci Pol Chem A,2014,52(2):188-199.
[42] He B,Wan Y,Bei J,etal.Synthesis and cell affinity of functionalized poly(L-lactide-co-beta-malic acid)with high molecular weight[J].Biomaterials,2004,25:5239-5247.
[43] Yan J,Zhang Y,Xiao Y,etal.Novel poly(ε-caprolactone)s bearing amino groups:Synthesis,characterization and biotinylation[J].Reactive and Functional Polymers,2010,70:400-407.
[44] Elisseeff J,Anseth K,Langer R,etal.Synthesis and characterization of photo-cross-linked polymers based on poly(L-lactic acid-co-L-aspartic acid)[J].Macromolecules,1997,30:2182-2184.
[45] Caponetti G,Hrkach JS,Kriwet B,etal.Microparticles of novel branched copolymers of lactic acid and amino acids:Preparation and characterization[J].Journal of Pharmaceutical Sciences,1999,88:136-141.
[46] Alice E V D Ende,Evan J Kravitz.Approach to formation of multifunctional polyester particles in controlled nanoscopic dimensions[J].Am Chem Soc,2008,130(27):8706-8713.
[47] Jiang X,Vogel E B.“Clickable” polyglycolides:Tunable synthons for thermoresponsive,degradable polymers[J].Macromolecules,2008,41:1937-44.
[48] Billiet L,Fournier D,Du Prez F.Combining “click” chemistry and step-growth polymerization for the generation of highly functionalized polyesters[J].Journal of Polymer Science Part A:Polymer Chemistry,2008,46:6552-6564.
[49] R Riva,Schmeits S,Jérme C,etal.Combination of ring-opening polymerization and “click chemistry”:Toward functional ization and grafting of poly(ε-caprolactone)[J].Macromolecules,2007,40:796-803.
[50] Mertzman J E,Kar S,Lofland S,etal.Surface attached manganese-oxo clusters as potential contrast agents[J].Chem Commun (Camb),2009,21(7):788-790.
[51] Su R J,Yang H W,Leu Y L,etal.Synthesis and characterization of amphiphilic functional polyesters by ring-opening polymerization and click reaction[J].Reactive and Functional Polymers,2012,72:36-44.
[52] Wurth J J,Shastri V P.Synthesis and characterization of functionalized poly(ε-caprolactone)[J].Polym Sci Part A Polym Chem,2013,51(16):3375-3382.
[53] S Målberg,P Plikk.A finne-wistrand[J].Chem Mater,2010,22(9):3009-3014.
[54] Parrish B,Emrick T.Aliphatic polyesters with pendant cyclopentene groups:Controlled synthesis and conversion to polyester-graft-PEG copolymers[J].Macromolecules,2004,37:5863-5865.
[55] Brooke A,Horn V,Wooley K L.Toward cross-linked degradable polyester materials:Investigations into the compatibility and use of reductive amination chemistry for cross-linking[J].Macromolecules,2007,40:1480-1488.
[56] Dwan′Isa J P L,Lecomte P,Philippe Duboisa,etal.Synthesis and characterization of random copolyesters ofε-caprolactone and 2-oxepane-1,5-dione[J].Macromolecules,2003,36:2609-2615.
[57] Tian D,Halleux O,Dubois P,etal.Poly(2-oxepane-1,5-dione):A highly crystalline modi fied poly(ε-caprolactone)of a high melting temperature[J].Macromolecules,1998,31:924-927.
[58] Taniguchi I,Mayes A M.A chemoselective approach to grafting biodegradable polyesters[J].Macromolecules,2005,38:216-219.
[59] A B,Horn V,Iha R K,etal.Sequential and single-step,one-pot strategies for the transformation of hydrolytically degradable polyesters into multifunctional systems[J].Macromolecules,2008,41:1618-1626.
[60] X W Yang,C Cui,Z Tonga.Novel unsaturated aliphatic polyesters:Synthesis,characterization,and properties of,multiblock copolymers composing of poly(butylene fumarate)and poly(1,2-propylene succinate)[J].Acta Biomater,2013,9(9):8232-8244.
[61] Zheng L,Wang Z,Li C.Novel unsaturated aliphatic polyesters:Synthesis,characterization,and properties of multiblock copolymers composing of poly(butylene fumarate)and poly(1,2-propylene succinate)[J].Ind Eng Chem Res,2012,51:14107-14111.
[62] Dai W,Huang H,Du Z,etal.Synthesis,characterization and degradability of the novel aliphatic polyester bearing pendantN-isopropylamide functional groups[J].Polymer Degradation and Stability,2008,93:2089-2095.
AdvancesinResearchforFunctionalAliphaticPolyester
SHEN Hui,LI Yang,LIU Yu
(Biological Materials Laboratory,School of Microelectronics and Solid-state Electronics,University of Electronic Science and Technology of China,Chengdu 610041,China)
This paper review introduced the research development of the functionalization such as halogenation,hydroxylation,amination and other functional groups of biodegradable linear aliphatic polyesters with 60references.
biodegradable linear polyester;functional group;synthesis;review
2014-03-26;
2014-06-12
沈慧(1988-),女,汉族,四川成都人,硕士研究生,主要从事生物材料和生物电子方面的研究。
刘钰,E-mail:liuyupoly@gmail.com
O621.3;O63
A
1005-1511(2014)04-0569-08

