新型3-季碳羟甲基(或3-季碳胺甲基)氧化吲哚的合成*
黄 璇,郭丰敏,刘雄伟,景德红,周 英,刘雄利,余章彪
(贵州大学 药学院 贵州省中药民族药创制工程中心,贵州 贵阳 550025)
·快递论文·
新型3-季碳羟甲基(或3-季碳胺甲基)氧化吲哚的合成*
黄 璇,郭丰敏,刘雄伟,景德红,周 英,刘雄利,余章彪
(贵州大学 药学院 贵州省中药民族药创制工程中心,贵州 贵阳 550025)
在无催化剂的条件下,3-取代氧化吲哚和甲醛在二氯甲烷中,于室温反应48h合成了7个新型的3-季碳羟甲基氧化吲哚(2a~2g),产率85%~92%。在无催化剂的条件下,以乙酸乙酯为溶剂,3-取代氧化吲哚通过和甲醛原位生成的亚胺正离子的加成反应合成了6个新型的3-季碳胺甲基氧化吲哚(4a~4f),产率57%~70%。2和4的结构经1H NMR,13C NMR和HR-ESI-MS表征。
3-羟甲基氧化吲哚;3-胺甲基氧化吲哚;多聚甲醛;仲胺;合成
3-季碳氧化吲哚普遍存在于生物活性的天然产物和药物分子中[1-2],是合成天然生物碱的起始原料[3-4]。由于其结构中的3-位羟甲基能够轻易地转换成胺甲基或由于胺甲基或羟甲基能轻易地被转换成其它活性官能团,所以3-季碳羟甲基或3-季碳胺甲基氧化吲哚已经成为人们合成的目标分子。3-取代氧化吲哚和甲醛或甲醛原位生成的亚胺正离子的加成反应提供了一条最有效最
直接合成3-季碳羟甲基或3-季碳胺甲基氧化吲哚的合成路线。到目前为止,尽管3-取代氧化吲哚作为亲核试剂和各种亲电试剂反应已经有大量的文献报道[5-15],但是,在无催化剂条件下,通过3-取代氧化吲哚和甲醛或甲醛原位生成的的亚胺正离子的加成反应,高效合成3-季碳羟甲基或3-季碳胺甲基氧化吲哚还未见文献报道。
为此,本文以3-取代氧化吲哚(1a~1g)和甲醛为原料,在无催化剂的条件下,在二氯甲烷中于室温反应48h合成了7个新型的3-季碳羟甲基氧化吲哚(2a~2g,Scheme 1),并研究了反应底物对反应速率的影响。在无催化剂的条件下,以乙酸乙酯为溶剂,3-取代氧化吲哚(3a~3f)通过和甲醛原位生成的亚胺正离子的加成反应合成了6个新型的3-季碳胺甲基氧化吲哚(4a~4f,Scheme 2),并考察了溶剂对反应选择性的影响。2和4的结构经1H NMR,13C NMR和HR-MS表征。
该方法提供了一种高效合成3-季碳羟甲基或3-季碳胺甲基氧化吲哚的方法,具有反应条件温和、时间短、收率高等优点,而且实验操作简单,分离纯化简单。
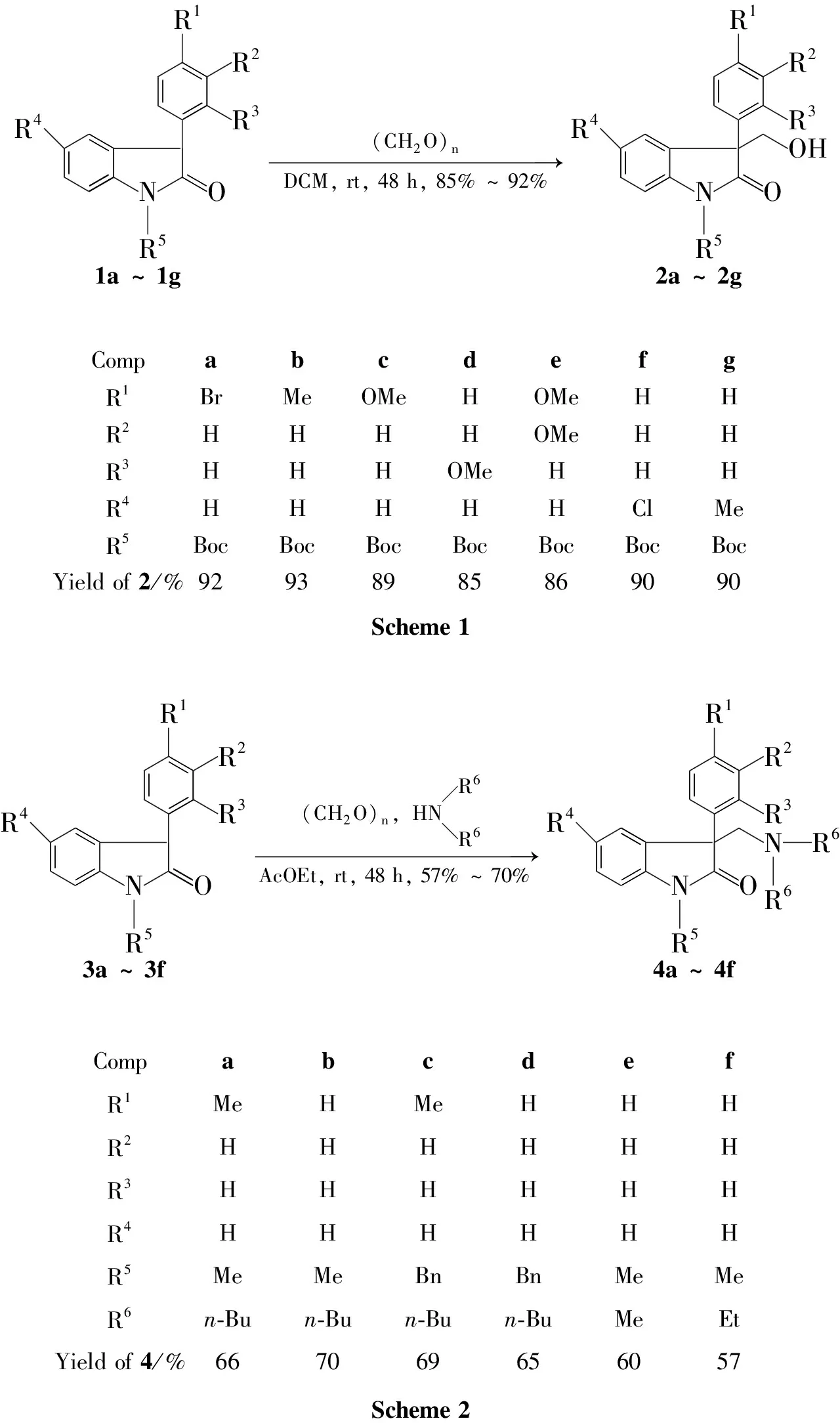
R4NR5 R1R3R2 O1a~1g(CH2O)nDCM,rt,48h,85%~92%→ R4NR5 R1R3R2 OOH2a~2gCompabcdefgR1BrMeOMeHOMeHHR2HHHHOMeHHR3HHHOMeHHHR4HHHHHClMeR5BocBocBocBocBocBocBocYieldof2/%92938985869090Scheme1 R4NR5 R1R3R2 O3a~3f(CH2O)n,HNR6R6AcOEt,rt,48h,57%~70%→ R4NR5 R1R3R2 ONR6R64a~4fCompabcdefR1MeHMeHHHR2HHHHHHR3HHHHHHR4HHHHHHR5MeMeBnBnMeMeR6n⁃Bun⁃Bun⁃Bun⁃BuMeEtYieldof4/%667069656057Scheme2
1 实验部分
1.1 仪器与试剂
Bruker-300MHz型核磁共振仪(氘代丙酮为溶剂,TMS为内标);Bruker BIO TOF III Q型高分辨质谱仪。
1a~1h和3a~3f按文献[16]方法合成;其余所用试剂均为分析纯;无水溶剂按标准方法脱水处理。
1.2 合成
(1)2a~2g的合成(以2a为例)
在反应试管中依次加入N-Boc-3-对溴苯基氧化吲哚(1a)343mg(1.0mmol),多聚甲醛150mg(5.0mmol)和二氯甲烷5mL,搅拌下于室温反应48h(TLC检测)。反应液经硅胶层析柱[洗脱剂:A=V(乙酸乙酯)∶V(石油醚)=1∶3]纯化得白色固体2a383mg。
按类似的方法合成白色固体2b~2g。
1-叔丁氧羰基-3-对溴苯基-3-羟甲基氧化吲哚(2a):1H NMRδ:1.60(s,9H),2.13(dd,J=4.5Hz,9.0Hz,1H),4.21(dd,J=4.5Hz,11.4Hz,1H),4.46(dd,J=9.0Hz,11.4Hz,1H),6.81~7.66(m,8H);13C NMRδ:28.2,56.1,66.4,84.7,115.0,123.7,124.5,127.6,128.1,128.9,131.3,132.8,133.0,140.1,148.6,177.9;HR-ESI-MS:m/z:Calcd for C20H20NO4BrNa{[M+Na]+}440.0470,found 440.0475。
1-叔丁氧羰基-3-对甲基苯基-3-羟甲基氧化吲哚(2b):1H NMRδ:1.59(s,9H),2.24(s,3H),2.14(dd,J=4.5Hz,9.0Hz,1H),4.22(dd,J=4.5Hz,11.4Hz,1H),4.56(dd,J=9.0Hz,11.4Hz,1H),6.78~7.67(m,8H);13C NMRδ:21.0,27.9,55.9,66.4,84.1,114.7,123.4,124.2,128.1,128.6,129.3,129.4,129.8,131.4,136.2,140.2,148.7,178.1;HR-ESI-MS:m/z:Calcd for C21H23NO4Na{[M+Na]+}376.1521,found 376.1526。
1-叔丁氧羰基-3-对甲氧基苯基-3-羟甲基氧化吲哚(2c):1H NMRδ:1.58(s,9H),2.11(dd,J=4.5Hz,9.0Hz,1H),3.70(s,3H),4.23(dd,J=4.5Hz,11.4Hz,1H),4.46(dd,J=9.0Hz,11.4Hz,1H),6.61~7.66(m,8H);13C NMRδ:28.1,55.1,56.4,66.3,84.0,113.1,115.0,123.4,124.2,126.8,128.0,128.1,131.4,140.2,148.7,158.1,178.0;HR-ESI-MSm/z:Calcd for C21H23NO5Na{[M+Na]+}392.1472,found 392.1474。
1-叔丁氧羰基-3-邻甲氧基苯基-3-羟甲基氧化吲哚(2d):1H NMRδ:1.62(s,9H),2.10(dd,J=4.5Hz,9.0Hz,1H),3.54(s,3H),4.19(dd,J=4.5Hz,11.4Hz,1H),4.36(dd,J=9.0Hz,11.4Hz,1H),6.63~7.69(m,8H);13C NMRδ:28.1,54.4,55.0,66.8,84.1,109.9,114.5,119.9,123.4,123.8,124.1,128.2,128.6,128.5,131.3,139.5,149.0,157.5,178.4;HR-ESI-MSm/z:Calcd for C21H23NO5Na{[M+Na]+}392.1471,found 392.1476。
1-叔丁氧羰基-3-3′,4′-二甲氧基苯基-3-羟甲基氧化吲哚(2e):1H NMRδ:1.55(s,9H),2.09(dd,J=4.5Hz,9.0Hz,1H),3.57(s,3H),3.74(s,3H),4.09(dd,J=4.5Hz,11.4Hz,1H),4.26(dd,J=9.0Hz,11.4Hz,1H),6.28~7.62(m,8H);13C NMRδ:28.0,55.2,55.4,56.1,66.6,84.4,110.6,112.1,115.6,122.5,123.3,124.5,127.5,128.1,128.4,140.6,147.4,148.3,148.5,178.3;HR-ESI-MSm/z:Calcd for C22H25NO6Na{[M+Na]+}422.1584,found 422.1579。
1-叔丁氧羰基-3-苯基-3-羟甲基-5-氯氧化吲哚(2f):1H NMRδ:1.55(s,9H),2.11(dd,J=4.5Hz,9.0Hz,1H),4.10(dd,J=4.5Hz,11.4Hz,1H),4.27(dd,J=9.0Hz,11.4Hz,1H),6.86~7.54(m,8H);13C NMRδ:27.8,56.4,66.6,84.4,116.6,117.3,126.5,127.5,127.3,129.4,130.2,131.3,134.5,139.5,148.3,176.8;HR-ESI-MSm/z:Calcd for C20H20NO4ClNa{[M+Na]+}396.0974,found 396.0980。
1-叔丁氧羰基-3-苯基-3-羟甲基-5-甲基氧化吲哚(2g):1H NMRδ:1.56(s,9H),2.10(dd,J=4.5Hz,9.0Hz,1H),4.11(dd,J=4.5Hz,11.4Hz,1H),4.28(dd,J=9.0Hz,11.4Hz,1H),6.86~7.51(m,8H);13C NMRδ:21.5,27.5,55.7,66.3,84.1,114.7,124.0,126.7,127.8,127.9,129.0,130.4,133.7,134.8,137.2,148.5,178.0;HR-ESI-MSm/z:Calcd for C21H23NO4Na{[M+Na]+}376.1521,found 376.1525。
(2)4a~4f的合成(以4a为例)
在反应试管中依次加入N-甲基-3-对甲基苯基氧化吲哚(3a)237mg(1.0mmol),多聚甲醛150mg(5.0mmol),二叔丁基胺258mg(2.0mmol)及乙酸乙酯5mL,搅拌下于室温反应48h(TLC检测)。反应液经硅胶柱层析(洗脱剂:A=1∶6)纯化得黄色油状物4a249mg。
按类似的方法合成黄色油状液体4b~4f。
1-甲基-3-(二叔丁基氨甲基)-3-对甲基苯基氧化吲哚(4a):1H NMRδ:0.76(t,J=6.6Hz,6H),0.94~1.13(m,8H),2.14(s,3H),2.17~2.32(m,4H),3.20(s,3H),3.21(d,J=13.2Hz,1H),3.49(d,J=13.2Hz,1H),6.85(t,J=9.0Hz,3H),7.10(t,J=7.5Hz,1H),7.24~7.41(m,4H);13C NMRδ:14.2,20.4,20.8,26.1,28.8,54.4,57.4,64.5,107.7,113.5,121.2,125.7,127.9,128.4,131.2,131.9,144.7,158.7,178.8;HR-ESI-MSm/z:Calcd for C25H34N2ONa{[M+Na]+}401.2564,found 401.2568。
1-甲基-3-(二叔丁基氨甲基)-3-苯基氧化吲哚(4b):1H NMRδ:0.78(t,J=6.6Hz,6H),0.95~1.14(m,8H),2.18~2.31(m,4H),3.21(s,3H),3.25(d,J=13.2Hz,1H),3.20(d,J=13.2Hz,1H),6.86~7.44(m,9H);13C NMRδ:14.1,20.3,26.0,28.7,54.6,57.1,64.1,107.5,113.4,121.1,125.5,127.7,128.3,131.1,131.7,144.5,158.4,178.7;HR-ESI-MS:m/z:Calcd for C24H32N2ONa{[M+Na]+}387.2410,found 387.2414。
1-苄基-3-(二叔丁基氨甲基)-3-对甲基苯基氧化吲哚(4c):1H NMRδ:0.74(t,J=6.6Hz,6H),0.94~1.03(m,6H),1.14~1.20(m,2H),2.17(s,3H),2.24~2.36(m,4H),3.25(d,J=13.2Hz,1H),3.64(d,J=13.2Hz,1H),4.81(d,J=15.6Hz,1H),5.07(d,J=15.6Hz,1H),6.65~6.68(m,1H),6.82~6.87(m,2H),7.03(t,J=7.2Hz,1H),7.11(t,J=7.2Hz,1H),7.10~7.44(m,8H);13C NMRδ:14.0,20.1,20.4,28.243.4,54.2,57.2,63.5,109.2,113.3,121.5,125.6,127.5,127.5,128.7,128.9,131.2,131.8,135.9,143.9,158.7,178.5;HR-ESI-MSm/z:Calcd for C31H38N2ONa{[M+Na]+}477.2887,found 477.2883。
1-苄基-3-(二叔丁基氨甲基)-3-苯基氧化吲哚(4d):1H NMRδ:0.71(t,J=6.9Hz,6H),0.92~1.04(m,6H),1.16~1.22(m,2H),2.25~2.36(m,4H),3.23(d,J=13.5Hz,1H),3.61(d,J=13.5Hz,1H),4.81(d,J=15.9Hz,1H),5.04(d,J=15.9Hz,1H),6.62~7.43(m,9H);13C NMRδ:14.1,20.3,28.2,43.7,54.7,57.9,63.8,109.1,113.8,121.7,125.6,127.2,127.7,128.3,128.5,131.1,131.8,135.9,143.8,158.6,178.6;HR-ESI-MSm/z:Calcd for C30H36N2ONa{[M+Na]+}463.2721,found 463.2726。
1-甲基-3-(二甲基氨甲基)-3-苯基氧化吲哚(4e):1H NMRδ:2.08(s,6H),3.21(s,3H),3.22(d,J=13.5Hz,1H),3.50(d,J=13.5Hz,1H),6.57(d,J=7.8Hz,1H),6.76~6.81(m,2H),6.96~7.07(m,4H),7.15(t,J=7.8Hz,1H),7.24(d,J=6.9Hz,1H);13C NMRδ:25.8,47.7,55.6,65.2,107.8,121.9,123.8,126.4,127.2,127.8,129.4,130.5,135.6,144.1,178.8;HR-ESI-MSm/z:Calcd for C18H20N2ONa{[M+Na]+}303.1471,found 303.1475。
1-甲基-3-(二乙基氨甲基)-3-苯基氧化吲哚(4f):1H NMRδ:0.75(t,J=7.2Hz,6H),2.33~2.45(m,4H),3.20(s,3H),3.24(d,J=13.5Hz,1H),3.51(d,J=13.5Hz,1H),6.54(d,J=7.5Hz,1H),6.82~6.87(m,2H),6.94~7.02(m,4H),7.10~7.16(m,1H),7.28(d,J=7.5Hz,1H);13C NMRδ:11.4,25.5,48.1,56.5,61.0,107.3,121.8,124.5,126.1,127.4,127.4,129.5,130.9,136.4,144.2,178.7;HR-ESI-MSm/z:Calcd for C20H24N2ONa{[M+Na]+}331.1782,found 331.1787。
2 结果与讨论
2.1 合成
(1)2的合成
通过对底物的扩展发现,无论氧化吲哚3-位苯环上的取代基是吸电子基还是给电子基(2a~2c),还是邻位、间位或对位取代基(2d~2e),都能在无催化剂二氯甲烷中,于室温反应即得到很好的产率(85%~92%)。此外,吲哚环上的R4无论是给电子还是吸电子基(2f~2g),对产率没有影响,也可以得到很好的产率(90%)。
(2)4的合成
以合成4a为例,考察了溶剂对该反应的影响,结果见表1。从表1可以看出,以乙酸乙酯为溶剂时,产率最好(66%),副产物3-季碳羟甲基氧化吲哚(5,Chart 1)的产率只有8%。以二氯甲烷、甲苯等为溶剂时,4a的产率很低(7%~14%),而5的产率反而很高,说明该反应所选溶剂对反应的选择性影响较大。

表1 溶剂对4a产率的影响*Table1 Effect of solvent on yield of 4a
*3a1.0mmol,paraformaldehyde 5.0mmol,solvent 5.0mL,at room temperature for 48h;isolatd yield after chromatographic purification
[1] Galliford C V,Scheidt K A.Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents[J].Angew Chem,Int Ed,2007,46:8748-8758.
[2] Zhou F,Liu Y L,Zhou J.Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3position[J].Adv Synth Catal DOI:10.1002/adsc.201000161.
[3] ounay A B,Overman L E.The asymmetric intramolecular Heck reaction in natural product total synthesis[J].Chem Rev,2003,103:2945-2964.
[4] Lin H,Danishefsky S J,Gelsemine.A thought-provoking target for total synthesis[J].Angew Chem,Int Ed,2003,42:36-51.
[5] Hamashima Y,Suzuki T,Takano H,etal.Catalytic enantioselective fluorination of oxindoles[J].J Am Chem Soc,2005,127:10164-10165.
[6] Ishimaru T,Shibata N,Nagai J,etal.Lewis acid-catalyzed enantioselective hydroxylation reactions of oxindoles andβ-keto esters using DBFOX ligand[J].J Am Chem Soc,2006,128:16488-16489.
[7] Tomita D,Yamatsugu K,Kanai M,etal.Enantioselective synthesis of SM-130686based on the development of asymmetric Cu(I)F catalysis to access 2-oxindoles containing a tetrasubstituted carbon[J].J Am Chem Soc,2009,131:6946-6948.
[8] Trost B M,Zhang Y.Mo-catalyzed regio-,diastereo,and enantioselective allylic alkylation of 3-aryloxindoles[J].J Am Chem Soc,2007,129:14548-14549.
[9] Hills I D,Fu G C.Catalytic enantioselective synthesis of oxindoles and benzofuranones that bear a quaternary stereocenter[J].Angew Chem,Int Ed,2003,42:3921- 3924.
[10] Shaw S A,Aleman P,Christy J,etal.Enantioselective TADMAP-catalyzed carboxyl migration reactions for the synthesis of stereogenic quaternary carbon[J].J Am Chem Soc,2006,128:925-934.
[11] Ogawa S,Shibata N,Inagaki J,etal.Cinchona-alkaloid-catalyzed enantioselective direct Aldol-type reaction of oxindoles with ethyl trifluoropyruvate[J].Angew Chem,Int Ed,2007,46:8666-8669.
[12] Ishimaru T,Shibata N,Horikawa T,etal.Cinchona alkaloid catalyzed enantioselective fluorination of allyl silanes,silyl enol ethers,and oxindoles[J].Angew Chem,Int Ed,2008,47:4157-4161.
[13] He R,Ding C,Maruoka K.Phosphonium salts as chiral phase-transfer catalysts:Asymmetric Michael and Mannich reactions of 3-aryloxindoles[J].Angew Chem,Int Ed,2009,48:4559-4561.
[14] Bui T,Candeias N R,Barbas C F.III.Dimeric quinidine-catalyzed enantioselective aminooxygenation of oxindoles:An organocatalytic approach to 3-hydroxyoxindole derivatives[J].J Am Chem Soc,2010,132:5574- 5575.
[15] Trost B M,Frederiksen M U.Palladium-catalyzed asymmetric allylation of prochiral nucleophiles:Synthesis of 3-allyl-3-aryl oxindoles[J].Angew Chem,Int Ed,2005,44:308-310.
[16] Duan S W,An J,Chen J R,etal.Facile synthesis of enantioenriched Cγ-tetrasubstituted r-amino acid derivatives via an asymmetric nucleophilic addition/protonation cascade[J].Organic Letter,2011,13(9):2290-2293.
SynthesisofNovel3-SubstitutedHydroxymethyl(orAminomethyl)Oxindoles
HUANG Xuan, GUO Feng-min,LIU Xiong-wei,JING De-hong, ZHOU Ying, LIU Xiong-li, YU Zhang-biao
(Guizhou Engineering Center for Innovative Traditional Chinese Medicine and Ethnic Medicine,College of Pharmacy,Guizhou University,Guiyang 550025,China)
Seven unreported 3-substituted hydroxymethyl oxindoles(2a~2g)in yield of 85%~92% were synthesized by addition reaction of 3-substituted oxindoles with paraformaldehyde under the conditions of catalyst-free in CH2Cl2at the room temperature for 48h.Six unreported 3-aminomethyl oxindoles(4a~4f)in yield of 57%~70% were synthesized by addition reaction of 3-substituted oxindoles with imine positive ion and secondary amines under the conditions of catalyst-free in AcOEt at the room temperature for 48h.The structures of2and 4were characterized by1H NMR,13C NMR and HR-ESI-MS.
3-aminomethyl oxindole;3-hydroxymethyl oxindole;paraformaldehyde;secondary amine;synthesis
2014-01-26;
2014-04-01
国家自然科学基金青年基金资助项目(21302024);教育部“新世纪人才支持计划”项目[教技函(2011)95号];贵州省中药现代化科技产业研究开发专项项目[黔科合ZY字(2013)3010号]
黄璇(1988-),女,汉族,贵州六盘水人,硕士研究生,主要从事天然活性物质的全合成及结构修饰研究。E-mail:447579828@qq.com
余章彪,教授,硕士生导师,E-mail:gym.zbyu@gzu.edu.cn
O626;O623.731
A
1005-1511(2014)04-0499-05

