新型3-(3,4,5-三甲氧基苯基)-4-氨基-5-取代苄砜基-1,2,4-三唑的合成及其抑菌活性*
杜 婕,杜海堂,桑维钧,张元富,潘发丹
(1.贵阳学院 化学系,贵州 贵阳 550005;2.贵州大学 农学院,贵州 贵阳 550025)
·研究论文·
新型3-(3,4,5-三甲氧基苯基)-4-氨基-5-取代苄砜基-1,2,4-三唑的合成及其抑菌活性*
杜 婕1,杜海堂1,桑维钧2,张元富2,潘发丹2
(1.贵阳学院 化学系,贵州 贵阳 550005;2.贵州大学 农学院,贵州 贵阳 550025)
以3-(3,4,5-三甲氧基苯基)-4-氨基-5-巯基-1,2,4-三唑为原料,经醚化和氧化反应合成了11个新型的的3-(3,4,5-三甲氧基苯基)-4-氨基-5-取代苄砜基-1,2,4-三唑(3a~3k),其结构经1H NMR,IR,MS和元素分析表征。初步生物活性测试结果表明,在用药量为50mg·L-1时,部分化合物对小麦赤霉菌、立枯菌、镰刀菌和链格孢菌有一定的抑菌活性。
1,2,4-三唑砜;合成;抑菌活性
1,2,4-三唑及其衍生物是一类具有广谱生物活性和药理活性的五元芳香杂环化合物,如有抗菌[1-3]、抗癌[4]、抗炎[5]、抗惊厥[6]等。在农药方面,1,2,4-三唑类化合物主要用于杀菌剂,其中已有几十个商业化的品种。
本课题组前期[7]的研究结果表明,在3-(3,4,5-三甲氧基苯基)-4-氨基-5-巯基-1,2,4-三唑的5-位引入苄基硫醚单元后,所得到的目标化合物表现出一定的抑菌活性。而砜类化合物在农药和医药领域也具有广泛应用显示出杀菌和抗癌等生物活性[8-10],目前已商品化的砜类农药有杀菌剂氧化萎锈灵、杀虫剂氟虫酰胺和除草剂磺草酮等。
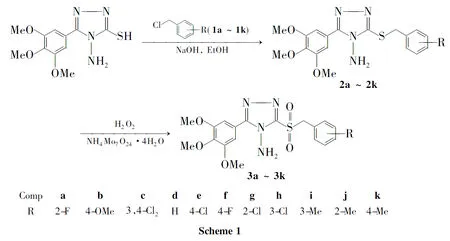
为了寻找新的具有抑菌活性的1,2,4-三唑化合物,本文以3-(3,4,5-三甲氧基苯基)-4-氨基-5-巯基-1,2,4-三唑为原料,经醚化和氧化反应合成了11个新型的3-(3,4,5-三甲氧基苯基)-4-氨基-5-取代苄砜基-1,2,4-三唑(3a~3k,Scheme 1),其结构经1H NMR,IR,MS和元素分析表征。并测定了3a~3k对小麦赤霉菌、立枯菌、镰刀菌和链格孢菌的抑制活性。
1 实验部分
1.1 仪器与试剂
YRT-3型熔点仪(温度未校正);INOVA-400MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);Affinity-1型红外光谱仪(KBr压片);Agilent 1100LC-MSD-Trap型质谱仪(ESI-MS);Elementar Vario EL CUBE型元素分析仪。
2a~2k按文献[7]方法合成;其余所用试剂均为分析纯。
1.23a~3k的合成通法
在圆底烧瓶中加入2a~2k1mmol和无水乙醇20mL,搅拌下于室温缓慢滴加钼酸铵3.7g(3mmol)的H2O2(15mL)溶液,滴毕,于室温反应至终点(TLC跟踪)。立即用饱和NaHSO3溶液淬灭反应,加水至体系中有大量白色沉淀析出,静置,抽滤,滤饼用混合溶剂[V(DMF)∶V(EtOH)=1∶3]重结晶得白色晶体3a~3k。
3a:收率64%,m.p.196℃~197℃;1H NMRδ:3.91(s,9H),4.90(s,2H),4.95(s,2H),7.14~7.19(m,2H),7.32~7.36(m,1H),7.39(s,2H),7.41~7.46(m,1H);IRν:3365,3124,2972,1587,1434,1128cm-1;ESI-MSm/z:423.0{[M+H]+},445.0{[M+Na]+};Anal.calcd for C18H19N4O5SF:C 51.18,H 4.53,N 13.26,S 7.59;found C 51.07,H 4.54,N 13.11,S 7.50。
3b:收率70%,m.p.141℃~143℃;1H NMRδ:3.78(s,3H),3.90(s,6H),3.91(s,3H),4.52(s,2H),4.71(s,2H),6.88(d,J=8.8Hz,2H),7.18(d,J=8.8Hz,2H),7.31(s,2H);IRν:3354,3205,2937,1587,1486cm-1;ESI-MSm/z:435.0{[M+H]+},457.1{[M+Na]+};Anal.calcd for C19H22N4O6S:C 52.52,H 5.10,N 12.90,S 7.38;found C 52.46,H 4.98,N 12.82,S 7.42。
3c:收率52%,m.p.99℃~101℃;1H NMRδ:3.91(s,6H),3.92(s,3H),4.83(s,2H),5.09(s,2H),7.25(dd,J=8.4Hz,2.4Hz,1H),7.35(s,2H),7.46(d,J=8.0Hz,1H),7.50(d,J=2.0Hz,1H);IRν:3321,3199,2922,1591cm-1;ESI-MSm/z:473.0{[M+H]+};Anal.calcd for C18H182N4O5SCl:C 45.67,H 3.83,N 11.84,S 6.77;found C 45.59,H 3.88,N 11.71,S 6.76。
3d:收率72%,m.p.143℃~144℃;1H NMRδ:3.89(s,6H),3.91(s,3H),4.44(s,2H),4.77(s,2H),7.18(s,1H),7.31(s,2H),7.36~7.46(m,4H);IRν:3344,3184,2924,1583cm-1;ESI-MSm/z:405.0{[M+H]+},427.0{[M+Na]+};Anal.calcd for C18H20N4O5S:C 53.45,H 4.98,N 13.85,S 7.93;found C 53.21,H 4.84,N 13.77,S 7.84。
3e:收率78%,m.p.165℃~166℃;1H NMRδ:3.91(s,6H),3.92(s,3H),4.81(s,2H),4.84(s,2H),7.28(d,J=8.8Hz,2H),7.34(s,2H),7.38(d,J=8.0Hz,2H);IRν:3367,3192,2930,1585cm-1;ESI-MSm/z:439.0{[M+H]+};Anal.calcd for C18H19N4O5SCl:C 49.26,H 4.36,N 12.77,S 7.31;found C 48.88,H 4.28,N 12.65,S 7.23。
3f:收率73%,m.p.143℃~145℃;1H NMRδ:3.83(s,6H),3.86(s,3H),5.01(s,2H),5.07(s,2H),6.97~7.01(m,2H),7.27~7.30(m,4H);IRν:3346,3201,2939,1589cm-1;ESI-MSm/z:423.0{[M+H]+},445.0{[M+Na]+};Anal.calcd for C18H19N4O5SCl:C 51.18,H 4.53,N 13.26,S 7.59;found C 51.11,H 4.58,N 13.01,S 7.44。
3g:收率65%,m.p.185℃~186℃;1H NMRδ:3.91(s,6H),3.92(s,3H),4.86(s,2H),5.08(s,2H),7.31(dd,J=7.4Hz,1.2Hz,1H),7.37~7.41(m,3H),7.44(dd,J=7.6Hz,1.6Hz,1H),7.48(dd,J=8.2Hz,1.2Hz,1H);IRν:3352,3153,2933,1589cm-1;ESI-MSm/z:439.0{[M+H]+},461.0{[M+Na]+};Anal.calcd for C18H19N4O5SCl:C 49.26,H 4.36,N 12.77,S 7.31;found C 49.05,H 4.28,N 12.62,S 7.25。
3h:收率67%,m.p.179℃~181℃;1H NMRδ:3.91(s,6H),3.92(s,3H),4.81(s,2H),4.85(s,2H),7.23~7.24(m,1H),7.31(s,1H),7.32(s,1H),7.34(s,2H),7.39~7.42(m,1H);IRν:3346,3190,2958,1585cm-1;ESI-MSm/z:439.0{[M+H]+},461.0{[M+Na]+};Anal.calcd for C18H19N4O5SCl:C 49.26,H 4.36,N 12.77,S 7.31;found C 48.38,H 4.25,N 12.66,S 7.25。
3i:收率73%,m.p.158℃~159℃;1H NMRδ:2.27(s,3H),3.89(s,6H),3.91(s,3H),4.38(s,2H),4.71(s,2H),6.99(s,1H),7.06(d,J=7.2Hz,1H ),7.23~7.28(m,2H),7.30(s,2H);IRν:3327,3197,2933,1583cm-1;ESI-MSm/z:419.0{[M+H]+},441.0{[M+Na]+};Anal.calcd for C19H22N4O5S:C 54.53,H 5.30,N 13.39,S 7.66;found C 54.42,H 5.27,N 13.26,S 7.48。
3j:收率69%,m.p.109℃~111℃;1H NMRδ:2.38(s,3H),3.89(s,6H),3.91(s,3H),4.33(s,2H),4.84(s,2H),7.04(d,J=7.2Hz,1H),7.14~7.18(m,1H),7.28~7.29(m,1H),7.31(s,2H),7.33~7.37(m,1H);IRν:3363,3280,2970,1589cm-1;ESI-MSm/z:419.0{[M+H]+},441.1{[M+Na]+};Anal.calcd for C19H22N4O5S:C 54.53,H 5.30,N 13.39,S 7.66;found C 54.31,H 5.31,N 13.22,S 7.54。
3k:收率77%,m.p.125℃~127℃;1H NMRδ:2.36(s,3H),3.89(s,6H),3.91(s,3H),4.44(s,2H),4.72(s,2H),7.14(d,J=8.0Hz,2H),7.18(d,J=8.0Hz,2H),7.32(s,2H);IRν:3354,3292,2935,1589cm-1;ESI-MSm/z:419.0{[M+H]+},441.0{[M+Na]+};Anal.calcd for C19H22N4O5S:C 54.53,H 5.30,N 13.39,S 7.66;found C 54.51,H 5.23,N 13.25,S 7.52。
2 结果与讨论
2.13的表征
以3a为例对目标化合物进行结构表征。在3a的IR谱中,3365cm-1和3124cm-1处的吸收峰归属N-H伸缩振动峰;1587cm-1附近吸收峰为杂环C=N伸缩振动峰。在3a的1H NMR谱图中,3.91附近的单峰归属苯环上OCH3的9个氢质子;4.90和4.95的单峰是硫醚亚甲基SCH2和杂环氨基NH2上两个氢的质子吸收峰;7.39处的单峰是3,4,5-甲氧基苯环上的两个氢的质子吸收峰;其它苯环的特征吸收峰的化学位移在7.14~7.46。3a的元素分析实测结果与理论计算值相符。
3a~3k的ESI-MS谱图均出现强的准分子离子峰{[M+H]+},这表明其结构较为稳定。其它化合物的结构和3a类似,所有化合物的氢谱数据、质谱数据和元素分析与其结构相吻合。
2.23的抑菌活性
采用生长速率法测定3a~3k对病原菌菌丝生长的抑制作用。供试病原菌:小麦赤霉菌、立枯菌、镰刀菌和链格孢菌;培养基:PDA(马铃薯、葡萄糖和琼酯),测试用药量为50mg·L-1。以商品化药物丙环唑为对照药剂,等量DMSO为空白对照。
将待测菌株接种在PDA平板上,于25℃培养3d,用内径为0.5mm的打孔器把菌落打成菌饼,在无菌操作下移植到含有不同药剂的PDA平板上,在25℃恒温下培养3d后用十字交叉法测量菌落直径,计算相对抑菌率(表1)。
从表1可见,3a~3k对链格孢菌都表现出较明显的抑制活性(>50%)。其中,3a和3g对链格孢菌的抑制率均为67.16%。
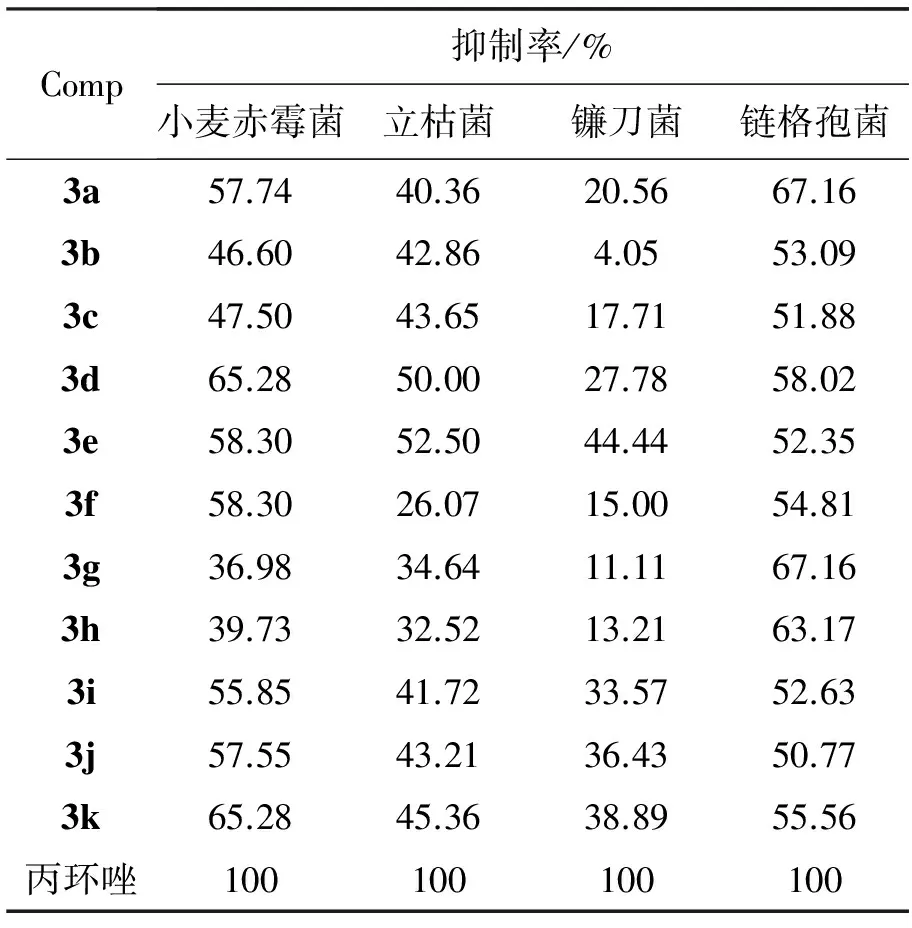
表1 3的抑菌活性*Table1 Inhibitory activities of 3
*c(3)=50mg·L-1
3 结论
合成了11个新型的的3-(3,4,5-三甲氧基苯基)-4-氨基-5-取代苄砜基-1,2,4-三唑(3a~3k)。初步生物活性测试结果表明,在用药量为50mg·L-1时,部分化合物对小麦赤霉菌、立枯菌、镰刀菌和链格孢菌有一定的抑菌活性。3a和3g对链格孢菌的抑制率均为67.16%,但低于阳性对照药丙环唑。
[1] Lu W C,Cao X F,Hu M,etal.A highly enantioselective access to chiral 1-(β-arylalkyl)-1H-1,2,4-triazole derivatives as potential agricultural bactericides[J].Chemistry &Biodiversity,2011,8:1497-1511.
[2] Li W J,Li Q,Liu D L,etal.Synthesis,fungicidal activity and sterol 14α-demethylase binding interaction of 2-azolyl-3,4-dihydroquinazolines on penicillium digitatum[J].J Agric Food Chem,2013,61:1419-1426.
[3] Vijesh A M,Isloor A M,Shetty P,etal.New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents[J].Eur J Med Chem,2013,62:410-415.
[4] Zhang Y B,Liu W,Yang Y S,etal.Synthesis,molecular modeling and biological evaluationof 1,2,4-triazole derivatives containing pyridine as potential anti-tumor agents[J].Med Chem Res,2013,22:3193-3203.
[5] Abdel Aziz M,Abuo Rahma G E D A A,Beshr E A M,etal.New nitric oxide donating 1,2,4-triazole/oxime hybrids:Synthesis,investigation of anti-inflammatory,ulceroginic liability and antiproliferative activities[J].Bioorg Med Chem,2013,21:3839-3849.
[6] Plech T,Luszczki J J,Wujec M,etal.Synthesis characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles[J].Eur J Med Chem,2013,60:208-215.
[7] 杜海军,杜海堂,桑维钧,等.3-(3,4,5-三甲氧基苯基)-4-氨基-6-取代苄硫基-1,2,4-三唑的合成及其生物活性[J].合成化学,2012,20(1):76-79.
[8] 杨超,杨松,宋宝安,等.2-取代硫醚(砜)-5-(4-硝基或4-甲氧基)-1,3,4-噻二唑类化合物的合成及抑菌活性[J].有机化学,2010,30(9):1327-1334.
[9] Chen C J,Song B A,Yang S,etal.Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives[J].Bioorg Med Chem,2007,15(12):3981-3989.
[10] Liu F,Luo X Q,Song B A,etal.Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety[J].Bioorg Med Chem,2008,16(7):3632-3640.
SynthesisandFungicidalActivitiesofNovel3-(3,4,5-Trime-thoxyphenyl)-4-amino-5-substituted-benzylsulfone-1,2,4-thiazoles
DU Jie1,DU Hai-tang1,SANG Wei-jun2,ZHANG Yuan-fu2,PAN Fa-dan2
(1.Department of Chemistry,Guiyang College,Guiyang 550005,China;2.College of Agriculture,Guizhou University,Guiyang 550025,China)
Eleven novel 3-(3,4,5-trimethoxyphenyl)-4-amino-5-substituted-benzylsulfone-1,2,4-thiazoles(3a~3k)were synthesized by etherification and oxidation using 3-(3,4,5-trimethoxybenzyl)-4-amino-5-mercapto-1,2,4-triazole as the starting material.The structures were characterized by1H NMR,IR,MS and elemental analysis.The preliminary bioassay indicated that some compounds exhibited certain fungicidal activities againstGibberellazeae,Rhizoctoniasp,Fusariumsp,andAlernariasp.
1,2,4-triazole sulfone;synthesis;fungicidal activity
2014-05-06
贵州省科学技术基金资助项目{黔科合J字LKG[2013]02号};贵州省教育厅自然科学基金资助项目{黔科教[2008]050}
杜婕(1965-),女,汉族,贵州贵阳人,讲师,主要从事有机合成的研究。
杜海堂,教授,E-mail:haitangdu@163.com
O626
A
1005-1511(2014)04-0485-04

