Selection of Chelated Fe (III)/Fe (II) Catalytic Oxidation Agents for Desulfurization Based on Iron Complexation Method
Luo Ying; Liu Youzhi; Qi Guisheng; Guo Huidong; Zhu Zhengfeng
(1. Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering, North University of China, Taiyuan 030051; 2. Research Center of Shanxi Province on High Gravity Chemical Engineering Technology, North University of China, Taiyuan 030051)
Selection of Chelated Fe (III)/Fe (II) Catalytic Oxidation Agents for Desulfurization Based on Iron Complexation Method
Luo Ying1,2; Liu Youzhi1,2; Qi Guisheng1,2; Guo Huidong1,2; Zhu Zhengfeng1,2
(1. Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering, North University of China, Taiyuan 030051; 2. Research Center of Shanxi Province on High Gravity Chemical Engineering Technology, North University of China, Taiyuan 030051)
Optimization of factors influencing the experiments on reactions involving 8 different chelating agents and soluble Fe (III)/Fe (II) salts was carried out to yield chelated iron complexes. A combination of optimized influencing factors has resulted in a Fe chelating capacity of the iron-based desulfurization solution to be equal to 6.83—13.56 g/L at a redox potential of 0.185—0.3. The desulfurization performance of Fe (III)/Fe (II) chelating agents was investigated on a simulated sulfur-containing industrial gas composed of H2S and N2in a cross-flow rotating packed bed. Test results have revealed that the proposed iron-based desulfurization solution showed a sulfur removal efficiency of over 99% along with a Fe chelating capacity exceeding 1.35 g/L. This desulfurization technology which has practical application prospect is currently in the phase of commercial scale-up study.
hydrogen sulfide; chelated iron; catalytic oxidation; factor analysis; redox
1 Introduction
Hydrogen sulfide gas generated during petroleum refining, natural gas processing, city gas production and other chemical synthesis processes, which can corrode metallic piping and equipment, cause environmental pollution and even affect product quality, is a pollutant with nasty smell that must be disposed of according to the government regulations. Therefore, the removal of H2S is one of the urgent issues during purification of gases generated from chemical treatment process[1].
Among a variety of desulfurization technologies, the iron complexation method has been finding extensive application thanks to its simple process scheme, high desulfurization efficiency and innoxiousness[2].
The desulfurization process by means of complexed iron species is based on the mechanism of redox reactions, which can adopt the recycled utilization of Fe3+—Fe2+—Fe3+species to recover sulfur. The redox process of hydrogen sulfide proceeds as follows:
a) Absorption of H2S by caustic (for example Na2CO3) solution:

b) Formation of elemental sulfur via the reaction of chelated iron on HS-:
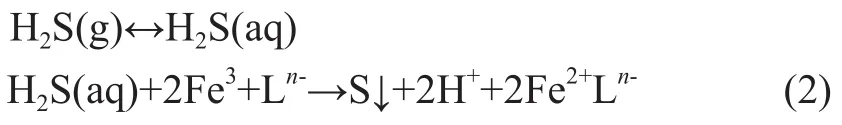
c) Regeneration of desulfurization solution via oxidation of Fe2+to Fe3+in the presence of oxygen (or air):
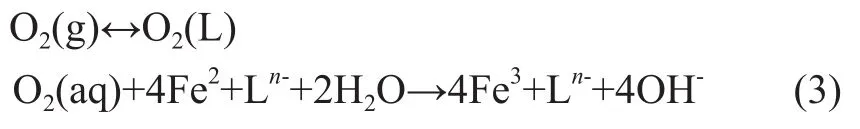
The overall reaction proceeds as follows:

In the course of the above-mentioned liquid-phase redox reaction, ‘n’ stands for the electric charges of the organic ligand L, which is an organic polyaminocarboxylic acid such as ethylenediaminetetraacetic acid (EDTA), hydroxyethylenediaminetetraacetic acid (HEDTA), or nitrilotriacetic acid (NTA). The key role of the chelated iron reagent is associated with its catalytic oxidation function.
In the whole reaction process the Fe ions play two roles. Firstly, the Fe ions work as the donor and accepter of electric charges to convert the sulfur ions into elemental sulfur, and secondly, iron ions functioning as the catalyst can speed up the reaction, while the organic ligand L as a chelating agent does not take part in chemical reaction but can make Fe ions dissolved in water to reach a definite concentration[4].
Over the recent years the process of desulfurization by means of chelated iron has been developing quickly in the overseas after having made great strides in improving the solvent composition and optimizing the process flow diagram and equipment configuration. The number of units for desulfurization with chelated iron method is increasing continuously to gradually replace the traditional wet redox units. Currently the Merichem’s LO-CAT technology, the French Sulfint technology, and the SulFerox technology[5]have made great progress among the overseas commercial desulfurization processes. In China there is also progress in studying sulfur removal technology by means of chelated iron technology albeit still with some problems related with complexed iron desulfurization solution, such as the relatively low concentration of catalytic oxidizing agent of chelated iron and low sulfur capacity[2,5].
The available technical literature information only describes the desulfurization efficiency of the selected chelating agents without referring to the research on selection of chelating agents, the structural characteristics of chelating agents, the interaction between the compound chelating agents, and the iron chelating capacity of various chelating agents at different pH values. This paper has made an optimal combination of factors of 42 chelating systems composed of compound desulfurization agents in an attempt to experimentally study the relationship between the dosage of various chelating agents and their iron chelating capacity and check the desulfurization performance of the selected novel Fe-based desulfurization solutions in an attempt to develop in China the novel Fe-based desulfurization solutions featuring high iron chelating capacity, high sulfur removal rate and high sulfur capacity to provide a theoretical guidance on catalytic oxidation via chelated Fe (III)/Fe (II) catalytic agents in the course of desulfurization by means of chelated iron species.
2 Experimental
2.1 Experimental instruments and reagents
The instruments used in experiments included:
A PHS-3C type pH-meter, and a L5 type ultraviolet spectrophotometer, manufactured by the Shanghai Yidian Scientific Instruments Co., Ltd.; a HZ85-2 magnetic stirrer, manufactured by the Beijing Zhongxing Weiye Instrument Co., Ltd.; an AUY120 type electronic balance, manufactured by the Japanese Shimadzu Company; a GWA-UN type ultra-pure water device, manufactured by the Beijing Puxi General Instrument Co., Ltd.; a LG10-2.4A type high-speed centrifuge, manufactured by the Beijing Jingli Centrifuge Co., Ltd.; a CHI660E type electrochemical work station, manufactured by the Shanghai Zhenghua Instrument Co., Ltd.; and a M-40 type hydrogen sulfide detector, manufactured by the INSCO Group Inc. of USA.
Chemical reagents included: EDTA, NTA , and HEDTA, provided by the Tokyo Kasei Kogyo Co., Ltd.; (NH4)2SO4·Fe2(SO4)3·24H2O and (NH4)2SO4·FeSO4·6H2O, provided by the Tianjin Red Rock Reagent Plant; ascorbic acid ando-phenanthroline, provided by the Tianjin Hengxing Chemical Reagent Manufacturing Company; glacial acetic acid, zinc acetate and KOH, provided by the Tianjin Tianli Chemical Reagents Co., Ltd., with all reagents being analytically pure.
2.2 Experimental equipment and process flow diagram
The process flow diagram for measuring the sulfur capacity of the desulfurization solution used in the experiments is presented in Figure 1.
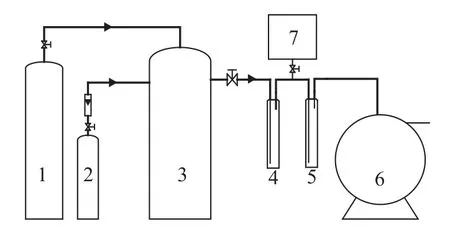
Figure 1 Experimental unit for measuring the sulfur capacity
H2S and N2were mixed to prepare the simulated gas, which contained a mass concentration of H2S gas (ρ1) ranging from 0.1 g/m3to 6 g/m3. The mixed gas prepared in a stationary gas blending tank was routed in the formof gas bubbles to the absorber A, which contained 30 mL of desulfurization solution, and the tail-gas after sulfur removal was regularly monitored on its H2S concentration (ρ2) by a H2S analyzer prior to entering the absorber B containing zinc acetate solution. The volume of tail-gas leaving the absorber B was measured by a wet type gas flow-meter. In the course of experiments the gas bubbling rate in the absorbers was maintained at 6 min/L in order to make hydrogen sulfide completely absorbed.
The experimental unit for removal of H2S by chelated iron in the cross-flow rotating packed bed is presented in Figure 2. H2S and N2streams were mixed in a surge drum to form the simulated sour gas containing H2S in the range of between 0.1—6 g/m3. The sour gas after having been measured by a rotameter entered the cross-flow rotating packed bed, in which the sour gas was kept in contact with the Na2CO3solution pumped into the bed by the recycle pump from the circulating tank. Under the conditions of high turbulence, large contact surface and highspeed renewal of interfaces between the gas and liquid the hydrogen sulfide gas was readily absorbed by the Na2CO3solution, with the desulfurized gas after passing through an additional absorbing tank filled with NaOH solution for further sulfur removal was vented into the atmosphere. The sodium carbonate solution after absorbing H2S was sent back to the circulating tank for repeated use.

Figure 2 Experimental unit for absorption of H2S in a cross-flow rotating packed bed
2.3 Experimental method
During the experiments 8 kinds of chelating agents with different structures were used with their names and structures presented in Scheme 1[6]:

Scheme 1 Structure and nomenclature of 8 chelating agents
The experiments measured the iron chelating capacity of eight chelating agents at a pH value of 7.0, 8.0, 9.0, 10.0, 11.0 and 12.0, respectively. On the basis of single factor tests, EDTA functioning as the main chelating agent wascompounded respectively with other 7 chelating agents to form 7 chelating systems of different structure. Six experiments were designed to test each chelating system and a total of 42 chelating systems were studied. The Fe chelating capacity and redox potential of 42 complex chelating systems were investigated at different pH values, different Fe (III)/Fe (II) molar ratios and different proportions of two chelating agents in order to obtain the optimized combination of these chelating agents. Multivariate linear regression analysis of the test results was conducted by means of the regress function in the MATLAB language in order to optimize the desulfurization solution selected from 42 chelating systems. Finally the selected iron-based desulfurization solution was checked on its sulfur removal performance in terms of its sulfur removal rate and its working sulfur capacity upon treating the simulated industrial sour gas consisting of H2S and N2mixture in the cross-flow rotating packed bed.
During the experiments the Fe-chelating capacity of the desulfurization solution was determined by ultraviolet spectrophotometry with o-phenanthroline, and the redox potential of the desulfurization solution was determined using the cyclic voltammetry in a three-electrode cell system. The working sulfur capacity of the desulfurization solution was determined using the equipment shown in Figure 1, while the sulfur removal rate of the desulfurization solution was determined by the equipment shown in Figure 2. The hydrogen sulfide concentration (6 g/m3) in the simulated sour gas was determined by iodometry, while the H2S concentration (<0.1 g/m3) in the desulfurized gas was determined by the gas detector.

Table 1 Experimental plan for formulating chelating systems
2.4 Processing of experimental data
The experimental design module in the MATLAB software package was used in designing experiments as well as in the statistical analyses.
The desulfurization rate E is used to characterize the effect for removal of H2S[1], as defined by:

in whichE= H2S removal rate, %; andρ1andρ2denote the H2S concentration (g/m3) at the inlet and outlet of the cross-flow rotating packed bed, respectively.
The sulfur capacityCSas a main indicator for characterizing the performance of the desulfurization solution can be calculated according to the following equation[7]:

in whichρrepresents the H2S concentration in the gas mixture, g/L;Qis the gas flowrate, m3/h;tis the duration for removal of H2S, min;vis the volume of desulfurization solution, m3; andCsis the sulfur capacity, g/L.
Fe chelating capacity[8]: This indicator can be used to characterize the ability to dissolve the chelated iron, which means the mass concentration of chelated iron in one liter of desulfurization solution. Visible ultraviolet spectrophotometry can be adopted to determine the Fe chelating capacity in the complexed iron solution usingo-phenanthrolineas the chromogenic agent[9].
Regression equation:

in whichXis the absorbance, which is recorded by the visible UV spectrophotometer, andYis the iron chelating capacity, g/L.
The redox potential is determined by the electrochemical work station at a KCl concentration of 2 mol/L with a scanning rate of 0.08 V/s.
3 Results and Discussion
3.1 Research and development of novel iron-based desulfurization solution
3.1.1 Single factor tests—iron chelating capacity of different chelating agents at different pH values
In order to compare the chelating performance of eight chelating agents, the iron chelating capacity of each che-lating agent was determined at a pH value of 7, 8, 9, 10, 11 and 12, respectively, with the results shown in Figure 3.

Figure 3 Iron chelating capacity of different chelating agents at different pH values
It can be seen from Figure 1 that within the easy range of testing, the phosphonic acid salt HEDP had the strongest iron species chelating ability along with a highest alkali tolerance. At a pH value of 10.0, the iron chelating capacity of HEDP could reach a maximum of 30.8 g/L, but the solution after chelating was extremely unstable and prone to severe degradation, which could greatly limit the applicability of HEDP. The chelating ability of TEA and HEDTA was not affected by the changes in pH value and these chelating agents had good alkali tolerance along with relatively large chelating capacity. The iron chelating ability of the aminocarboxylic acid type chelating agents, viz. EDTA, DPTA and NTA, decreased with an increasing pH value, and these chelating agents showed poor alkali tolerance at a pH value exceeding 10.0, but they were suited to a pH range of between 8 and 10.0.
Nassar[10]has discovered after study that with regard to the iron species which has been chelated by NTA, EDTA, or DTPA, the chelated Fe(III)-NTA showed a best effect for absorbing hydrogen sulfide. Investigation conducted by Martell, et al.[6]has revealed that NTA is so far the comparatively excellent ligand, the longer degradation duration and cheap cost of which make itself the catalyst of choice for desulfurization process. Citric acid and sulfosalicylic acid among hydroxycarboxylic acid homologues contain a definite amount of hydroxyl and carboxyl radicals which are biodegradable and non-toxic to serve as the subject of current hot spots in R&D activities. However, the ability of citric acid to chelate Fe ions drops quickly with an increasing pH value and shows the worst performance in terms of its alkali tolerance, whereas the sulfosalicylic acid is less affected by pH value changes along with a relatively stable alkali tolerance. In the pH range of between 8.0 and 9.0 the iron chelating capacity of other seven chelating agents lies between 4.37 g/L and 14 g/L.
3.1.2 Dual factors duplicate tests – Influence of different chelators blending ratio
(1) Synergism between various influencing factors
During the experiments the synergistic coefficient of each compounded system was based on 6 experimental results, which were processed by means of the MATLAB software. Every two chelating agents chosen out of EDTA, NTA and HEDTA were blended to form compounded systems and the synergism of each factor was studied based on the range analysis of duplicate tests of dual factors, with the study results shown in Figure 4.

Figure 4 Synergism of every factor in the compounded system
It can be seen from Figure 4 that the pH value in each chelating system shows the greatest synergistic effect, because the high pH value in alkaline solution can promote absorption of hydrogen sulfide. However, only the pH value of two compounded chelating systems consisting of NTA and HEDTA demonstrates positive effect, while the pH value of other two systems displays negative effect. Simon[11]upon investigating the absorption of H2S by CDTA with chelated iron species in an alkaline solution revealed that its pH value showed the highest synergistic effect, which showed that our research outcome is in good agreement with Simon’s study results.
The synergistic effect of the chelators blending ratio inevery chelating system only assumes the second place. However, the synergism of chelators blending ratio in the EDTA and HEDTA compounded system showed a negative effect, while the other two chelating systems demonstrated positive effects. Among various factors influencing the iron chelating capacity, the Fe3+/Fe2+molar ratio showed a minimum synergism, while the compounded system composed of HEDTA and NTA displayed a more active effect, and a extremely minimal active effect was seen in the compounded system composed of EDTA and NTA, but the chelators blending ratio only imposed negative effect on the compounded system consisting of EDTA and HEDTA. The overall influence of the Fe3+/Fe2+molar ratio was relatively low despite the synergism it possessed, which was in agreement with the research outcome of Simon[11].
(2) Interaction of various factors
During the experiments it is necessary to consider not only the influence of each factor on the indicators tested, but also the interaction between every factor on the experimental results. When two chelators were commingled to form a compounded system, the influence of the interaction between every factor on the iron chelating capacity was established based on 6 experimental results and was processed with the MATLAB software. For instance, EDTA, NTA and HEDTA were blended to form dual compounded systems in order to analyze the effect of interaction through range analysis via the duplicate testing of dual factors, with the results presented in Figures 5, 6 and 7.
It can be seen from Figure 5 that in the compunded system containing EDTA and NTA the three factors, viz. the pH value, the Fe3+/Fe2+molar ratio and the chelators blending ratio, had interactions on each other. According to the range analysis of experimental results, the interaction between factors of the EDTA/NTA compunded system was mainly incarnated by the pH value and the chelators blending ratio which showed reverse interaction. Since the interaction was less affected by the Fe3+/Fe2+molar ratio, the influence of interactions of the pH value and the chelators blending ratio on the iron chelating capacity should be studied preferentially. It is shown that at a pH value of 9.0 the interaction had a best effect with a chelator blending ratio of 4:1.

Figure 5 Interaction of various factors involved in the EDTA and NTA compounded system

Figure 6 Interaction between various factors in the EDTA and HEDTA compounded system

Figure 7 Interaction between various factors in the NTA andHEDTA compounded system
It can be seen from Figure 6 that in the compunded system comprising EDTA and NTA the three factors, viz. the pH value, the Fe3+/Fe2+molar ratio and the chelators blending ratio, had interaction between each other. According to the range analysis of experimental results,the interaction between factors of the EDTA/HEDTA compunded system belonged to a positively regressive type of interaction. The optimal combination of the EDTA/HEDTA compunded system was achieved at a pH value=8.5, a chelator ratio=1:3, and a Fe3+/Fe2+molar ratio of 3:1. Since the interaction of Fe3+/Fe2+molar ratio was greater than that of the chelators blending ratio and the pH value, it is suggested to preferentially consider the chelator blending ratio and the pH value other than the factor of Fe3+/Fe2+molar ratio.
It can be seen from Figure 7 that in the compunded system comprising NTA and HEDTA the three factors, viz. the pH value, the Fe3+/Fe2+molar ratio and the chelators blending ratio, had interaction between each other. According to the range analysis of experimental results, the interaction between factors of the NTA/HEDTA compunded system is mainly embodied in the interaction of the Fe3+/Fe2+molar ratio with the other two factors, indicating to a positively regressive type of interaction. The interaction between the chelators blending ratio and the pH value could be neglected. The optimal combination of the NTA/HEDTA compounded system is obtained at a pH value=9.5, a chelator blending ratio of 5:1 and a Fe3+/Fe2+molar ratio of 5:1.
3.1.3 Relationship between iron chelating capacity and dosage of chelating agent
The optimized combination of various factors can provide the basis for the selection level and step length for designing the response surface of the compounded chelating agents. As regards the compounding of two chelators referred to by Marcus[12], the synergistic system consisting of a strong chelator and a weak chelator has a more apparent action for synergistic extraction of metal ions than that of the synergistic system consisting of either two strong chelators or two weak chelators. We have studied the iron chelating capacity of 42 chelating systems and the dosage of each combination of two chelating agents, with the study results presented in Figure 8.
The multivariate linear regression analysis of experimental data was conducted by means of the regress function in the MATLAB language to establish a relationship between the iron chelating capacity and the dosage of two different chelators, with the regression equation shown below:
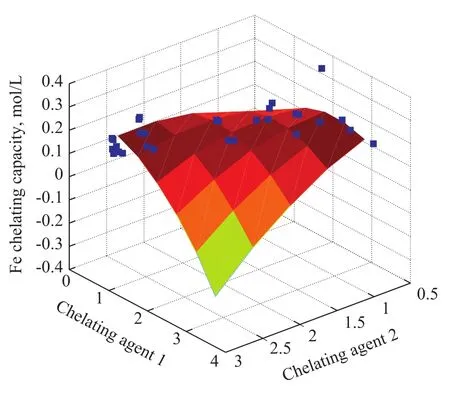
Figure 8 The iron chelating capacity of 42 compounded chelators

in whichyis the iron chelating capacity (g/L), andX1orX2represents the dosage of two different chelating agent (g/L), respectively.
When the eight selected chelating agents with different structures were compounded, the synergistic effect of these chelating agents was similar[12]in the ability to form mixed complex despite the difference in their coordination groups and the magnitude of steric hindrance among different chelating agents. The investigation conducted by our team has revealed that since the solubility of Fe chelate in the complexation system formed from compounding of two chelators is much greater in organic solvent than the single Fe chelate, the partition ratio of this system is significantly increased to confirm the apparent synergistic extraction function of different chelating agents in the compounded system. The iron-based desulfurization solutions used in the following experiments were formulated based on the results of regression equation.
3.2 Performance of novel iron-based desulfurization solution
3.2.1 Iron chelating capacity and redox potential of novel iron-based desulfurization solution
Three novel iron-based desulfurization solutions preferentially selected by the regression equation were checked on their iron chelating capacity and redox ability with the results depicted in Table 2.

Table 2 The Fe chelating capacity and redox potential of 3 novel Fe-based desulfurization solutions
It can be seen from Table 2 that at a pH value of 8.5, the Fe chelating capacity of three novel Fe-based desulfurization solutions was greater than 6 g/L that could effectively alleviate iron deposition in caustic solution and increase the solubility of Fe ions. In comparison with the iron chelating capacity of the desulfurization solution adopted in the LO-CAT process which is equal to 1.0 g/L[2]at a pH value in the range of 8—9, the desulfurization solution developed by our team can meet the technical goal of larger iron chelating capacity. The redox potentialφof the desulfurization solution with chelated iron species should meet the following condition:φS/H2S<φFe3+L/Fe2+L<φO2/H2O, and according to calculation the actual potential of the desulfurization solution is -0.21<φdesulfurizationsolution<0.59. In the LO-CAT process the redox potentialφis maintained between 0.15 and 0.25[5]at a pH value of 8—9. The redox potential of three novel Fe-based desulfurization solutions referred to in this paper is in the range of 0.185—0.3, which is in the range of equilibrium potentials between two reaction systems and can carry out oxidation of H2S and reduction of oxygen, respectively, while in the meantime it can verify the great depth for sulfur removal along with recycling of iron ions during desulfurization by means of the chelated iron.
3.2.2 Sulfur removal performance of novel ironbased desulfurization solutions
A gas mixture composed of H2S and N2used as the simulated industrial gas was subjected to sulfur removal by three Fe-based desulfurization solutions in the cross-flow rotating packed bed in order to check the sulfur removal performance and sulfur capacity of novel desulfurization solutions, with the test results presented in Figure 9.
It can be seen from Figure 9 that among three desulfurization solutions the best one could achieve a sulfur removal rate of over 99%, and all three novel desulfurization solutions could reach a sulfur removal rate of 98% within 10 minutes, which could meet or surpass the standard for sulfur removal rate specified by the world’s advanced countries[13]. During stable operation of this desulfurization process for 40 minutes, the sulfur removal rate achieved by these novel desulfurization solutions could be as high as 90%. Qi Guisheng[14]reached a sulfur removal rate of over 95% by using PDS as the desulfurization catalyst in a cross-flow rotating packed bed at a gas-liquid contact time of less than one second. In comparison with that case, the iron chelating capacity of the desulfurization solutions referred to in this paper has greater iron chelating capacity along with high sulfur removal rate in the crossflow rotating packed bed, which is conducive to reducing the consumption of desulfurization solution and chemical reagents used in the desulfurization process to achieve remarkable economic benefits at a relatively low operating cost.

Figure 9 Desulfurization rate of three novel desulfurization solutions

Figure 10 Comparison between laboratory desulfurization solutions and three novel Fe-based desulfurization solutions on their sulfur capacity[3]
The experimental data on sulfur capacity of three novel ironbased desulfurization solutions are presented in Figure 10.It can be seen from Figure 10 that the sulfur capacity of three novel Fe-based desulfurization solutions all exceeds 1.35 g/L, which is higher than the sulfur capacity referred to in the technical literature. The choice desulfurization solutions with high sulfur capacity can be conducive to decreasing the energy consumption of the circulation pump and the reduction of desulfurization solution consumption to cut the operating cost of the desulfurization process and enhance the economic benefits.
4 Conclusions
(1) The iron chelating capacity and the alkali tolerance of 8 chelating agents were compared at different pH values. HEDP showed a largest iron chelating capacity, which was 30.8 g/L at a pH value of 10, and the other 7 chelating agents had their iron chelating capacity in the range of between 4.37 g/L and 14 g/L.
(2) Among the three factors influencing the iron chelating capacity of the chelating system, the pH value showed the highest synergistic function, with the chelator blending ratio occupying the second place and the Fe3+/Fe2+molar ratio having the minimum synergistic function. All factors involved in the chelating system could be combined to reach an optimized level.
(3) The relationship between iron chelating capacity and chelators dosage during blending of two chelating agents was established through multivariate regression analysis based on the regress function of the MATLAB language according to the following regression equation:
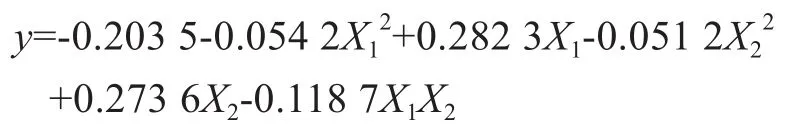
(4) The Fe-based desulfurization solutions formulated according to the regression equation had the iron chelating capacity in the range of between 6.83—13.56 g/L, with their redox potential reaching 0.185—0.3, the sulfur removal rate exceeding 99% in the cross-flow rotating bed, and the sulfur capacity reaching in excess of 1.35 g/L, making themselves relatively ideal Fe-chelated desulfurization solutions.
Acknowledgement:This work was financially supported by the Natural Science Fundation of China (No. 21376229) and the Science and Technology Development Plan of Shanxi Province, China (No. 20130321035-02).
[1] Qi Guisheng, Liu Youzhi, Jiao Weizhou. Study on industrial application of hydrogen sulfide removal by wet oxidation method with high gravity technology [J]. China Petroleum Processing and Petrochemical Technology, 2011, 13(4): 29-34
[2] Xiao Jiugao, Yang Jianping, Hao Aixiang. Abroad research progress of H2S removing process with chelated iron [J]. Journal of Chemical Industry & Engineering, 2003, 24(5): 41-44 (in Chinese)
[3] Wubs H J, Beenackers A A C M. Kinetics of the oxidation of ferrous chelates of EDTA and HEDTA in aqueous solution [J]. Industrial & Engineering Chemistry Research, 1993, 32(11): 2580-2594
[4] Hu Yaoliang. High-efficiency H2S removal process—LO-CAT[J]. Petroleum Refinery Engineering, 2007, 37(11): 30-35(in Chinese)
[5] Zhang Wu, He Jinglong, Chang Honggang, et al. The applications status and prospect analysis of iron-redox desulfurization technology[J]. Chemical Engineering of Oil & Gas, 2008, 37: 130-133(in Chinese)
[6] Martell A E, Motekaitis R. J, Chen D. Selection of new Fe (III)/Fe (II) chelating agents as catalysts for the oxidation of hydrogen sulfide to sulfur by air [J]. Canadian Journal of Chemistry, 1996, 74(10): 1872-1879
[7] Shang Hairu. Basic research on high gravity desulfurization technology by complex iron[D]. Taiyuan: North University of China, 2010 (in Chinese)
[8] Yu Yingzhen, Jin Xianhua, Fu Jiaya. Test method for cation chelating power and comparison thereof [J]. Textile Auxiliaries, 2006, 23(12): 40-42 (in Chinese)
[9] Luo Ying, Liu Youzhi, Qi Guisheng, et al. Application of UV-Vis spectrophotometry for determination of chelated iron concentration[J]. Natural Gas Chemical Industry, 2013, 38(5): 81-87 (in Chinese)
[10] Nassar N N, Husein M M, Pereira-Almao P. Ultradispersed particles in heavy oil: Part II, sorption of H2S (g) [J]. Fuel Processing Technology, 2010, 91(2): 169-174
[11] Piche S, Ribeiro N, Bacaoui A. Assessment of a redox alkaline/iron-chelate absorption process for the removal of dilute hydrogen sulfide in air emissions [J]. Chemical Engineering Science, 2005, 60(22): 6452-6461
[12] Marcus Y, Kertes A. Ion Exchange and Solvent Extraction of Metal Complexes [M]. New York: Wiley-Interscience, 1969
[13] Wang Jiaming, Mo Hongbiao. LO-CAT sulfur recovery technique and application prospect thereof [J]. Natural Gas and Oil, 2011, 29(3): 30-34 (in Chinese)
[14] Qi Guisheng, Liu Youzhi, Pan Hongxia, et al. Hydrogen sulfide removal by wet oxidation method in a cross-flow rotating packed bed[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2012, 28(2): 195-199 (in Chinese)
Recieved date: 2014-01-27; Accepted date: 2014-03-10.
Prof. Liu Youzhi, Telephone: +86-351-3921986; E-mail: lyzzhongxin@126.com.
- 中国炼油与石油化工的其它文章
- Research on New Silica Sol Matrix Used in Fluid Catalytic Cracking Reaction
- Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
- Experimental Study on Liquid-Liquid Equilibria of Alcohol- Ester-Water-CaCl2System
- Study on Relationship between Microstructure of Active Phase and HDS Performance of Sulfided Ni-Mo Catalysts: Effect of Metal Loading
- Friction Characteristics of Space Lubricating Oil No. 4129 in Rolling and Sliding Contact
- Research on Hydrolysis and Saccharification of Corn Stover

