Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
Yu Mengshan; Nie Yanpei; Zhou Lu; Yi Jianjun; Huang Qigu; Gao Kejing; Yang Wantai
(1. State Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029; 2. Lab for Synthetic Resin Research Institution of Petrochemical Technology, China National Petroleum Corporation)
Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
Yu Mengshan1; Nie Yanpei1; Zhou Lu1; Yi Jianjun2; Huang Qigu1; Gao Kejing2; Yang Wantai1
(1. State Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029; 2. Lab for Synthetic Resin Research Institution of Petrochemical Technology, China National Petroleum Corporation)
Two kinds of cycloalkoxy silane compounds were synthesized and used as the internal electron donors (IEDs) of supported Ziegler-Natta catalyst for ethylene polymerization to produce polyethylene with broader molecular weight distribution (MWD). The effect of the structure and the amount of these IEDs on the polymerization performance was investigated. The results implied that the molecular weight distribution of the obtained polyethylene could be adjusted by the incorporation of IEDs. SEM result showed that the morphology of catalyst particle was spherical and uniform in size distribution. The titanium content of these catalysts was higher, the active TiCl4species were easily anchored on the support than that without adding IED, which was determined by ICP. The GPC result confirmed that the polyethylene with broader molecular weight distribution in the range of from 23.4 to 25.6 was obtained using triethoxy-(-cyclopentyloxy)-silane (ED1) and triethoxy-(-cyclohexyloxyl)- silane (ED2) as the internal electron donors.
supported Ziegler-Natta catalyst; polyethylene; broader molecular weight distribution; electron donor
1 Introduction
Since the discovery of the Ziegler-Natta catalyst in the 1950s, the polyolefin industry has experienced rapid development worldwide. A variety of polyolefin products with good properties have emerged. For example, the polyethylene with broad molecular weight distribution has been attracting much attention thanks to its excellent processability and mechanical property[1-5]. The molecular weight distribution (MWD) of polyethylene plays an important role in determining its mechanical and rheological properties. Many approaches have been used to tailor the required MWD, including physical blending[6-7], synthesis through hybrid or mixed catalysts[8-9], and multi-pot processes[10]. Wang[11]reported that polyethylene with broad MWD and high weight average molecular weight (Mw) was obtained by the hybrid or mixed catalysts, Fe(acac)3-bis(imino)pyridine/MAO catalyst system. The bimodal MWD of polyethylene produced over the supported ironbased catalyst was well tailored by varying polymerization conditions, such as the ethylene pressure and molar ratio of Al/Fe. Upon considering the production cost and energy consumption, the one-pot strategy based on current industry technology is more reasonable. In our previous work[12]based on organosiloxane compounds employed as internal electron donors of the Ziegler-Natta catalyst, (co-)polyethylene with broad MWDs was successfully prepared on these modified catalysts. However, the relationship between the structure of electron donor and MWD of the obtained polyethylene is not clear, which implies that the further work on the preparation of polyethylene with broader MWD is necessary through adjusting the structure of the IEDs.
In this research, we synthesized two kinds of cycloalkoxy silane used as IEDs of the Ziegler-Natta catalysts for ethylene polymerization. These Ziegler-Natta catalysts showed high catalytic activity for producing polyethylene featuring broader MWD (23.4—25.6). The influence of the structure and the amount of IEDs on the polymerization behavior was discussed as well. Currently in industry the preparation of PE100 featured broader MWDs in therange of between 23 and 26, a useful product in application is based on the multi-pot technology.
2 Experimental
2.1 General procedures and materials
Tetraethoxysilane, Si(OEt)3Cl, cyclopentanol, cyclohexanol, 2-ethylhexanol, anhydrous magnesium dichloride and AlEt3(at a concentration of 1.0 mol/L in hexane) were purchased from the Acros Organics Agent in China. Triethylamine, anhydrous ethanol, alcohol, hydrochloric acid, TiCl4, toluene,n-heptane andn-hexane were purchased from the Beijing Chemicals Company. Toluene andn-hexane were further purified by refluxing over sodium at normal pressure for 48 h prior to use. Alcohols were treated with activated 5 A molecular sieves in nitrogen atmosphere for one week before use. Decane was purchased from the Beijing Chemical Industry Company Ltd. Ethylene (polymerization grade) obtained from the Beijing Guangming Chemical and Engineering Company, Ltd. was used without further purification.
2.2 Ethylene slurry polymerization
All polymerization processes were carried out in a 300 mL glass reactor after evacuating all moisture and oxygen by a high-vacuum pump. Under a nitrogen atmosphere, freshly distilled solvent (100 mL), along with a required amount of catalysts (Cat. e1—Cat. e9) and co-catalyst AlEt3were introduced successively into the reactor at room temperature. The nitrogen atmosphere in the reactor was replaced with ethylene and the temperature was increased to 70 ℃ rapidly. The polymerization was carried out for 1 h. Finally, the reaction was terminated with addition of 10% HCl dissolved in alcohol. The product was obtained after filtration and washing, followed by drying in a vacuum oven at 80 ℃. The catalytic activity was calculated according to the product amount divided by polymerization time.
2.3 Characterization
1H NMR spectra were recorded on a Varian INOVA600 MHz spectrometer in CDCl3solution at 25 ℃ with TMS used as the reference. All H chemical shifts were reported relative to proton resonance in CDCl3at a chemical shift (δ) of 7.26. Elemental analysis was performed on a Perkin-Elmer 2400 microanalyzer. The Ti content of catalyst was measured using a Shimadzu ICPS-5000 instrument. About 10 mg of the supported catalyst was dissolved in HF and HNO3acid completely. The solution was diluted with distilled water and used for the ICP analysis. The average molecular weight and molecular weight distribution were measured by a PL-GPC200 instrument using standard polyethylene as the reference and 1,2,4-trichlorobenzene as the solvent at 150 ℃.
2.4 Preparation of electron donor
Under nitrogen atmosphere, the solution containing 1.0 mL of cyclopentanol in 100 mL ofn-hexane was added into a 300-mL Schlenk flask equipped with a stirring bar. After 1.5 mL of triethylamine and 2.2 mL of Si(OEt)3Cl were dripped slowly into the reactor successively by a syringe at room temperature, the mixture was stirred for 2 h at ambient temperature. Then the mixture was filtered. The filtrate was purified under vacuum to give a colorless transparent liquid, triethoxy-(-cyclopentyloxy)-silane (ED1) at a yield of 87.3%. ED1, C11H24O4Si (MW: 248 g/mol)1H NMR analysis (Figure 1):δ1.24 (tri,J=7.025 Hz, 9H—OCH2CH3);δ1.51 (m, 2H —CH2CH2CH2CH2—);δ1. 68 (m, 2H —CH2CH2CH2CH2—);δ1.75 (m, 4H —CH2CH2CH2CH2—);δ3.85 (q,J=6.975 Hz, 6H —OCH2CH3);δ4.48 (m, 1H—CH(O)—). Elemental analysis: calcd: C=53.23%; H=9.68%, and O=25.81%; found: C=53.21%; H=9.67%, and O=25.80%.

Figure 1 The1H NMR spectra of ED1
Under nitrogen atmosphere, the solution of 1.0 mL of cyclohexanol in 100 mL ofn-hexane was added into a 300 mL Schlenk flask equipped with a stirring bar. After 1.3 mL of triethylamine and 1.9 mL of Si(OEt)3Clwere dripped slowly into the reactor in succession by a syringe at room temperature, and then the mixture was stirred for 2 h at room temperature. Furthermore, the mixture was filtered. The filtrate was purified under vacuum to give a colorless transparent liquid, triethoxy-(-cyclohexyloxyl)-silane (ED2) at a yield of 90.7%. ED2, C12H26O4Si (MW: 262 g/mol)1H NMR analysis (Figure 2):δ1.20 (m, 1H —CH2CH2CH2CH2CH2—CH(O)—);δ1.24 (tri,J=7.025 Hz, 9H —OCH2CH3);δ1.32—1.40 (m, 4H —CH2CH2CH2CH2CH2—CH(O)—);δ1.51 (m, 1H —CH2CH2CH2CH2CH2—CH(O)—);δ1.75—1.87 (m, 4H —CH2CH2CH2CH2CH2—CH(O)—);δ3.85 (q,J=6.975 Hz, 6H —OCH2CH3);δ3.81 (m, 1H—CH(O)—). Elemental Analysis: calcd: C=54.96%; H=9.92%, and O= 24.43%; found: C=54.95%; H=9.90%, and O=24.41%.
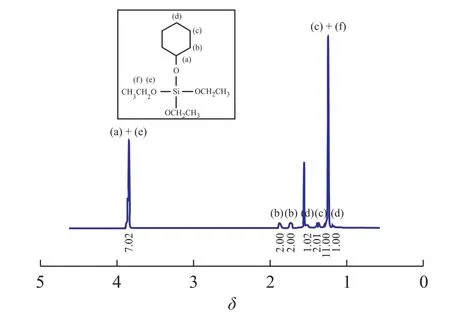
Figure 2 The1H NMR spectra of ED2
Diethoxy-isopropoxy-(t-butoxy)-silane (ED3) was prepared according to the procedure mentioned in our previous work[12].
2.5 Preparation of polyethylene catalyst
1.0 g of MgCl2(10.5 mmol), 6.0 mL of 2-ethylhexanol in 16.0 mL of decane, 2.1 mmol of ED1 along with a required amount of epichlorohydrin (ECH) and tributyl phosphate (TBP) were added successively at room temperature into a 300-mL Schlenk flask equipped with a stirring bar. Upon being preheated to 110 ℃, the mixture was maintained for 3 h in a nitrogen atmosphere. The mixture then turned into a homogeneous solution. After being cooled down to -10 ℃, excess TiCl4(nTi:nMg=30:1) was dripped into the solution slowly by a syringe. After that, the solution was warmed to 60 ℃ and maintained for 3 h. More precipitates were produced during this process[13-16]. The residue after filtration was washed twice with decane (20 mL×2) and three times with n-hexane (20 mL×3), and a brown powder,i.e. catalyst (Cat. e1), was obtained at a yield of 1.23 g.
Cat. e2-Cat. e9 were prepared following the same procedure after merely changing the type and amount of IEDs, as shown in Table 1.

Table 1 Property of Cat. e1 to Cat. e9 for ethylene polymerization
3 Results and Discussion
3.1 Ethylene polymerization
The results of ethylene polymerization catalyzed by Cat. e1—Cat. e9 are listed in Table 1. It can be seen from Table 1 that all these catalysts are favorable for ethylene polymerization. However, there is a noticeable difference between the catalysts using tetraethoxysilane as IEDs (Cat.e8) and two new kinds of cycloalkoxy silanecompounds serving as IEDs (Cat. e1—Cat. e7). The molecular weight distribution of polyethylene prepared over Cat. e1—Cat. e7 exhibits much broader than that prepared over Cat. e8. The polyethylene catalyzed by Cat. e3 has a broadest MWD (MWD=48.0), which has been reported in our previous work[12], indicating that the MWD of polyethylene is broadened by the addition of the IED during the preparation of catalysts. But further work on the MWD adjustment through electron donor is necessary. Therefore, two IEDs (ED1 and ED2) were adopted and expected to realize the specific molecular weight distribution of polymer. It can be seen from Table 1 that Cat. e1 and Cat. e2 were used for ethylene polymerization, exhibiting high catalytic activity equating to 7.3×106g PE/(mol Ti h) and 6.9×106g PE/(mol Ti h), respectively. It can be noticed from Table 1 that the obtained polyethylene samples feature broader MWDs in the range of 25.6—23.4. The influence of the amount of electron donor was also investigated in Table 1. As regards the samples ED1 and ED2, the Ti content of the catalysts shows a slight decrease when the molar ratio of [ED] to [MgCl2] decreases from 0.2 to 0.1, and the catalytic activity shows an obvious reduction (Cat .e1 and Cat .e4, Cat. e2 and Cat. e6 in Table 1). However, the catalytic activity becomes obviously lower when the molar ratio of [ED] to [MgCl2] increases from 0.2 to 0.3 (Cat. e1 and Cat. e5, Cat. e2 and Cat. e7 in Table 1). It is possible that the vacancies of Ti atoms are occupied by the excess ED, resulting in reduction of the active sites. It can be seen from Table 1 that the change in the molar ratio of [ED] to [MgCl2] from 0.3 to 0.1 makes a slight change in MWD, implying that the active centers are not mainly affected by the amount of ED.
3.2 Characterization of catalyst particles
The SEM images of Cat. e1 and Cat. e2 are shown in Figure 3. It can be found from Figure 3 that the catalyst particles are spherical in shape and uniform in size with a diameter of 10 μm—18 μm. According to the morphology duplication theory[17], the spherical catalyst particles can lead to spherical polymer particles. Spherical morphology of polyolefins is important in industrial application.

Figure 3 SEM images of catalyst particles
3.3 Characterization of polyethylene
Cat. e1 was used for ethylene polymerization under high pressure in order to obtain the expected morphology of polymer particles. The SEM images are given in Figure 4. It can be seen from Figure 4a that the polyethylene particles emerged in the form of blocks. The bulky polymer particles are composed of many small particles (Figure 4b), which has been explained in the literature[18]. Although the particles are not really spherical, the distribution in size is fairly uniform.
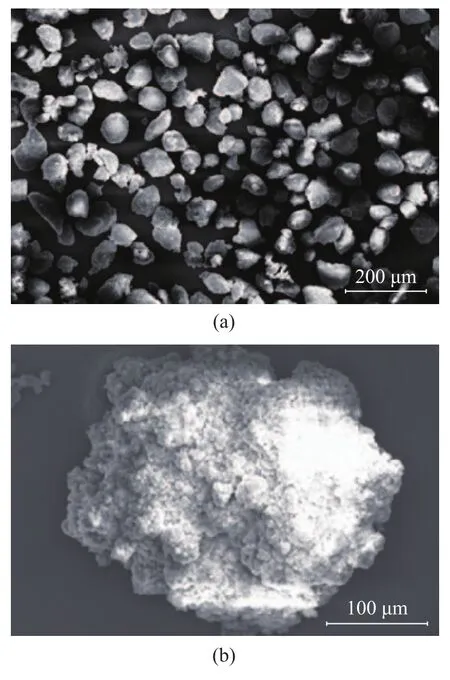
Figure 4 SEM images of polyethylene particles catalyzed by Cat. e1
GPC results are given in Figure 5. Figure 5 shows that the polyethylene catalyzed by Cat. e1 and Cat. e2 has broader MWD (MWD=25.6 and 23.4 in Figure 5a and 5b) than the polyethylene obtained over the catalyst without addition of ED (MWD=6.2 in Figure 5d). But the MWDs of these polyethylene samples catalyzed by Cat. e1 and Cat. e2 are narrower than that of polyethylene catalyzed by Cat. e3 (MWD=48.0 in Figure 5c). A possible coordina-tion mechanism was presented in previous report[19]. As regards the Cat. e1 and Cat. e2, these catalysts were prepared adopting two alkoxy silane compounds having two types of substituents, oxyethyl and cycloalkoxyl species. The alkoxy groups of the IEDs could be coordinated with the vacancies of Ti atoms, so that many kinds of active centers are produced.

Figure 5 GPC spectra of polyethylene catalyzed by Cat. e1-Cat. e4, Cat. e8 and Cat. e9 (in Table 1) (a) MWD=25.6 obtained over Cat. e1, (b) MWD=23.4 obtained over Cat. e2, (c) MWD=48.0 obtained over Cat. e3, (d) MWD=6.2 obtained over Cat. e9, (e) MWD=5.8 obtained over Cat. e8
4 Conclusions
This work reported the supported Ziegler-Natta catalysts coupled with novel IEDs for ethylene polymerization to form polymers featuring broader MWD. Based on the traditional Ziegler-Natta catalyst, two compounds of cycloalkoxy silane were synthesized and used as IEDs for ethylene polymerization. Through optimizing reaction conditions, the catalysts with activities reaching up to 6.9×106—7.3×106gPE/(mol Ti h) could produce polyethylene characteristic of a broader MWDs ranging from 23.4 to 25.6 when the EDs serving as IEDs were incorporated during the catalyst preparation process. But the amount of IED imposed a slight influence on the molecular weight distribution of the obtained polymer. These catalysts were effective for ethylene polymerization to produce PE100 product via the one-pot strategy.
Acknowledgements:We sincerely thank the National Natural Science Foundation of China (No. 21174011), the Natural Science Foundation of Beijing, (No. 2102036) and the PetroChina Innovation Fund (Grant No. 2011D-5006-0502).
[1] Heidemeyer P, Pfeiffer J. Special requirements on compounding technology for bimodal polyolefines and their industrial application[J]. Macromolecular Symposia, 2002, 181(1): 167-176
[2] Alt F P, Bohm L L, Enderle H F. Bimodal polyethylene-Interplay of catalyst and process[J]. Macromolecular Symposia, 2001, 163(1): 135-144
[3] Zacca J J, Debling J A, Ray W H. Reactor residence-time distribution effects on the multistage polymerization of olefins—II. Polymer properties: bimodal polypropylene and linear low-density polyethylene[J]. Chemical Engineering Science, 1997, 52(12): 1941-1967
[4] Lopez-Linares F, Barrios A D, Ortega H, et al. Toward the bimodality of polyethylene, initiated with a mixture of a Ziegler–Natta and a metallocene /MAO catalyst system[J]. Journal of Molecular Catalysis A: Chemical, 2000, 159(2): 269-272
[5] Cho H S, Chung J S, Lee W Y. Control of molecular weight distribution for polyethylene catalyzed over Ziegler–Natta/ metallocene hybrid and mixed catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2000, 159(2): 203-213
[6] Cho K, Lee B H, Hwang K M, et al. Rheological and mechanical properties in polyethylene blends[J]. Polymer Engineering & Science, 1998, 38(12): 1969-1975
[7] Premphet K, Paecharoenchai W. Polypropylene/metallocene ethylene-octene copolymer blends with a bimodal particle size distribution: Mechanical properties and their controlling factors[J]. Journal of Applied Polymer Science, 2002, 85(11): 2412-2418
[8] Zhang Y, Huang J L, Yang X X, et al. Some mixed cyclopentadienyl–indenyl zirconium complexes with PhCH2or PhCH2CH2substituents in ethylene polymerization[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2005, 43(6): 1261-1269
[9] Cho H S, Choi Y H, Lee W Y. Characteristics of ethylene polymerization over Ziegler–Natta/metallocene catalysts: Comparison between hybrid and mixed catalysts[J]. Catalysis Today, 2000, 63(2): 523-530
[10] Ali A H, Hsieh J T T, Kauffman K J, et al. Producing blown film and blends from bimodal high density high molecular weight film resin using magnesium oxide-supported Ziegler catalyst: The United States, US 5284613[P], 1994-02-08
[11] Wang L C, Ren H, Sun J Q. Fe(acac)3-bis(imino) pyridine/MAO: A new catalytic system for ethylene polymerization[J]. Journal of Applied Polymer Science, 2008, 108(1): 167-173
[12] Liu Z, Zhang X L, Huang H B, et al. Synthesis of (co-) polyethylene with broad molecular weight distribution by the heterogenous Ziegler–Natta catalysts via one-pot strategy[J]. Journal of Industrial and Engineering Chemistry, 2012, 18(6): 2217-2224
[13] Kong Y, Yi J J, Dou X L, et al. Heterogeneous Ziegler-Natta catalysts with different structure of ligands for the preparation of copolymer of ethylene and 1-octene with high comonomer incorporation. Polymer, 2010, 51(17): 3859-3866
[14] Huang Q G, Liu W J, Dou X L, et al. Olefin polymerization catalyst and preparation method: China, CN 201010186264.2[P], 2010-09-29
[15] Liu Z, Yu M S, Wang J, et al. Preparation and characterization of novel polyethylene/carbon nanotubes nanocomposites with core–shell structure[J]. Journal of Industrial and Engineering Chemistry, 10.1016/j.jiec.2012.08.034 (2013)
[16] Wang J, Yu M S, Jiang W H, et al. The preparation of nanosized polyethylene particles via carbon sphere nanotemplates[J]. Industrial & Engineering Chemistry Research, 2013, 52 (49): 17691-17694
[17] Huang Q G, Liu Z, Liu W J, et al. Preparation and self-assembly of global nano-particles of MgCl2-CH3CH2OH complex[J]. Acta Polymerica Sinica, 2012(8): 883-886 (in Chinese)
[18] Galli P, Luciani L, Gechi G. Advances in the polymerization of polyolefins with coordination catalysts[J]. Die Angewandte Macromolekulare Chemie, 1981, 94(1): 63-89
[19] Seppälä J V, Auer M. Factors affecting kinetics in slurry type coordination polymerization. Progress in Polymer Science, 1990,15(1): 147-176
Recieved date: 2013-12-24; Accepted date: 2014-2-28.
Huang Qigu, E-mail: huangqg@mail. buct.edu.cn.
- 中国炼油与石油化工的其它文章
- Deep Extractive Desulfurization of Gasoline with Ionic Liquids Based on Metal Halide
- Synthesis of Macro-Mesostructured γ-Al2O3with Large Pore Volume and High Surface Area by a Facile Secondary Reforming Method
- Performance of FCC Catalyst Improved with Vanadium Trapping Components
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 2. Single-Event Microkinetic (SEMK) Assessment
- Synthesis, Characterization and Evaluation of Sulfur Transfer Catalysts for FCC Flue Gas
- Selection of Chelated Fe (III)/Fe (II) Catalytic Oxidation Agents for Desulfurization Based on Iron Complexation Method

