Performance of FCC Catalyst Improved with Vanadium Trapping Components
Ren Fei; Liu Qianqian; Zhu Yuxia
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Performance of FCC Catalyst Improved with Vanadium Trapping Components
Ren Fei; Liu Qianqian; Zhu Yuxia
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
The vanadium species were contaminated on FCC catalysts by using the Mitchell method. After the hydrothermal deactivation of the FCC catalysts, the cracking reaction was performed on these catalyst samples. The test results revealed that the conversion of feedstock and the gasoline yield obtained over the FCC catalysts with vanadium trapping components were obviously higher than those without addition of vanadium trapping components. The results also showed that the dry gas and coke selectivity on the FCC catalysts containing vanadium trapping components was improved. The X-Ray diffraction results proved that the zeolite crystal structure was well protected by the vanadium trapping components during its hydrothermal deactivation step. The results of SEM-EDX mapping disclosed that the vanadium was enriched on the vanadium trapping components which verified the positive function of vanadium trapping components.
catalytic cracking; vanadium trapping components; distribution; vanadium poisoning
1 Introduction
During the operating cycles of “reaction-strippingregeneration”, FCC catalyst can be obviously poisoned by heavy metals such as vanadium and nickel elements most of which are present in the heavy feedstock or resid. The deposition of vanadium on the surface of FCC catalysts occurs with the cracking reaction, and it can block the pores of catalysts[1]. The blocked pores will prevent the diffusion of reactant molecules into the inner pores of zeolite, which will decrease the accessibility of the reactants to the active sites of the catalyst[2]. On the other hand, these vanadium components are deposited on catalysts during FCC process and show strong mobility after decomposition under the FCC catalyst regeneration condition, and they can destroy the zeolite framework by removing aluminum from the skeleton and thereby make the catalysts deactivated[3]. Research has been continuously aiming at reducing vanadium poisoning, and it has shown that the activity, selectivity and the metaltolerance ability of the catalyst can be improved by adding an appropriate amount of vanadium trapping components[4], and DTA results have shown that vanadium can be trapped by them to form components with high phase-transition temperature[5]. FCC catalysts containing vanadium trapping components were prepared, and the contaminant-vanadium was deposited on the catalysts by the Mitchell method. The mechanism for interaction between vanadium and vanadium trapping components was discussed based on the results by means of different characterization methods in this study.
2 Experimental
2.1 Samples preparation
Two FCC catalyst samples were selected for this study. Sample 1 was a fresh conventional bottom-cracking FCC catalyst originating from commercial production, sample 2 was the same catalyst which was blended with vanadium trapping components. Vanadium impregnation was performed on two catalyst samples according to the Mitchell method which used vanadium naphthenate dissolved in iso-octane, followed by drying and calcination. The physical and chemical properties of the vanadium contaminated sample 1 and sample 2 before deactivation are shown in Table 1.
Lab deactivation was performed according to the following procedure to simulate the E-cat performance. Thecontaminated samples 1 & 2 were deactivated at 700 ℃in the presence of 100% steam for 6 hours followed by treatment in ACE-Model D100 for two cycling process with alternative “oxidation-reduction” treatments. Then the simulated equilibrium catalyst samples were obtained and named as sample 3 and sample 4, respectively.

Table 1 Physical and chemical properties of sample 1 and sample 2
2.2 Characterization methods
The contents of vanadium in the samples were determined by the X-Ray fluorescence method. An X-ray diffraction instrument was utilized to determine the crystallinity and unit cell size of the samples. The distribution of vanadium on the surfaces of the samples was characterized by using scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) mapping techniques.
2.3 Catalytic performance testing
The micro-activity (MAT) of the catalyst samples was tested on the WFS-1D equipment provided with a fixed bed reactor operating at 460 ℃ using 5.0 g of catalyst and 1.56 g of feedstock. The feed injection time was 70 s.
The catalytic cracking performance of FCC catalyst was carried out in an advanced cracking evaluation (ACE) unit using 9.0 g of catalyst. A total of 2.24 g of feedstock with a density of 0.910 4 g/cm3and a CCR content of 3.1% (w) was injected to the system within 56 s. The reaction temperature was adjusted to 500 ℃. The composition of FCC gas product was analyzed by an online GC instrument, and the composition of liquid product was analyzed by an off-line GC instrument.
3 Results and Discussion
3.1 Cracking performance of catalyst samples
The micro-activity test and ACE results of sample 3 and sample 4 are listed in Table 2. The MAT results show that the micro-activity of sample 4 is higher than that of sample 3. The ACE results illustrate that the conversion and the yields of gasoline and LPG obtained on sample 4 are higher than those on sample 3. Meanwhile, the ACE results also show that the selectivity of dry gas and coke on sample 4 is better than that on sample 3. All the results mentioned above indicate that the catalyst containing vanadium trapping components shows better catalytic cracking property at a high content of vanadium contaminants, indicating that the FCC catalyst can be protected by vanadium trapping components.
3.2 Distribution of vanadium on the catalyst samples
The morphology and the elemental composition of the catalyst particle profiles were examined with a SEM and an X-ray EDS analyzer as referred to by Lapps[6], in which the techniques of spot analysis and line scanning were utilized to determine the variations in the content of the component elements. The results of vanadium profiles showed that the distribution of vanadium on different catalyst particles and different components of catalyst (e.g. zeolite, matrix and additives) can be observed clearly. Vanadium is enriched at the particle edges, especially in samples with low vanadium loadings (and consequently relatively low number of aging cycles), and tends to become progressively almost even with an increasing reaction-regeneration cycles. In this paper, the distribution of Al, Si, Mg and V elements in sample 3 and sample 4 wereexamined by means of SEM and EDX mapping techniques, and the results are shown in Figure 1 and Figure 2.
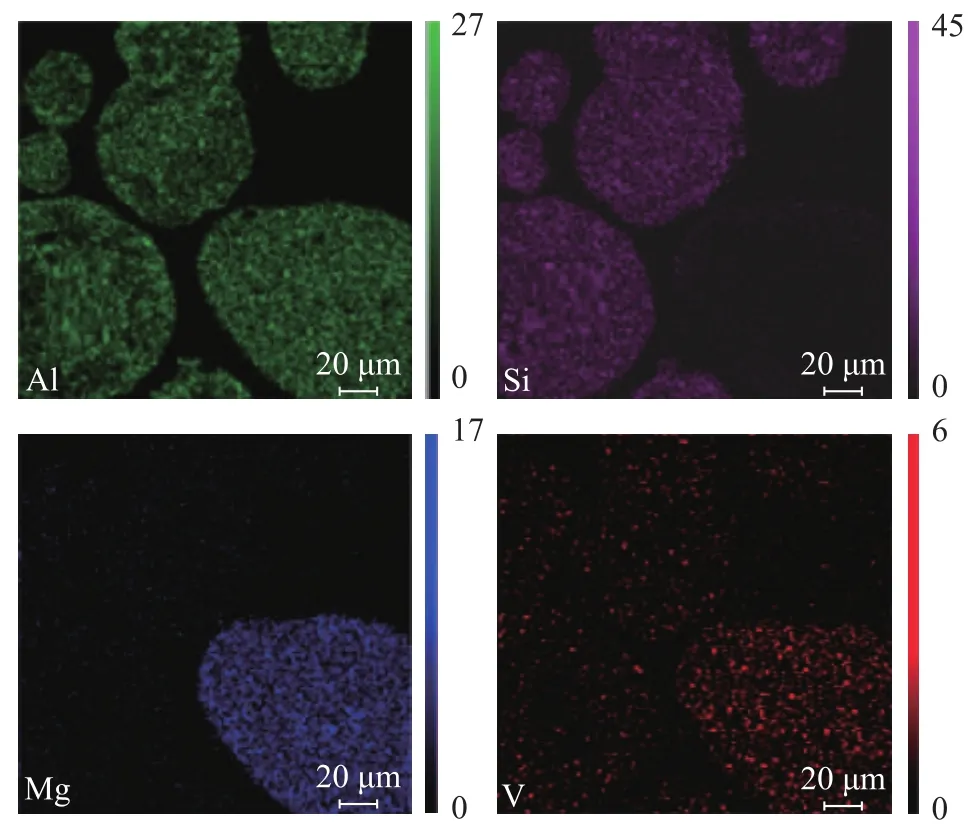
Figure 2 Mapping of the sample 4
It can be seen from Figure 1 that the distribution of Al, Si and V elements on catalyst profiles are nearly uniform, which is greatly different from that of Figure 2. The FCC catalyst particles and the vanadium trapping components particles can be distinguished by their difference in composition and the mapping results of Si and Mg elements. The particles with a much higher content of Si can be attributed to FCC catalyst particle, and the particles with a much higher content of Mg can be attributed to particles of vanadium trapping components. It can be seen from Figure 2 that the V content on the particles of vanadium trapping components is clearly much higher than that on the FCC catalyst particles.

Table 3 SEM-EDX analysis of sample 4
The contents of various elements in the catalyst and vanadium trapping components profiles of sample 4 are analyzed by SEM-EDX, with the results shown in Table 3. The vanadium content in the vanadium trapping components is 4.1%, which is much high than that in the main catalyst. Above results illustrate that most of the vanadium species deposited on samples 4 is mainly trapped by the vanadium trapping components so that the FCC catalyst is protected. Therefore, the cracking performance of sample 4 is improved by the addition of vanadium trapping components.
3.3 Mechanism of the reaction between V and V trapping components
It is very hard to observe the reaction process of vanadium and catalyst because the process is quite complicated and vanadium has different valence values and high mobility[7]. The ACE results and the EDX mapping results show that vanadium can be mostly trapped by vanadium trapping components, especially during the process of hydrothermal deactivation step, in which the steam reacts on vanadium oxides to form vanadic acid[8]. Vanadic acid is prone to volatilization at high temperature, but the vanadium trapping components can react upon it to form some V-containing compounds, which are very stable at the operating temperature in the regenerator, and this is the mechanism of vanadium trapping. The comparisonbetween the crystallinity and unit cell size of sample 1 and sample 2 before and after hydrothermal deactivation is illustrated in Figure 3. It can be seen that the decrease in unit cell size of sample 4 is much less than that of sample 3. Moreover, the crystallinity retention of sample 4 is much higher than that of sample 3. According to the results of Figure 3, it can be determined that the vanadium trapping components can counteract the dealumination of the zeolite framework by vanadium destruction to a large extent, which can be attributed to the compounds with high phase-transition temperature generated from the reaction of vanadium trapping components on vanadium species[5]. The mobility of vanadium species is remarkably decreased by formation of the compounds with high phase-transition temperature, so there is less chance for the vanadium species to further attack the zeolite framework.
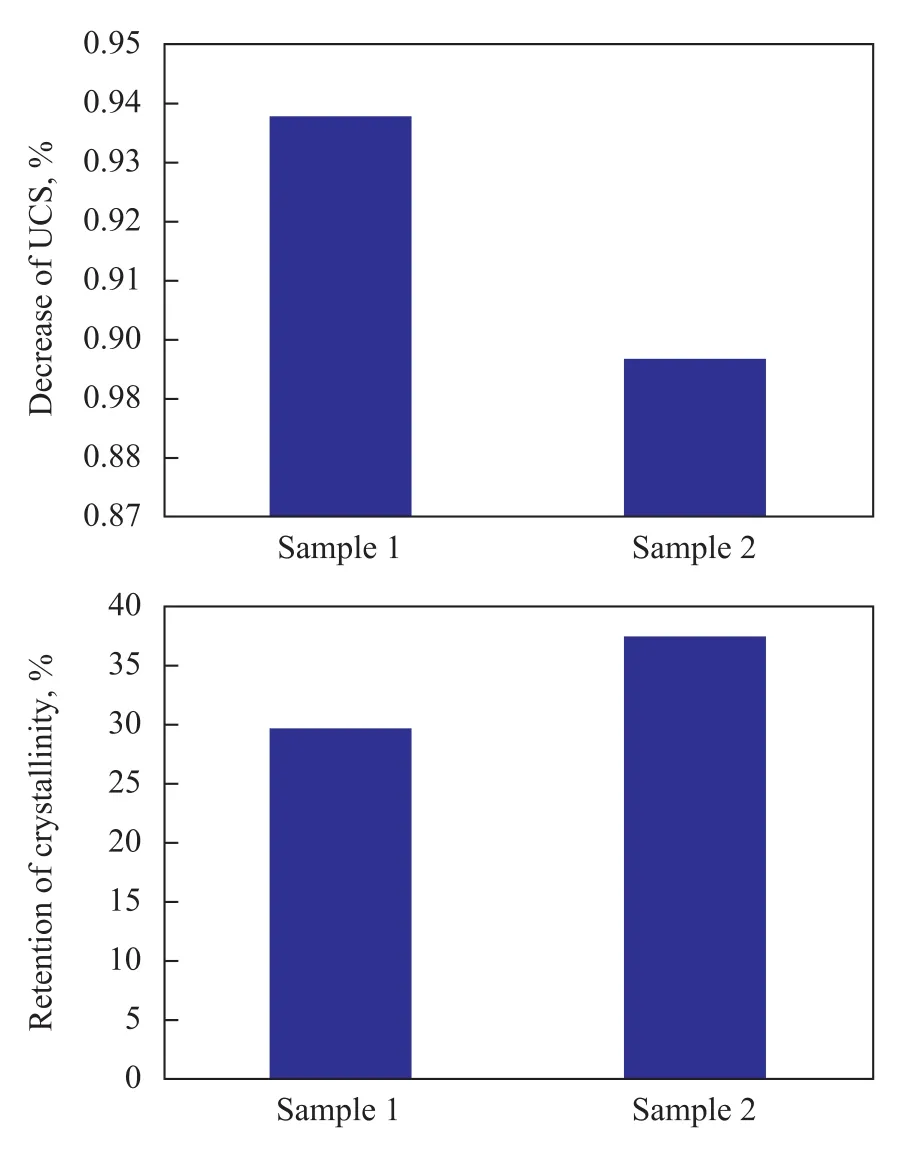
Figure 3 Decrease of UCS and retention of crystallinity for two catalyst samples.
4 Conclusions
(1) The MAT and ACE results have verified that in comparison with conventional FCC catalyst, the catalyst containing vanadium trapping components showed better catalytic cracking performance at a vanadium contamination level of 1.8%, which could be attributed to the function of vanadium trapping components.
(2) The results of SEM-EDX mapping and quantitative characterization of FCC catalysts indicated that vanadium could be mostly captured by the vanadium trapping components. The XRD results revealed that vanadium trapping components could counteract to a large extent the dealumination of the zeolite framework inflicted upon by vanadium destruction.
Acknowledgement:The project was supported by the National Basic Research Development Program “973” Project of China(2010CB732301) and the SINOPEC Research and Development Program(No.112034)
Reference
[1] Liu Yujian, Long Jun, Zhu Yuxia, et al. Temperatureprogrammed reduction characterization of vanadium oxides with different oxidation numbers and vanadium deposits on FCC catalyst[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2005, 21(5): 28-35 (in Chinese)
[2] Escobar A S, Pereira M M, Pimenta R D M, et al. Interaction between Ni and V with USHY and rare earth HY zeolite during hydrothermal deactivation [J]. Appl Catal, 2005, 286: 196-201
[3] Du Xiaohui, Tang Zhicheng, Zhang Haitao, et al. Effect of vanadium and nickel contamination on the property of FCC catalyst [J]. Chemical Engineering & Equipment, 2011 (5): 1-5 (in Chinese)
[4] Li Xiao, Qian Feng, Huang Jiazhen, et al. Study on the vanadium poisoning & V-trap in FCC catalyst II. Employing inorganic minerals & rare earth metals as V-trap [J]. Journal of East China University of Science and Technology, 2000, 26(3): 269-273 (in Chinese)
[5] Zhu Yuxia, Wang Xieqing. Study on thermal stability of vanadate [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2003, 19(3): 78-82 (in Chinese)
[6] Lappas A A, Nalbandian L, Iatridis D K, et al. Effect of metals poisoning on FCC products yields: Studies in an FCC short contact time pilot plant unit [J]. Catalysis Today, 2001, 65: 233-240
[7] Shen Yanfei, Suib S L, Occelli M L. Vanadium migration between model components of fluid cracking catalysts: SEMEDX studies [J]. ACS Symposium Series, 1993, 517: 185-203
[8] Yu Jiyong, Lu Shanxiang, Chen Hui. Study on vanadiumpoisoning affecting catalysts in FCC and vanadium-traps and their applications [J]. Modern Chemical Industry, 2007, 27(S1): 60-64 (in Chinese)
Recieved date: 2013-11-08; Accepted date: 2014-2-12.
Prof. Zhu Yuxia, Telephone: +86-10-82368233; E-mail: zhuyuxia.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
- Deep Extractive Desulfurization of Gasoline with Ionic Liquids Based on Metal Halide
- Synthesis of Macro-Mesostructured γ-Al2O3with Large Pore Volume and High Surface Area by a Facile Secondary Reforming Method
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 2. Single-Event Microkinetic (SEMK) Assessment
- Synthesis, Characterization and Evaluation of Sulfur Transfer Catalysts for FCC Flue Gas
- Selection of Chelated Fe (III)/Fe (II) Catalytic Oxidation Agents for Desulfurization Based on Iron Complexation Method

