Synthesis of Macro-Mesostructured γ-Al2O3with Large Pore Volume and High Surface Area by a Facile Secondary Reforming Method
Meng Xiuhong; Duan Linhai; Xie Xiaohua; Wang Qiang; Wang Haiyan
(1. College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001; 2. College of Chemical Engineering, China University of Petroleum, Qingdao 266555; 3. College of Environmental Science and Engineering, Beijing Forestry University, Beijing 100083)
Synthesis of Macro-Mesostructured γ-Al2O3with Large Pore Volume and High Surface Area by a Facile Secondary Reforming Method
Meng Xiuhong1,2; Duan Linhai2; Xie Xiaohua2; Wang Qiang3; Wang Haiyan1,2
(1. College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001; 2. College of Chemical Engineering, China University of Petroleum, Qingdao 266555; 3. College of Environmental Science and Engineering, Beijing Forestry University, Beijing 100083)
Through improving the aging process during synthesis of the support, γ-Al2O3with large pore volume and high surface area was synthesized by a facile secondary reforming method. The synthesis parameters, such as the reaction temperature, the first aging temperature and the second aging temperature, were investigated. The textural properties of γ-Al2O3were characterized by means of N2adsorption-desorption isotherms, X-ray powder diffractometry (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy and thermogravimetry (TG). The experimental results indicated that AACH and amorphous AlOOH were the precursors of alumina, which were formed via precipitation from solutions after reaction of aluminum sulphate with ammonium hydrogen carbonate. The precursor nanocrystallites grew and re-assembled during the secondary reforming process, which resulted in an increased pore size and pore volume and a decreased bulk density. The as-synthesized γ-Al2O3materials featured meso/macroporosity, large pore volume (2.175 cm3/g), high surface area (237.8 m2/g), and low bulk density (0.284 g/mL).
γ-Al2O3; macro-mesostructured; reforming method; large pore volume; high surface area.
1 Introduction
γ-Al2O3has important applications as industrial catalyst support, catalyst, adsorbent or ceramic raw material because of its low cost, high surface area and porosity, good thermal stability, high mechanical strength and extensive variability of acid-base properties[1-3]. Macro-mesostructured γ-Al2O3support is important and useful for the treatment of bulky molecules, such as heavy petroleum fractions and residue, coal liquids, etc.[4]However, along with increasing the pore volumes, the specific surface areas usually decrease for most of the previously reported alumina samples, which is undesirable for their catalytic applications. For example, H. S. Potdar[5]successfully synthesized two kinds of γ-Al2O3by a precipitation/digestion route, the first one had a pore volume of 0.377 cm3/g and a surface area of 261 m2/g, and the second one possessed a pore volume of 1.02 cm3/g and a surface area of 133.9 m2/g. Thus, in order to prepare the macro-mesostructured γ-Al2O3, several attempts such as introducing pore forming additives[6], using the surfactant as template, and adopting hydrothermal or glycothermal treatment[7-9]have been implemented.
Ammonium aluminium carbonate hydroxide (AACH, with formula NH4Al(OH)2CO3has a structure similar to the mineral dawsonite (NaAl(OH)2CO3), which is composed of the octahedral structure of AlO2(OH)4[10-11]. Because AACH can be simply transformed into aluminium oxide with different crystal structures at a moderate thermal treatment temperature, it could be a promising precursor for synthesis of aluminium oxide with special properties. AACH is typically prepared by precipitation of aqueous solutions of aluminium salts (NH4Al(SO4)2, Al(NO3)3, AlCl3) or Al(OH)3suspension with aqueous solutions of (NH4)2CO3or NH4HCO3[12-17]. (NH4)2CO3orNH4HCO3possess a particular bulky functional group which can change the pore size merely by physical separation of particles the “steric” or “spatial” filling factor. The addition of (NH4)2CO3or NH4HCO3to a boehmite gel before calcination can result in the development of a well-defined pore structure in the range of 2—15 nm, but the pore volume is ca. 1.0 cm3/g. It seems more likely that either microcrystalline inclusions of the ammonium salts (NH4
+and HCO3-) in the crystal are removed to leave voids with appropriate size or the ammonium salts favour the formation of agglomerates of such size. However, due to the low crystallinity and small size of AACH particles reported in the literature, it was difficult to obtain the meso/macroporous γ-Al2O3materials with large pore volume for processing heavy oil in petrochemical industry. Li G. C.[18]prepared rod-like AACH via a facile hydrothermal method, while the obtained γ-Al2O3materials possessed meso/macroporosity, but the pore volumes were below 0.85 cm3/g. Therefore, it is extremely worthwhile to find a method for preparing highly crystalline AACH with large crystal size.
Herein, we report a novel method for preparation of meso/ macroporous γ-Al2O3via a facile secondary reforming. The reaction parameters (the reaction temperature, the first aging temperature and the second aging temperature, and the aging time) during the synthesis of AACH were studied. Both AACH and aluminium oxide were characterized by N2adsorption-desorption, XRD, SEM, FT-IR, TG, and other methods. The well-ordered alumina material prepared from our starting materials had meso/ macroporous porosity with super large pore volume, high surface area, low bulk density and uniform connecting pore size, which is an attractive feature for making potential catalysts and filter materials.
2 Experimental
2.1 Preparation procedures
Synthesis of AACH was carried out in a reactor equipped with a mechanical stirrer and a temperature controller. The starting materials for the synthesis of AACH covered AS (Al2(SO4)3·18H2O, provided by Sinopharm) and AHC (NH4HCO3, provided by Sinopharm). AACH was prepared by simultaneously adding dropwise aqueous solutions of AS (0.5 mol/L) and AHC (NH4HCO3, 1.5 mol/L) at a flow rate of 5 mL/min. The molar ratio of NH4HCO3/Al was 6, and the pH value of the solution was controlled at around 8.5 by using aqueous NH4OH, and the reaction temperature was controlled at 65 ℃, 75 ℃, or 85 ℃. Then the precipitate was subjected to aging for one hour at different temperatures (T=50, 60, 70, or 80 ℃) in the first aging process. The precipitates aged once were filtered and washed several times with hot water until no more sulfate ion was detected in the effluent (by means of barium sulfate test) prior to drying at 40 ℃ for 24 h. This method could obtain the as-dried samples by precipitation (once aging) and was named as the ‘once aging method’ in this paper.
Then the as-dried sample was re-dispersed in a dilute AHC solution (at an NH4HCO3/Al molar ratio of 0.75), and the suspension was aged for two hours at different temperatures (T=70, 80, or 90 ℃) in the second aging process. The samples aged twice were then filtered, washed and dried at 40 ℃ for 24 h. This method was named as the “secondary reforming method” in this paper. All the samples prepared by once aging method and “secondary reforming method” were calcined in a programmable furnace at 650 ℃for 5 h in air at a heating rate of 2 ℃/min.
2.2 Characterizations
To identify phases and their crystallinity of as-synthesized materials, X-ray diffraction (XRD) studies were carried out with a Rigaku diffractometer using Cu Kα radiation. The microstructures of calcined samples were studied by scanning electron microscope (SEM) with images obtained by a Hitachi S-4800 instrument (field emission scanning electron microscope) operated at 5.0 kV. The BET surface areas and pore volumes were estimated from nitrogen adsorption and desorption isotherm data obtained at 77 K on a constant-volume adsorption apparatus (Micromeritics, type ASAP-2400). The pore volumes were determined at a relative pressure (p/p0) of 0.99. The pore size distribution and average diameter of calcined materials were verified by BJH (Barrett-Joyner-Halenda) model from the desorption branches of the nitrogen isotherm. The Fourier transform infrared (FT-IR) spectroscopy was carried out in a Bruker Optics Tensor 27 spectrometer equipped with a Golden Gate Diamond ATR unit. Spectra were collected at 298 K in the range of 400—4 000 cm-1by co-addition of 32 scans at a nominal resolution of 4 cm-1by taking the spectrum of the empty cell at ambientconditions as the background. TG was carried out in a Netzsch STA 449 C analyzer, while increasing the temperature from 50 ℃ to 500 ℃ at a heating rate of 10 K/min.
3 Results and Discussion
Hydrolysis of AHC in aqueous medium generates OH-ions to obtain the precipitate. The as-obtained precipitate can be converted into crystalline AlOOH precursor by reaction (1) during the ripening and drying process. Under alkaline condition, basic AlO(OH)2-ions are generated by reaction (2), which is the controlling step for preparing AACH. With an increasing [NH4HCO3]/[Al2(SO4)3] molar ratio (>3) or pH value (>9), AACH will be readily formed by reaction (3)[5]. The crystallites of AlOOH and AACH can be transformed to the phase of γ-Al2O3by reactions (4) and (5) when they are calcined at 650 ℃.
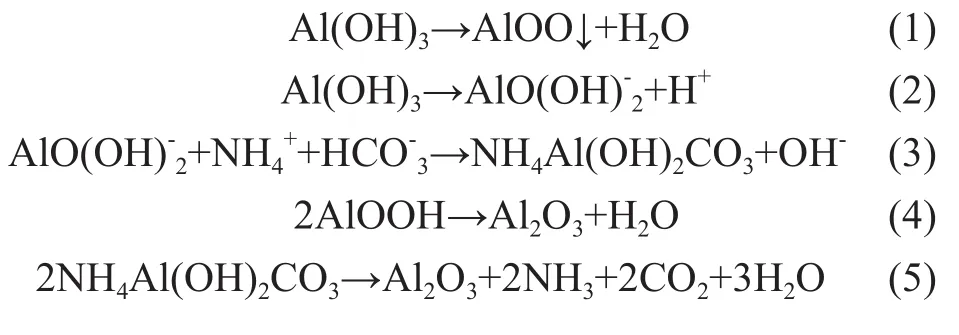
3.1 Effect of reaction temperature on texture properties of aluminas
Nitrogen adsorption-desorption isotherms and pore distribution of the alumina samples are shown in Figure 1. It can be seen that both of the samples exhibit the shape of a classical isotherm of type IV according to the IUPAC classification. The existence of the hysteresis loops in the isotherms is ascribed to the capillary condensation of N2gas occurring in the pores[19]. Their hysteresis loops are of type H2, suggesting that the as-prepared alumina samples have heterogeneous pore size distribution and non-uniform particle size. There are two hysteresis loops in all isotherms, which is consistent with a double pores distribution (Figure 1(b)). It can be seen that the smaller pores bear a concentrated distribution and the larger pores display a broad distribution. Such difference is attributed to different pore formation mechanisms. Aluminum species are connected with hydrogen bond after removal of water between the crystal planes, and the smaller pores are formed to show their concentrated distribution pattern. The pores with large size are determined by AACH which is an intermediate product in the synthesis of the alumina. During the calcination process, AACH can be decomposed to release the gases, such as CO2, NH3and H2O, and the diameters of some primary smaller mesopores are broadened owing to the release of these gases.

Figure 1 (a) Isotherms and (b) pore diameter distribution of alumina prepared with different temperature
Table 1 lists the textural properties of calcined samples at different reaction temperatures, and both the first and second aging temperatures are set at 70 ℃. It shows that the reaction temperature can affect the modal pore diameter of alumina significantly. The sample prepared at a reaction temperature of 75 ℃ has a larger pore volume of 2.175 cm3/g with an average pore diameter of 36.6 nm, which is larger than that of samples prepared at the reaction temperature of 65 ℃ and 85 ℃ (equating to 1.331 cm3/g and 0.784 cm3/g, respectively) and that of the conventional γ-Al2O3(ca. 1.0 cm3/g). Other studies[20-21]showed that the more the amount of AACH was formed in the meso-structure, the more large pores and greater pore volume could be obtained. Under this experimental condition, the aluminum sulfate solution and the precipitants (NH4HCO3and NH4OH) were broughtinto the system drop by drop, while the precipitates increased continuously with the particle size distribution of alumina narrowed (the small pores), and these results were confirmed by N2adsorption. At low reaction temperature (65 ℃), the nucleation rate was greater than the growth rate, so the proportion of small pores was large. On the contrary, at high reaction temperature (85 ℃), the growth rate was greater than the nucleation rate, so the proportion of small pores decreased and the particle size increased (Figure 1(b)). Surprisingly, both the average pore diameters and pore volumes of samples prepared at reaction temperature of 65 ℃ and 85 ℃ were all smaller than those of the sample prepared at 75 ℃. The reason may be that mild reaction condition was beneficial to both the nucleation rate and the growth rate of AACH particles. In addition, large irregularly shaped particles could be expected to be packed loosely, leaving considerable voidage between the particles, so that the BET surface area (237.8 m2/g) and bulk density (0.284 g/mL) of the alumina prepared at 75 ℃ were smaller.

Table 1 Texture properties of alumina samples prepared at different reaction temperatures
3.2 Effect of the first aging temperature on texture properties of alumina samples
The precipitates after entering into reaction for 1 h were aged at a certain temperature to homogenize the gel because of the slow ripening process, and the aging step was essential for converting the precipitates to crystalline AACH precursor[5]. Table 2 lists the properties of alumina samples prepared at different first aging tempretures, while the reaction temperature and second aging temperature are equal to 75 ℃ and 70 ℃, respectively. It can be seen that when using AACH as a precursor, the pore volumes, pore diameters and BET surface areas actually increased and bulk densities decreased with an increasing first aging temperature (from 50 ℃ to 70 ℃). The corresponding N2adsorption and desorption isotherms and pore diameter distribution are shown in Figure 2. It can be seen that during aging process, the size of the primary particles of AACH increased due to the Ostwald ripening[22]. The Ostwald ripening means that the number of smaller particles continues to shrink, while larger particles continue to grow. So it is recognized that the volume and the size of the small pores increased. This increase was also due, at least partially, to further removal of the NH3and CO2at these temperatures along with the formation of more void space. But when the first aging temperature increased from 70 ℃ to 80 ℃, all the texture properties were still untouched, suggesting that 70 ℃ is an optimal temperature for homogenizing the gel stably.
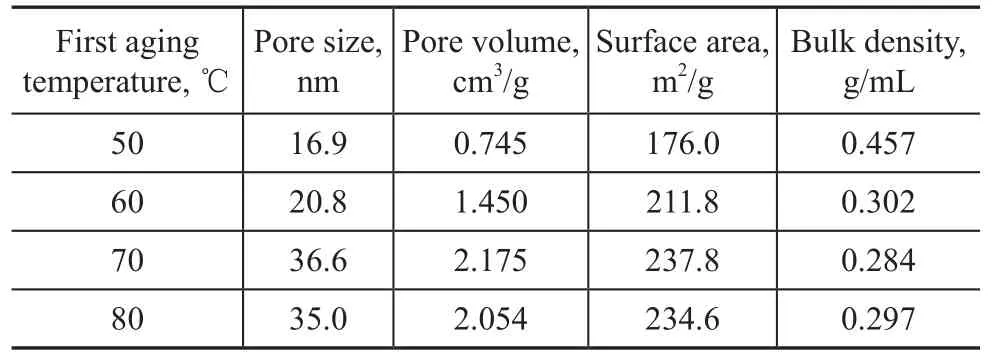
Table 2 Texture properties of alumina samples prepared at different first aging temperatures
3.3 Effect of the second aging temperature on texture properties of alumina samples
Table 3 lists the properties of alumina samples prepared at different second aging temperatures, while the reaction temperature and the first aging temperature are equal to 75 ℃ and 70 ℃, respectively. It is interesting that all the samples prepared at different second aging temperatures show approximately similar surface area, pore volume, pore diameter and bulk density, suggesting that 70 ℃ is an optimal temperature in the second aging process. The corresponding N2adsorption and desorption isotherms and pore diameter distribution of the samples are shown in Figure 3.
3.4 Effect of the second aging process on texture properties of alumina samples
The as-synthesized sample and calcined sample prepared by once aging method are labeled as A-1 and C-1, respectively. Correspondingly, the as-synthesized sample and calcined sample prepared by the secondary reforming method are labeled as A-2 and C-2, respectively. Figures 4(a) and (b) show the isotherms and pore distribution of alumina samples C-1 and C-2. There is one hysteresis loop in the isotherms of sample C-1, which is consistent with one overlapping broad peaks of mesopore and macropore distribution. Meanwhile, the sample C-2 has double apparent peaks. Table 4 lists the properties of the alumina samples prepared by the once aging method and the secondary reforming method at the same reaction temperature (75 ℃) and a same first aging temperature of 70 ℃, with the second aging temperature equating to 70 ℃. It can be seen from Table 4 that the texture properties of sampleC-1 and sample C-2 are markedly different. The pore volume, specific surface area, pore size and bulk density of γ-Al2O3obtained by the once aging method is 1.16 cm3/g, 253.3 m2/g, 18.0 nm and 0.464 g/mL, respectively, which agrees well with or surpass those of the conventional alumina sample[2,5,23]. The sample C-2 has a large pore volume (2.175 cm3/g) and average pore size (36.6 nm), which are much larger than those of the sample C-1 (1.16 cm3/g and 18.0 nm), and greatly surpass those of the conventional alumina. The bulk density of sample C-2 (0.284 g/mL) is much lower than that of C-1 (0.464 g/mL). The sample C-2 also maintains a high BET surface area of 237.8 m2/g. It means that the second aging process plays an important role in the crystal growth, resulting in large pore size and pore volume, and small bulk density.

Figure 4 (a) Isotherms and (b) pore diameter distribution of alumina prepared with different second aging temperature

Table 4 Properties of alumina samples prepared by either the once aging method or the twice aging method
The samples were characterized by the FT-IR, XRD, TG and SEM techniques in order to further identify the different characteristics of the samples prepared by either the once aging method or the secondary reforming method. The XRD patterns of alumina samples A-1 and A-2 (Figure 5) show that both of them bear the phase of AACH (JCPDS 01-076-1923). The corresponding FT-IR spectra of sample A-1 and A-2 are identified (Figure 6). It can be seen clearly that both of the FT-IR spectra are very similar and show a broad adsorption band at 3 420 cm-1which is attributed to OH stretching in AlOOH, and a band at 1 640 cm-1corresponding to OH bending. The Al-O-Al bending stretching vibrations observed at 1 073 cm-1and a shoulder at 1 163 cm-1are formed due to symmetric and asymmetric bending modes, respectively. The NH4
+bending stretching vibrations observed at 3 016—3 174 cm-1overlap the stretching mode of adsorbed water. The CO32-asymmetric stretching mode is observed at around 1 452—1 539 cm-1. The asymmetric stretching mode observed at around 635 cm-1is assigned to Al–O stretching vibrations. These fingerprints are the unequivocal evidence for the presence of AACH and amorphous AlOOH corresponding to the amorphous species anticipated by thermogravimetry, which is also in good agreement with XRD analyses because no apparent peaks appear in the XRD pattern of amorphous AlOOH[24]. Our assignment is based on the infrared characterization communicated by several authors[23,25], who explicitly reported the amorphous nature of this compound.

Figure 5 XRD patterns of sample A-1 and A-2
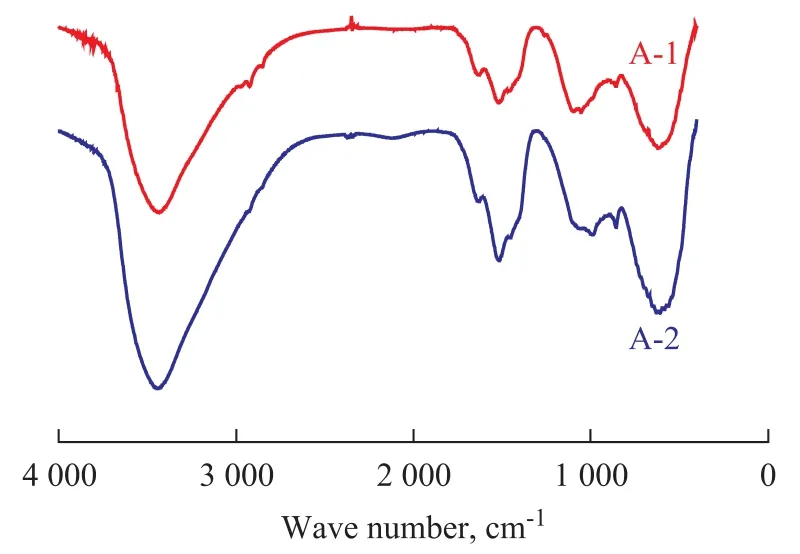
Figure 6 FT-IR spectras of sample A-1 and A-2
In addition, the thermal behaviors of sample A-1 and A-2 were studied by TG and DTG as depicted in Figure 7. It indicates that dehydration processes occur gradually. It is assumed that if only AACH phase is precipitated by reaction (3) relating to samples A-1 and A-2, a total weight loss of about 63.3% would have been expected due to reaction (5). If samples A-1 and A-2 only contained amorphous AlOOH phase formed by reaction (2), then in this case, a total theoretical weight loss of about 15% would have been expected due to reaction (4). However, a total weight loss of 35.8% and 41.2% is determined from the TG curves of sample A-1 and A-2, respectively, suggesting that the as-dried precursor consists of amorphous AlOOH and AACH, which is in good agreementwith the FT-IR and XRD results. It is confirmed[26]that the precipitation of basic salts occurs rapidly after mixing with the counter anions in the presence of anions that exhibit strong af finity for Al, such as sulphate, phosphate and silicate. Because of the af finity for Al species, these anions can prevent or at least retard the A1-OH polymers from further hydrolysis and polymerization into crystalline AACH. Therefore, a part of the basic salts formed is amorphous in nature. The amorphous AlOOH would obviously be present as small particles and could lead to small-pored activated alumina upon calcination, so it can be safely concluded that the AACH is readily generated by reaction (3) under these experimental conditions of a high pH value (8.5) and a high molar ratio of NH4HCO3/Al (6) accompanied by the formation of amorphous AlOOH in reaction (1). It can be seen that the weight loss of sample A-2 (41.2%) was larger than that of sample A-1 (35.8%), suggesting that more amount of AACH existed in the sample A-2 than in the sample A-1. It can be seen from the DTG curves in Figure 7 that the major weight loss took place below 270 ℃, which originated from two events, one was the decomposition of AACH according to the literature information[20,27-28], indicating thatin samples associated by weaker bonds were easily decomposed, the other event was desorption of the physically adsorbed water in the pores of amorphous AlOOH[24]and AACH. Furthermore, the slow weight loss of samples above 300 ℃ was ascribed to the decomposition of AlOOH in the composition of alumina crystallites.
Figure 8 indicates the SEM images of samples C-1 and C-2. The structure of AACH crystal is composed of chains of Al–O octahedrons[15]linked by shared oxygen atoms with strong covalent bonds, andare located between the chains associated by weak bonds, such as hydrogen bonds. During the decomposition of AACH, the chains of Al–O cannot be broken, and only the bonds associated withare broken with the emission of gases of CO2, NH3, and H2O, then leaving gaps between the chains, resulting in many disordered and nonuniform pores ranging from several nanometers to several hundred nanometers in the morphologies of samples. The two kinds of alumina samples all have broad pore size distribution in the large pore sections, which is in agreement with the results obtained by N2adsorption/ desorption isotherms. It is obvious that the morphology of the samples changed from tiny nanofibers to large stick-like particles measuring over 100 nm in length and tens of nanometers in width. The sample C-1 was fluffy and flocculent with piled up tiny fibers. The morphology of the sample C-2 with irregular stick-like particles was short, thick and was much larger than that of the sample C-1 because the pH value in this system (8.5) induced nanocrystallites to acquire negative charges due to its isoelectric point of 9.2[19]. In our secondary reforming method, because the sulfate ions that exhibit strong af finity for Al species have been removed by washing with hot water following the first aging process, thus, in the second aging process,ions can easily be adsorbed on the surface of Al species through electrostatic force, which can greatly contribute to the reassembly of AACH nanocrystallites to form a multilayer structure[3]. The reassem-bly of AACH evolves toward a more thermodynamically favored acicular crystals characteristic of the dawsonite mineral[29-30]. As a result, these nanocrystallites can grow and assemble along a preferential direction to form sticklike AACH particles.
4 Conclusions
A simple and effective way to synthesize γ-Al2O3with super large pore volume, low bulk density and high surface area was systematically elaborated. AACH and amorphous AlOOH were the precursors of alumina, which formed via precipitation form the solution reaction of aluminum sulphate on ammonium hydrogen carbonate. The amorphous AlOOH would obviously be present as small particles and could lead to small-pored activated alumina upon calcination. AACH can be decomposed to discharge the gases such as CO2, NH3and H2O during the calcination process, which can lead to the formation of large pored alumina. The sample prepared by the secondary reforming method has a greater proportion of AACH than that prepared by the once aging method. After calcination, the as-synthesized alumina by the secondary reforming method (sample C-2) has more large pores than that formed by the once aging method (sample C-1).
The reaction temperature and the first aging temperature have significant effect on textural properties of γ-Al2O3, especially the porosity volume and pore size of small pores, but the temperature in the second aging process has a slight effect on the properties of γ-Al2O3. The texture properties of sample C-1 and sample C-2 are also markedly different. The pore volume, specific surface area, pore size and bulk density of sample C-1 is equal to 1.16 cm3/g, 253.3 m2/g, 18.0 nm, and 0.464 g/mL, respectively, which are in agreement with those of conventional alumina. The sample C-2 has a larger pore volume (2.175 cm3/g) and average pore size (36.6 nm), and lower bulk density (0.284 g/mL) as compared to those of the sample C-1, and it also maintains a high BET surface area (237.8 m2/g). The morphology of the samples changed from tiny nanofibers (sample C-1) to large stick-like particles (C-2) measuring over 100 nm in length and tens of nanometers in width. All the test results imply that the secondary reforming method plays an important role in the growth and reassembly of AACH nanocrystallites, which results in a large pore size and pore volume, and small bulk density. Our test results can be of significance to improve the mass transfer (adsorption and diffusion) property of sorbates in alumina due to the great macroporosity distribution, especially for the large molecules, which may have potential application in heavy oil catalytic cracking process.
Acknowledgement:The authors are pleased to acknowledge the financial support by the Natural Science Foundation of Liaoning Province of China (Grant No. 2013020122), the National Natural Science Foundationof China (Grant No. 21076100 and 51308045), and the financial support by the PetroChina Company Limited (Grant No. 10-01A-01-01-01).
Reference
[1] Zhang Z, Pinnavaia T J. Mesostructured γ-Al2O3with a lathlike framework morphology[J]. J Am Chem Soc, 2002, 124(41): 12294-12301
[2] Krokidis X, Raybaud P, Gobichon A E, et al. Theoretical study of the dehydration process of boehmite to γ-alumina[J]. J Phys Chem B, 2001, 105(22): 5121-5130
[3] Guzmán-Castillo M, Bokhimi X, Toledo-Antonio A, et al. Effect of boehmite crystallite size and steaming on alumina properties[J]. J Phys Chem B,, 2001, 105(11): 2099-2106
[4] Blin J L, Léonard A, Yuan Z Y, et al. Hierarchically mesoporous/macroporous metal oxides templated from polyethylene oxide surfactant assemblies[J]. Angew Chem, 2003, 42(25): 2872-2875
[5] Potdar H, Jun K W, Bae J W, et al. Synthesis of nano-sized porous γ-alumina powder via a precipitation/digestion route[J]. Appl Catal A: General, 2007, 321(2): 109-116
[6] Trimm D, Stanislaus A. The control of pore size in alumina catalyst supports: A review[J]. Appl Catal, 1986, 21(2): 215-238
[7] Stanislaus A, Al-Dolama K, Absi-Halabi M. Preparation of a large pore alumina-based HDM catalyst by hydrothermal treatment and studies on pore enlargement mechanism[J]. J Mol Catal A: Chemical, 2002, 181(1/2): 33-39
[8] Inoue M, Kominami H, Inui T. Synthesis of large pore-size and large pore-volume aluminas by glycothermal treatment of aluminium alkoxide and subsequent calcination[J]. J Mater Sci, 1994, 29(9): 2459-2466
[9] Zhu Z, Liu H, Sun H, et al. PEG-directed hydrothermal syn-thesis of multilayered alumina microfibers with mesoporous structures[J]. Micropor and Mesopor Mater, 2009, 123(1/3): 39-44
[10] Frueh A, Golightly J. The crystal structure of dawsonite NaAl(CO3)(OH)2[J]. The Canadian Mineralogist, 1967, 9(1): 51-56
[11] Li Z, Feng X, Yao H, Guo X. Ultrafine alumina powders derived from ammonium aluminum carbonate hydroxide[J]. J Mater Sci, 2004, 39(6): 2267-2269
[12] Morinaga K, Torikai T, Nakagawa K, et al. Fabrication of fine α-alumina powders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH) [J]. Acta Materialia, 2000, 48(18/19): 4735-4741
[13] Kim S W, Shin D C. Fabrication and synthesis of α-alumina nanopowders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH) [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 313(1): 415-418
[14] Santiago M, Yalfani M S, Pérez-Ramírez J. In-line dispersion–precipitation method for the synthesis of metalsubstituted dawsonites. Genesis of oxide materials with superior properties[J]. J Mater Chem, 2006, 16(28): 2886-2889
[15] Ma C C, Zhou X X, Xu X, et al. Synthesis and thermal decomposition of ammonium aluminum carbonate hydroxide (AACH)[J]. Mater Chem Phys, 2001, 72(3): 374-379
[16] Zhang X, Wen Z, Gu Z, et al. Hydrothermal synthesis and thermodynamic analysis of dawsonite-type compounds[J]. J Solid State Chem, 2004, 177(3): 849-855
[17] Stoica G, Pérez-Ramírez J. Reforming dawsonite by memory effect of AACH-derived aluminas[J]. Chem of Mater, 2007, 19(19): 4783-4790
[18] Li G C, Liu Y Q, Guan L L, et al. Meso/macroporous γ-Al2O3fabricated by thermal decomposition of nanorods ammonium aluminium carbonate hydroxide[J]. Mater Res Bull, 2012, 47(4): 1073-1079
[19] Wu Y S, Ma J, Li M C, et al. Synthesis of γ-Al2O3with high surface area and large pore volume by reverse precipitation-azeotropic distillation method[J]. Chemical Research in Chinese Universities, 2013, 29(2): 1-4
[20] Vogel R, Marcelin G, Kehl W. The preparation of controlled pore alumina[J]. Appl Catal, 1984, 12(2): 237-248
[21] Liu X M, Xue H X, Li X, et al. Synthesis and hydrodesulfurization performance of hierarchical mesoporous alumina[J]. Catal Today, 2010, 158(3/4): 446-451
[22] Chuah G, Jaenicke S, Xu T. The effect of digestion on the surface area and porosity of alumina[J]. Micropor and Mesopor Mater, 2000, 37(3): 345-353
[23] Scholtz E C, Feldkamp J R, White J L, et al. Properties of carbonate-containing aluminum hydroxide produced by precipitation at constant pH[J]. J Pharm Sci, 1984, 73(7): 967-973
[24] Hong T L, Liu H T, Yeh C T, et al. Electron microscopic studies on pore structure of alumina[J]. Appl Catal A: General, 1997, 158(1/2): 257-271
[25] Serna C J, White J L, Hem S L. Structural survey of carbonate-containing antacids[J]. J Pharm Sci, 1978, 67(3): 324-327
[26] Prodromou K, Pavlatou-Ve A. Formation of aluminum hydroxides as influenced by aluminum salts and bases[J]. Clays and Clay Minerals, 1995, 43(1): 111-115
[27] Shen X Q, Li Z J, Yao H, et al. Preparation of nanosized alumina powders by pyrolysis of ammonium aluminium carbonate hydroxide[J]. Chinese Journal of Inorganic Chemistry, 2003, 19(6): 650-654 (in Chinese)
[28] Jia Q L, Jia X L, Zhang H J, et al. Thermal decomposition kinetics of ammonium aluminum carbonate hydroxide[J]. Key Engineering Materials, 2008, 368(1): 1577-1579
[29] Stoica G, Groen J C, Abello S, et al. Reconstruction of dawsonite by alumina carbonation in (NH4)2CO3: Requisites and mechanism[J]. Chem Mater, 2008, 20(12): 3973-3982
[30] Carrasco L F, Puertas F, Varela M B, et al. Synthesis and crystal structure solution of potassium dawsonite: An intermediate compound in the alkaline hydrolysis of calcium aluminate cements[J]. Cement and Concrete Research, 2005, 35(4): 641-646
Recieved date: 2013-12-19; Accepted date: 2014-01-09.
Wang Haiyan, Telephone: +86-24-56860958(O); E-mail: fswhy@126.com.
- 中国炼油与石油化工的其它文章
- Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
- Deep Extractive Desulfurization of Gasoline with Ionic Liquids Based on Metal Halide
- Performance of FCC Catalyst Improved with Vanadium Trapping Components
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 2. Single-Event Microkinetic (SEMK) Assessment
- Synthesis, Characterization and Evaluation of Sulfur Transfer Catalysts for FCC Flue Gas
- Selection of Chelated Fe (III)/Fe (II) Catalytic Oxidation Agents for Desulfurization Based on Iron Complexation Method

