Research on Hydrolysis and Saccharification of Corn Stover
Gao Lan; Liu Ying; Guo Yong; Liu Jinsheng; Lin Jianmin
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Research on Hydrolysis and Saccharification of Corn Stover
Gao Lan; Liu Ying; Guo Yong; Liu Jinsheng; Lin Jianmin
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
In this paper three methods (dilute acid pretreatment, aqueous ammonia/dilute acid pretreatment and alkaline pretreatment) were used to study the hydrolysis of corn stover and characteristics of each method were compared. The results showed that the lignin removal rate was 71.8% when the corn stover was treated with a caustic soda solution containing 1.5% of NaOH, at a temperature of 75 ℃ for 90 min with an initial solid-liquid ratio of 1:8 (w/v). Hydrolysis yield of the NaOH pretreated sample reached 78.5%, which was much higher than other control groups. These results are useful for evaluation of pretreatment technologies, and identification of key factors that limit cellulose hydrolysis, and can also serve as a basis for designing and screening appropriate pretreatment technologies.
cellulose; pretreatment; hydrolysis; saccharification
1 Introduction
Currently industrial biofuels such as ethanol and butanol are mainly produced from lignocellulosic sources which include sugar crops (sugarcane, beet, and starch materials), starch crops (wheat, barley, sorghum, corn, potatoes, cassava) and lignocellulosic materials (wood, agricultural and forestry waste fiber). The most abundant renewable resource among these raw materials is lignocellulose, which is a promising raw material albeit with low utilization efficiency[1-2]. Biofuels as clean alternative source of traditional fuels has raised considerable interest due to the diminishing fossil fuel reserves and increased air pollution. There are approximately 35%—45% of cellulose and 20%—40% of hemicellulose contained in lignocellulosic feedstock (on dry weight basis). Cellulose is primarily hydrolyzed to glucose, and hemicellulose is hydrolyzed to a variety of pentose and inhibitors. Xylose content can reach up to 35% in the monosaccharide of hydrolyzate[3-4]. In China, the corn stalks annually amount to 224 million tons[5]. Corn stalks contain a lot of cellulose, lignin, hemicellulose and other natural polymer materials. Currently only a small portion of corn stover is used as the feed, while a dominating portion is burned or abandoned on farmland, which is not only a waste of resources, but also causes serious environmental pollution[6]. Efficient pretreatment of cellulose and the applied research in industrial production will bring about huge benefits to the economy and ecology.
Enzymatic hydrolysis of cellulosic polysaccharide is a key step at the lignocellulose biore fineries. To enhance efficiency of cellulose hydrolysis, pretreatment is necessary for effectively breaking down its recalcitrant structure. So, great attention has been paid to the design of pretreatment technologies to crack the recalcitrant nature of lignocellulose. Pretreatment is an important technology for the utilization of lignocellulose biomass. Advanced pretreatment technologies can significantly enhance the hydrolysis of cellulose, and reduce the cost of biofuel production. Therefore, the main purpose of any pretreatment is to alter or remove structural and compositional impediments to hydrolysis and subsequent degradation processes in order to enhance digestibility, improve the rate of enzyme hydrolysis and increase the yields of intended products[7-8]. These methods cause mechanical, physical, chemical or biological changes in the plant biomass in order to obtain the desired products[9]. Technology for bioconversion of lignocellulosic wastes has long been considered to be rather expensive. Over the past few years a large number of pretreatment methods have been developed, including alkali treatment, ammonia explosion, and other methods[10]. Among these methods, physicochemical and biological treatment systems seem to bethe most favored options.
Dilute-acid hydrolysis has been successfully developed for pretreatment of lignocellulosic materials. Sulfuric acid has been of the most interesting means among such studies as it is inexpensive and effective. Hemicellulose can be readily hydrolyzed by dilute acids under moderate conditions[11-12]. Alkali pretreatment can be carried out at ambient conditions, but pretreatment times are of the order of several hours or days. Compared with acid treatment processes, alkaline processes cause less sugar degradation, and many of the caustic salts can be recovered or regenerated. Sodium, potassium, calcium, and ammonium hydroxides are suitable alkaline pretreatment agents. Among these four alkalis, sodium hydroxide has been studied the most[10,13]. Ammonia has also been used as a pretreatment medium to remove lignin. The ammonia pretreatment reduces lignin content and removes some hemicellulose while decrystallizing cellulose. It can have some effects on the rate of cellulose hydrolysis. The cost of ammonia, and especially of ammonia recovery, drives up the cost of this pretreatment[7].
Therefore, it is extremely important to carefully examine the appropriate pretreatment of lignocellulose biomass. In this paper, three methods (dilute acid pretreatment, aqueous ammonia/dilute acid pretreatment, and alkaline pretreatment) were used to study the hydrolysis of corn stover and characteristics of each method were compared. These measures are useful for evaluation of pretreatment technologies, and identification of key factors that limit cellulose hydrolysis, and can also serve as a basis for designing and screening the appropriate pretreatment technologies, as well as for understanding the hydrolysis mechanism of lignocellulose.
2 Experimental
2.1 Materials
Corn stover from local farms (Yanqing District, Beijing) was milled to pass 2.0 mm screen, washed thoroughly with tap water, filtered and air-dried. The sample was kept at room temperature for use. The main composition of raw corn stover was as follows (on dry weight basis): 36.8% of cellulose, 23.5% of hemicellulose, 19.8% of lignin, and 19.9% of other ingredients.
Novozymes commercially viable enzyme Cellic®CTec2 was used in this experiment. The enzyme activity (U/mL) was assayed according to the methods recommended by Ghose[14]and Bailey[15]: xylanase activity: 16 263.2, filter paper activity (FPA): 1 619.59, cellobiase activity (CBA): 4 184.9, and CMCase activity: 25 947.8.
2.2 Analytical methods
Samples for analysis of glucose, xylose, arabinose and other constituents were centrifuged at high speed for 10 minutes. The supernatant was filtered through membrane filters and analyzed on a HPLC apparatus (Agilent 1 260 Infinity) equipped with a ZORBAX carbohydrate column and a refractive index detector. The 80% acetonitrile solution was used as the mobile phase.
Three parallel samples were used in all analytical determinations, and data were presented as the mean value of three replicates.
2.4 Experimental method
The crushed corn stover sample was subjected to hydrolysis by three pretreatment methods to give different hydrolysates. The mixture was subjected to vacuum filtration to separate the solid residues from the filtrate fraction. The corn stover residue and hydrolysate were collected and analyzed separately.
2.4.1 Dilute acid pretreatment
For dilute acid pretreatment, 100 g of crushed corn stover sample was pretreated at 105 ℃ with 1% sulfuric acid solution for a de finite reaction time at an initial solid-liquid ratio of 1:10 (w/v). The filtrate was collected for analysis. The residue was washed with water until a neutral reaction on litmus paper was detected.
Dilute acid-pretreated hemicellulosic hydrolysate was detoxi fied and concentrated by evaporation. The concentrated hemicellulosic hydrolysate was regularly stirred at 75 ℃ in a water bath shaker; meanwhile the lime solution was added slowly to neutralize the remaining sulfuric acid. The hemicellulosic hydrolysate was adjusted to pH 6.5—7.0 by adding lime solution to remove the residualby forming CaSO4precipitate and to adjust the pH value suitable for other preparation process. The CaSO4precipitate was removed by vacuum filtration after 2 h, and the chemical composition of the liquor was analyzed.
2.4.2 Aqueous ammonia/dilute acid pretreatment
For conducting aqueous ammonia/dilute acid pretreatment, 100 g of crushed sample was soaked for 12 hours at room temperature, filtered and washed, then was pretreated at 105 ℃ with 1.0% sulfuric acid solution for 4 h at an initial solid-liquid ratio of 1:10 (w/v). The filtrate was collected for analysis. The residue was washed with water until a neutral reaction was reached.
2.4.3 Alkaline pretreatment
For conducting alkaline pretreatment, the corn stover was pretreated at 75 ℃ with 1.5% sodium hydroxide solution for 90 min at an initial solid-liquid ratio of 1:8 (w/v). The filtrate was collected for analysis. The residue was washed with water until a neutral reaction was reached.
2.4.4 Enzymatic hydrolysis
The batch enzymatic hydrolysis process was used to deal with the samples. The pretreated corn stover residue as the substrate was dissolved by citrate buffer. The enzymatic hydrolysis process was carried out in a batch 250-mL Erlenmeyer flask with the substrate concentration equating to 80 g/L, with the pH value of substrate mixture adjusted to 5.0.
Corn stover residues obtained after dilute acid pretreatment and alkaline pretreatment were both hydrolyzed by the enzyme (Novozymes Cellic®CTec2). The hydrolysates were concentrated by roto-evaporation method at 65 ℃, and then were analyzed respectively.
3 Results and Discussion
The weight loss of fiber material and the removal of hemicelluloses/lignin are important parameters to reflect the effect of pretreatment in this process. Hemicellulose component in lignocellulosic feedstock is hydrolyzed to monosaccharides via mineral acid catalysis. As hydrochloric acid is rarely used because of its corrosivity and volatileness, the most currently used in the pretreatment as catalyst is sulfuric acid. Corn stover was pretreated by dilute acid, aqueous ammonia/dilute acid, and sodium hydroxide, respectively, for enhancing the enzymatic susceptibility of substrate.
3.1 Effects of dilute acid pretreatment on major components and weight loss
For pretreatment of lignocelluloses, the addition of an acid catalyst is a prerequisite to make the substrate accessible to enzymes[16].
It can be found from several pretreatment experiments (Table 1) that the mass loss of the biomass increased with an increasing processing time. The fiber residue could be easily enzymatically saccharified after being pretreated by 1% H2SO4solution at 105 ℃ for 1—6 h, which was mainly ascribed to the continuous hydrolysis of hemicellulose in the corn stover as the reaction was going on. Removal of hemicellulose increased with an extended time also verified this conclusion. After taking into consideration the material loss and energy consumption, a 3 hour duration was specified as the pretreatment time of the following process.

Table 1 Weight loss of corn stover and removal rate of three main components in dilule H2SO4pretreatment process
3.2 Comparison of different methods for pretreatment of corn stover components
Initial corn stover sample and samples being treated by three methods mentioned above (dilute acid pretreatment, aqueous ammonia/dilute acid pretreatment, and alkaline pretreatment) are labeled as A, B, C, and D, respectively. It can be seen from Table 2 that great changes occurred in the chemical composition of samples after being treated with different methods. The concentration of the three main components in the sample D which had been subjected to alkaline pretreatment was the highest in these three groups.

Table 2 Composition analysis of raw and three pretreated corn stover samples
Dilute-acid hydrolysis has been successfully developed for pretreatment of lignocellulosic materials. Sulfuric acid at a concentration of usually below 4%, has been of the most heightened interest in such studies as it is inexpensive and effective. Dilute H2SO4solution has been used to commercially manufacture furfural from cellulosic materials. Although the concentrated acids are powerful agents for cellulose hydrolysis, they are toxic, corrosive, hazardous, and thus require reactors that are resistant to corrosion, which makes the pretreatment process very expensive. In addition, the concentrated acid must be recovered after hydrolysis to make the process economically feasible[11]. Dilute NaOH treatment of lignocellulosic materials has been found to cause swelling, leading to an increase in internal surface area, a decrease in the degree of polymerization, a decrease in crystallinity and separation of structural linkages between lignin and carbohydrates, and disruption of the lignin structure[17].
3.3 Comparison between samples on weight loss of the biomass and removal rate of main components
It can be seen from Table 3 that dilute acid pretreatment showed a high removal rate of hemicellulose (reaching 79.3%), but less effect on lignin removal (equating to 7.6%). Ammonia/dilute acid pretreatment was a combination of acid and alkali treating, the hemicellulose and lignin removal rate could reach 73.9% and 30.7%, respectively. Pretreatment of the sample with the 1.5% NaOH solution removed 71.8% lignin and almost all the acetate from the raw material, because lignin was more readily dissolved in alkaline solution. The pretreated corn stover residues were prone to degradation by cellulose complex. The alkaline liquor separated from the reaction mixture could be recycled for 3—4 times to be used in repeated pretreatment batches.
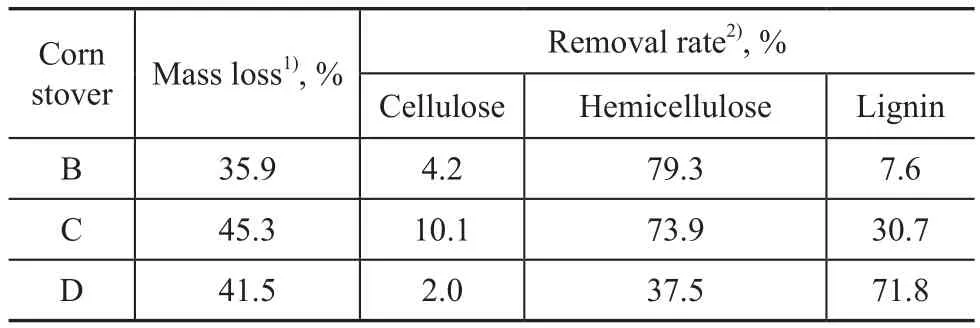
Table 3 Comparison of mass loss and removal rate of main components in three pretreatments
3.4 Comparison of different pretreatment methods on the effect of enzymatic hydrolysis
Different pretreatment methods have great impact on the efficiency of enzymatic hydrolysis of corn stover. As shown in Figure 1, after 48 h of enzymatic hydrolysis, the yield of hydrolysate from dilute acid pretreated fiber residue reached 38.9%, indicating that even though it could remove most hemicellulose of corn stover, but the enzymatic hydrolysis-promoting effect was still very limited. The yield of hydrolysate from ammonia/dilute acid pretreated sample reached 61.9%, which was a substantial improvement over the case using only dilute acid pretreatment method. The yield of hydrolysate from NaOH pretreated sample reached 78.5%, which was much higher than other pretreatment methods.
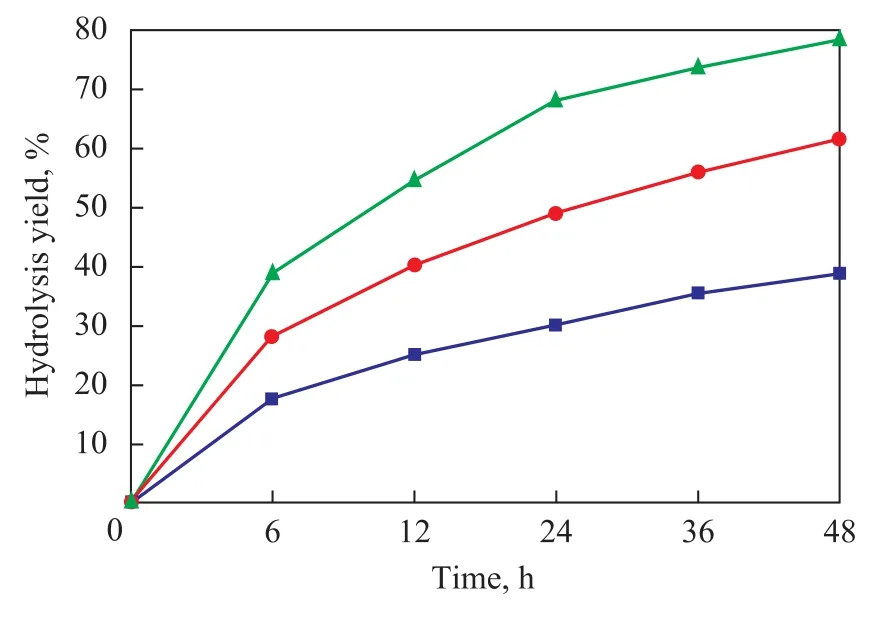
Figure 1 Time course of enzymatic hydrolysis of three pretreated corn stover samples
Some bases can be used for the pretreatment of lignocellulosic materials, and the effect of alkaline pretreatment depends on the lignin content of the materials. Alkali processes utilize lower temperatures and pressures than otherpretreatment technologies do. The digestibility of NaOH-treated hardwood was reported to increase from 14% to 55% with a decrease of lignin content to 20% from 24%—55%[18]. However, no effect of dilute NaOH pretreatment was observed for softwoods with the lignin content greater than 26%. Dilute NaOH pretreatment was also found to be effective for the hydrolysis of straws with a relatively low lignin content in the range of 10%—18%[10]. It can be seen from Figure 1 and Table 3 that there exhibited a positive correlation between the yield of enzymatic hydrolysis of the substrate and the lignin removal rate during the pretreatment of corn stover. Lignin removal can effectively promote the enzymatic hydrolysis efficiency. The extent of removal of hemicellulose of substrate showed no significant impact on hydrolysis yield, although dilute acid pretreatment could achieve a 79.3% removal of hemicellulose. Lignin was effectively removed in the NaOH pretreated sample. Fiber materials swelled, and its water holding capacity increased during the process, thereby the efficiency of enzymatic hydrolysis was greatly promoted by reactions mentioned above. The liquid composition analysis of three samples after enzymatic hydrolysis (in 48 h) is shown in Table 4. Glucose and xylose were the major monosaccharide components, yet the ratio of them was different depending on the pretreatment methods adopted. In addition, it also contained a small amount of arabinose degraded from hemicellulose. The amount of acetic acid varied in different hydrolysates. Furfural was only detected in the acid pretreated hydrolysate.

Table 4 Chemical composition of different corn stover hydrolysates
The content of glucose, xylose and arabinose were higher in the NaOH pretreated enzymatic hydrolysate, mainly because the cellulose from the corn stover pretreated by dilute alkali was susceptible to degradation, and hemicellulose left in the fibrous residue could be degraded by xylanase. Cellulose and hemicellulose in lignocellulose materials contain a lot of hydroxyl groups, and as a result, the hydrogen bond of intramolecular and intermolecular species is an important physical property of lignocellulose[19]. It is reported[14]that hydrogen bonds in lignocellulose have great contribution to the crystallinity of cellulose, therefore, the degree of crystallinity will be reduced with damage to the strength of hydrogen bonds (where intermolecular hydrogen bonding may be decisive) in wood fibers. This study had examined the changes in a variety of lignocellulose pretreatment processes and their impact on the strength of hydrogen bonds in cellulose hydrolysis. The results showed that reducing intermolecular hydrogen bonds could help promote hydrolysis of cellulose. Since the acid treatment process may interfere with the hydrogen ions and hydrogen bonds can be strengthened to a certain extent, subsequent enzymatic hydrolysis of cellulose was not significant. However, alkali treatment caused the destruction of intermolecular hydrogen bonding coupled with a corresponding increase in the rate of hydrolysis of cellulose[14].
It has been shown that materials that have been subjected to acid hydrolysis cannot be readily subjected to fermentation because of the presence of toxic substances. Furthermore, the acid pretreatment process requires costly materials of construction, high pressure, neutralization and conditioning of hydrolysate prior to biological steps, slower cellulose digestion by enzymes, and nonproductive binding of enzymes to lignin[7].
Upon considering the operating cost and cycle length as well, the alkaline-pretreated enzymatic hydrolysate could be chosen as a suitable source for carrying out further processes.
4 Conclusions
Glucose and xylose were the main monosaccharides in enzymatic hydrolysate which was obtained from lignocellulose materials. The ratio between them varied with different methods of pretreatment. The presence of xylose and arabinose indicated that the hemicellulose contained in residue was degraded by xylanase existing in the enzyme. The hydrolysate solution also contained a certain amount of acetic acid which was mainly generated via deacetylation of hemicellulose. The content of furfural detected in the alkaline-pretreated enzymatic hydrolysate was also low. The alkaline pretreatment method was simple, with a short process flow diagram, and the pro-cessing liquid could be repeatedly used for about 3 times. Fiber materials were sufficiently swelled, and its water holding capacity increased during the process, thereby the efficiency of enzymatic hydrolysis was greatly promoted by those reactions mentioned above. This hydrolysis solution had a high content of sugars (glucose and xylose) and less inhibitors, and was more suitable for the subsequent chemical process.
Acknowledgements:Financial support provided by the SINOPEC Research Program (No. S213070) is gratefully acknowledged.
[1] Balat M, Balat H, Oz C. Progress in bioethanol processing[J]. Prog Energy Combust Sci, 2008, 34(5): 551-573
[2] Naik S N, Goud V V, Rout P K, et al. Production of first and second generation biofuels: a comprehensive review[J]. Renewable Sustainable Energy Review, 2010, 14(2): 578-597
[3] Song A D, Pei G Q, Wang F Q, et al. Exploration on the diversified materials for fuel ethanol production in China[J]. Transactions of the CSAE, 2008, 24(3): 302-307
[4] Kim S, Dale B E. Global potential bioethanol production from wasted crops and crop residues[J]. Biomass and Bioenergy, 2004, 26(4): 361-375
[5] Li C G, Wang Y Q, Li N, et al. Study on Extraction of Cellulose and Removal of Hemicelluloses and Lignin from Corn Stalk[J]. Chinese Agricultural Science Bulletin, 2011, 27(1): 199-202 (in Chinese)
[6] Ouyang J, Li X, Dong Z W, et al. Study on preparation of sugar by alkali pretreatment and enzymatic hydrolysis of corn stalk[J]. Journal of Nanjing Forestry University (Natural Science Edition), 2010, 34(3): 1-5 (in Chinese)
[7] Mosiera N, Wymanb C, Dale B, et al. Features of promising technologies for pretreatment of lignocellulosic biomass[J]. Bioresource Technology, 2005, 96(6): 673-686
[8] Hendriks A T W M, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass[J]. Bioresource Technology, 2008, 100(1): 10-18
[9] Mtui G Y S. Recent advances in pretreatment of lignocellulosic wastes and production of value added products[J]. African Journal of Biotechnology, 2009, 8(8): 1398-1415
[10] Kumar P, Barrett D M, Delwiche M J, et al. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production[J]. Ind Eng Chem Res, 2009, 48 (8): 3713-3729
[11] Zeitsch, K. J. The Chemistry and Technology of Furfural and Its Many By-products: Sugar Series[M] New York: Elsevier, 2000, Vol. 13
[12] Esteghlalian A, Hashimoto A G, Fenske J J, et al. Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass[J]. Bioresour Technol, 1997, 59: 129-136
[13] Wyman C E, Dale B E, Elander R T, et al. Coordinated development of leading biomass pretreatment technologies[J]. Bioresour Technol, 2005, 96: 1959-1966
[14] Zhao H, Jones C I L, Baker G A, et al. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis[J]. Journal of Biotechnology, 2009, 139(1): 47-54
[15] Huang R L, Su R X, Qi W, et al. Understanding the key factors for enzymatic conversion of pretreated lignocellulose by partial least square analysis[J]. Biotechnology Progress, 2010, 26(2): 384-392
[16] Jorgensen H, Kristensen J B, Felby C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities[J]. Biofuels, Bioprod Bioref, 2007, 1(2): 119-134
[17] Soto M L, Dominguez H, Nunez M. J, et al. Enzymatic saccharification of alkali-treated sunflower hulls[J]. Bioresour Technol, 1994, 49(1): 53-59
[18] Kuhad R C, Singh A, Eriksson K E. Microorganisms and enzymes involved in degradation of plant fiber cell walls[M]. Adv Biochem Eng/Biotechnol, 1997, 57, 45-125
[19] RolIin J A, Zhu Z, Sathitsuksanoh N, et al. Increasing cellulose accessibility is more important than removing lignin: a comparison of cellulose solvent-based 1ignocellulose fractionation and soaking in aqueous ammonia[J]. Biotechnology and Bioengineering, 2011, 108(1): 22-30
Recieved date: 2014-03-20; Accepted date: 2014-04-16.
Dr. Gao Lan, Telephone: +86-10-82368017; E-mail: gaolan.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Experimental Study on Liquid-Liquid Equilibria of Alcohol- Ester-Water-CaCl2System
- Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
- Friction Characteristics of Space Lubricating Oil No. 4129 in Rolling and Sliding Contact
- Research on New Silica Sol Matrix Used in Fluid Catalytic Cracking Reaction
- Corresponding Factors Influencing Crude Oils Assay Using Low-field Nuclear Magnetic Resonance
- Study on Relationship between Microstructure of Active Phase and HDS Performance of Sulfided Ni-Mo Catalysts: Effect of Metal Loading

