Study on Sulfidation Degree and Morphology of MoS2Catalyst Derived from Various Molybdate Precursors
Zhang Le; Li Mingfeng; Nie Hong
(Research Institute of Petroleum Processing, SINOPEC, Beijing100083)
Study on Sulfidation Degree and Morphology of MoS2Catalyst Derived from Various Molybdate Precursors
Zhang Le; Li Mingfeng; Nie Hong
(Research Institute of Petroleum Processing, SINOPEC, Beijing100083)
The MoS2catalysts were prepared from various molybdate precursors including inorganic and organic molybdate compounds. The sulfidation degree and morphology of active phases of MoS2activated by various molybdate precursors in H2S/H2stream at different temperatures were studied by X-ray photoelectron spectroscopy (XPS) and high-resolution transmission electron microscopy (HRTEM). The organic molybdate precursors lead to MoS2catalysts with higher sulfidation degree and smaller active phases to demonstrate higher catalytic activity during hydrodesulfurizaiton (HDS) of 4,6-DMDBT.
MoS2; molybdate precursors; sulfidation degree; morphology
1 Introduction
Environmental concerns lead to increasingly tightening regulations on sulfur, nitrogen and aromatics content in fuels[1]. Conversion of these compounds is therefore of paramount importance, and such an objective needs the development of more active catalysts. Molybdenum sulfide materials have emerged as a class of promising catalysts for hydrotreating reactions.
Many researches have dealt with the activation treatment by means of gas phase (H2/H2S) and liquid phase (DMDS) activation of catalysts, with the effects on morphological and catalytic properties of treated catalysts properly reported[2-3]. Especially, the decomposition of thiosalts has been widely used in preparing molybdenum or tungsten disulfide with high surface area[4-5]. L. Alvarez[6]reported that the method of activation (in-situ or ex-situ) of tetraalkylammonium thiomolybdates and the nature of the thiosalt precursor (with or without C) can influence strongly the textural and catalytic properties of the final MoS2and Co/MoS2catalysts. The use of a tetraalkylammonium thiomolybdate precursor (with C) reduces significantly the formation of a MoS2-like intermediate and can lead to a final meso-structure of MoS2. H. Nava and co-workers[7]prepared unsupported nickel-molybdenum-tungsten sulphide catalysts from tri-metallic NiMoW alkyl precursors with tetraalkylammonium thiomolibdotungstates salts, (R4N)4MoWS8(where R=H, methyl, propyl, butyl, or cetyl-trimethyl). The nature of the alkyl group can strongly affect both the specific area and the HDS activity. The catalytic activity is strongly enhanced when the carbon-containing precursors are used. So the effect of molybdate precursors (with or without C) was warmly discussed with respect to their influence on the structure, morphology and activity of MoS2catalysts. However, this investigation is mainly aimed at thiosalt precursor and sulfidation degree, and the morphology of active phases of MoS2activated by various molybdate precursors at different temperatures is less studied. In the present work, the activation law, the difference in morphology, and performance of MoS2activated by various molybdate precursors have been studied and the effect of carbon contained in the molybdate precursors has been discussed.
2 Experimental
2.1 Catalyst preparation
MoS2catalysts were prepared from five different molybdates precursors listed in Table 1. The molybdates precursors Mo-1, Mo-2 and Mo-4 are chemical reagents. The molybdate precursor Mo-3 was prepared by heating a solution of citric acid and molybdenum trioxide (at a molar ratio of 1:1). The molybdate precursor Mo-5 was obtained by the following experiment. Under heating and stirring,the ammonium heptamolybdate tetrahydrate solution was added to a solution of hexadecyl trimethyl ammonium bromide (CTAB) prior to being refluxed at 373 K for 4 h. The white precipitate formed thereby was isolated by filtration, washed with water and dried at 393 K for 3 h to obtain Mo-5. The carbon content in the molybdates precursors Mo-3 and Mo-5 was analyzed by a carbon-sulfur analyzer. Then the molybdate precursors are all sulfided in a flow of 15% H2S/H2mixture at 473 K and 573 K, respectively, for 4 h to produce the MoS2catalysts (Table 2).

Table 1 The properties and resources of molybdate precursor
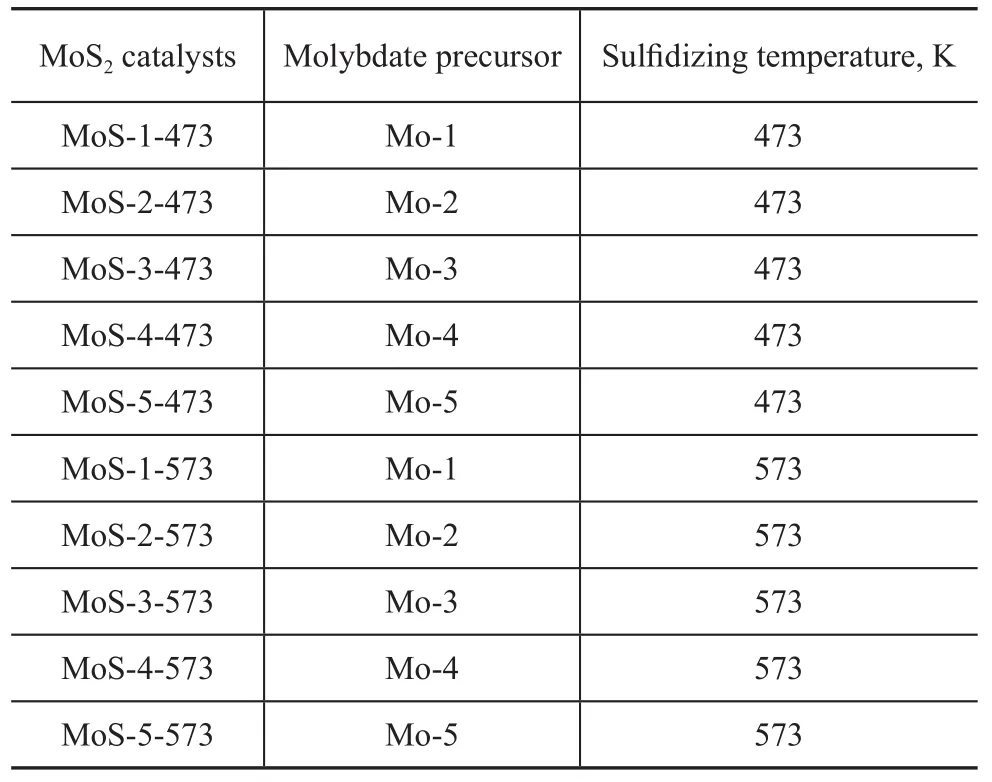
Table 2 MoS2catalysts prepared by various molybdate precursors under different activation conditions
The MoS2catalysts were characterized by X-ray photoelectron spectroscopy (XPS) and high-resolution transmission electron microscopy (HRTEM). The XPS experiments were performed in a VG Scientific ESCALab 220i-XL spectrometer, with source of X-rays, Al Kα (1486.6 eV) anode and 300 W of power. HRTEM was carried out on a TECNAI F20 G2 apparatus, made by the FEI Company with a resolution of 0.24 nm.
The hydrodesulfurization (HDS) of 4,6-dimethyldibenzothiophene (4,6-DMDBT) was carried out in a fixed-bed micro-reactor made by the American Autoclave Engineers Company. The molybdate precursors were in-situ sulfided with a solution of 5% CS2in cyclohexane at a flow rate of 0.4 mL/min at 360 ℃, 4.14 MPa of H2pressure and a H2flow rate of 400 mL/min for 3 h. Then the reactor was switched to treatment of the reactant feed (0.45% of 4,6-DMDBT in decane) at a flow rate of 0.2 mL/min in the same hydrogen atmosphere. The reaction products were analyzed by the on-line GC-FID directly. The conversion of 4,6-DMDBT was calculated using the internal standard method. The HDS activities were calculated using the following equation:
Total HDS activity:AHDS=F0×conversion/m
whereF0is the molar flow rate of the reactant (mol/s) andmis the mass of the catalyst (g).
3 Results and Discussion
3.1 The sulfidation laws of the catalysts
The XPS spectra of the MoS2catalysts were collected. Table 6 gives the binding energies of S2p and Mo3d derived from decomposition of the XPS spectra of MoS2catalysts. The XPS spectra of S2p on the MoS2catalysts exhibit only one peak at about 162.2 eV, which corresponds to S2-[8-9]. The absence of any signal at 169.0 eV after sulfidation indicates that no oxidation of the catalysts occurs during the transfer of the solid from the sulfidizing reactor to the XPS spectrometer. In the Mo3d spectra, the peaks are attributed to Mo4+species (229 eV and 232 eV for the 3d5/2and 3d3/2, respectively) and Mo6+species (233 eV and 236 eV)[10].
The sulfidation degree of surface species has been calculated based on the area of the XPS peaks of the various species as shown in Table 3. The peaks at around229 eV and 232 eV are attributed to Mo4+species and can be used to calculate the sulfidation degree of surface Mo species.

Table 3 Binding energy of elements and sulfidation degree of molybdenum in MoS2catalysts
There are great difference in the sulfidation degree for the molybdate precursors at a sulfidation temperature of 473 K. The sulfidation degree of inorganic molybdate precursors (Mo-1 and Mo-2) dips to as low as 50%—60% upon activation at 473 K. The organic molybdate precursors (Mo-3, Mo-4 and Mo-5) have much higher sulfidation degree than inorganic molybdate precursors, and especially Mo-4 and Mo-5 are almost totally sulfided upon activation at 473 K. Interestingly, the sulfidation degree increases with the increase in carbon content of the molybdate precursors (Figure 1).
Upon sulfidation at 573 K, the sulfidation degree of catalysts activated by different molybdate precursors shows no large difference. The sulfidation degree of catalysts activated by organic molybdate precursors is still higher than that of catalysts activated by inorganic molybdate precursors. The sulfidation degree of catalysts activated by inorganic molybdate precursors reach up to 82% under this condition, and the catalysts are almost totally sulfided by organic molybdate precursors.

Figure 1 Carbon content of molybdate precursors versus molybdenum sulfidation degree of MoS2upon activation at 473 K
3.2 Morphology of active phases of MoS2catalysts
Figure 2 presents the HRTEM photographs of the sulfided MoS2catalysts prepared from various molybdate precursors upon activation at different temperatures.
Interpretation of the HRTEM pictures of Mo sulfidebased catalysts was well documented[11-12]. The black lines in the pictures correspond to the lattice images of MoS2fragments. These short black lines might be either single or in packets of up to about seven parallel threads which correspond to the lamellar structure of the Mo dichalcogenides. Figure 2 presents some short black lines in the MoS2catalysts corresponding to the lamellar structure of random MoS2.
It is difficult to find the black lines in the MoS-1-473 and MoS-3-473 and there is a small amount of black lines in the MoS-2-473. However, in the HRTEM pictures of MoS-4-473 and MoS-5-473, a large amount of black lines can be observed. So it means that under mild activation at a low temperature of 473 K, seldom MoS2lamellar structure can be identified in precursors Mo-1, Mo-2 and Mo-3, while a large amount of MoS2layer stacks can be formed in precursors Mo-4 and Mo-5. Upon sulfidation at 573 K, it can be seen clearly that the number of MoS2layer stacks in MoS-1-573, MoS-2-573 and MoS-3-573 increases significantly. There are also many MoS2layer stacks in MoS-4-573 and MoS-5-573. In addition, the number of MoS2layer stacks in MoS-3-573, MoS-4-573 and MoS-5-573 is remarkably more than that in MoS-1-573 and MoS-2-573. In another word, MoS-3-573, MoS-4-573 and MoS-5-573 have a much higher density of active sites than that in MoS-1-573 and MoS-2-573.
According to the literature[13], the lengthLof the black lines, which roughly corresponds to the lateral dimension of the observed MoS2platelets, and the numberNof three-dimensional stacked layers can be calculated asfollows. In addition, the average number of MoS2stacks in an area of 1 000 nm2was also calculated. More than 200 slabs were examined on several HRTEM pictures taken from different parts of the same sample dispersed on the microscope grid.
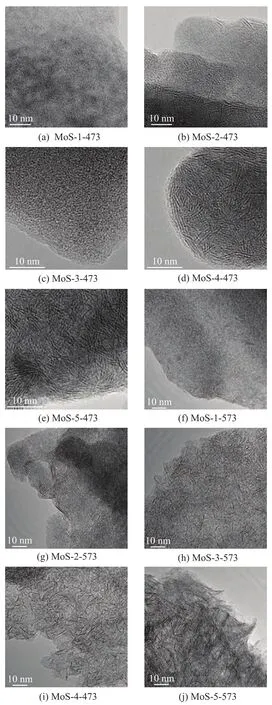
Figure 2 High-resolution TEM images of sulfided MoS2catalysts

Table 4 Mean lengthand numberof the stacked layers
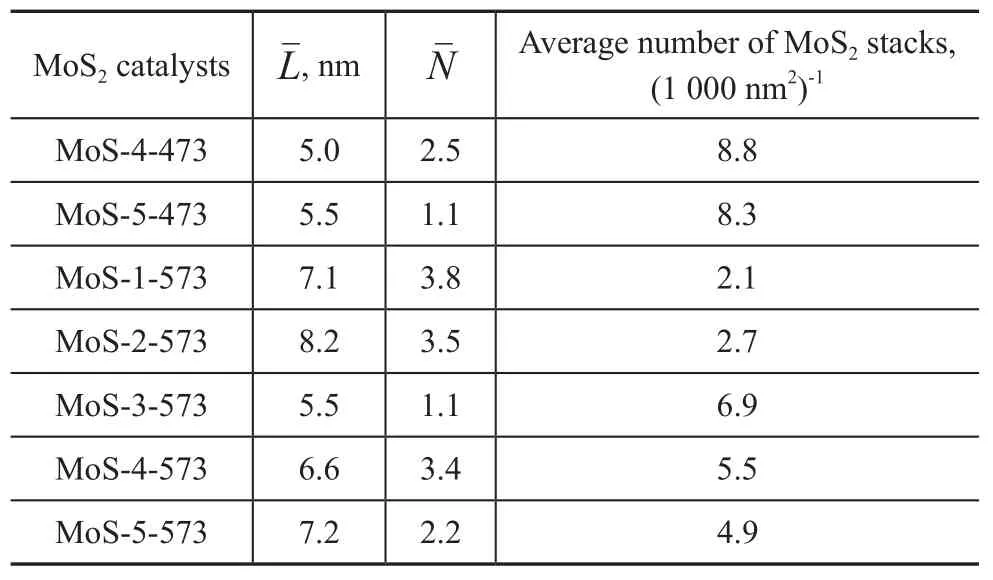
Table 4 Mean lengthand numberof the stacked layers
?
Because there are seldom MoS2layer stacks in MoS-1-473, MoS-2-473 and MoS-3-473, the length and number of the MoS2stacked layers are not calculated. After sulfidation at 473 K, MoS-4-473 and MoS-5-473 have 1—3 layers of MoS2slabs with a thickness of about 5 nm, and their average number of MoS2stacks in 1 000 nm2is high. After sulfidation at 573 K, MoS-1-573 and MoS-2-573 have a large MoS2layer stacks with 3—4 layers in thickness and 7—8 nm in length. After sulfidation at 573 K, the length and number of the MoS2stacked layers of MoS-3-573, MoS-4-573 and MoS-5-573 derived from the organic molybdate precursors are all smaller than those of MoS-1-573 and MoS-2-573 derived from the inorganic molybdate precursors. On the other hand, the average number of MoS2stacked layers in MoS-3-573, MoS-4-573 and MoS-5-573 is much greater. Especially, MoS-3-573 has the smallest MoS2slabs, albeit with a highest density. It is strange that the MoS-4-573 and MoS-5-573 derived from molybdate precursors with high carbon content have larger MoS2slabs and lower density of active sites than that derived from the molybdate precursor Mo-3 with low carbon content. It may cause a lower activity of MoS-4-573 and MoS-5-573 as compared to MoS-3-573.When the MoS2layer stacks of MoS-3-573 are compared with MoS-4-473 and MoS-5-473, it can be found out that MoS-4-473 and MoS-5-473 have higher density of active sites than that of MoS-3-573 with almost the same size of MoS2slabs. So there is a corresponding suitable sulfidation temperature for the different molybdate precursors. For molybdate precursors Mo-4 and Mo-5, a temperature of 473 K is more appropriate for their sulfidation which can produce smaller size of MoS2slabs and higher density of active sites. However, if molybdate precursors Mo-4 and Mo-5 are transformed to sulfides at a temperature which is higher than their proper temperature, the active phases will grow into slightly larger slabs and the average number of MoS2stacks will decrease.
In general, if the molybdate precursors are all transformed into sulfides at a suitable temperature, the size of MoS2slabs will decrease and the density of active sites will increase with an increasing carbon content in the molybdate precursors. Therefore, the organic molybdate precursors will promote a small size of MoS2stacking layers and a much high density of active sites after proper sulfidation.
3.3 Evaluation of the catalysts
It is generally accepted that the HDS of 4,6-DMDBT occurs through two parallel reaction pathways as follows: (i) direct desulfurization (DDS) which gives 3,3´-dimethlybiphenyl (DMBiPh); and (ii) desulfurization through hydrogenation (HYD) which yields 3-(3´-methylcyclohexyl)-toluene (DMCHT) with a tetrahydrogenated compound as an intermediate[14]. In all reactions for HDS of 4,6-DMDBT over MoS2catalysts derived from various molybdate precursors, there is no DDS product, so only the total HDS activity is given in Table 5.

Table 5 Catalytic activity of MoS2catalyst for HDS of 4,6-DMDBT
The results show that the sulfidized MoS-1, MoS-2 catalysts have the lowest HDS activity, while the HDS activity of MoS-3 increases by about 100% as compared to that of MoS-1 catalyst. Among all these catalysts, the HDS activity of sulfidized MoS-4 and MoS-5 catalysts are the highest which is about 6 times higher than that of MoS-1 catalyst. By taking into account the above evaluation results, the carbon content in the molybdate precursors is quite consistent with their measured catalytic activity (Figure 3). The MoS2catalyst prepared from the organic molybdate precursor Mo-5 has a highest activity along with a highest carbon content in the precursor at the same time. Comparably, the MoS2catalysts prepared from the inorganic molybdate precursors Mo-1 and Mo-2 have the lowest activity.
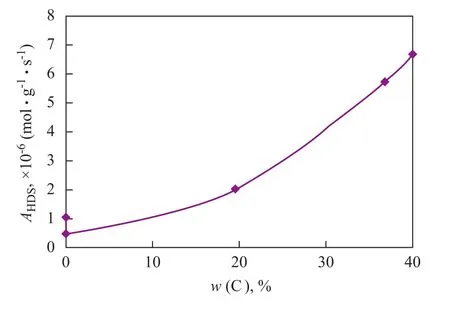
Figure 3 Activity of MoS2catalysts for HDS of 4,6-DMDBT as a function of carbon content of their molybdate precursors
4 Discussion
The nature of the molybdate precursor influences strongly the sulfidation degree, morphology of active sites and catalytic properties of the final MoS2catalysts. The presence of initial carbon in the molybdate precursor has a beneficial effect on the final MoS2structure and performance. Compared with the inorganic molybdate precursor, the carbon-containing molybdate precursor (with C) can form MoS2catalyst with higher sulfidation degree, smaller MoS2slabs and higher HDS activity. With the increase of the carbon content in the molybdate precursor, firstly the activation of catalyst would become easier because the temperature of activation decreases greatly. The Mo-4-473 and Mo-1-573 have almost the same sulfidation degree while their activation temperature differs by 100 K. Secondly, the MoS2slabsbecome smaller and the density of active sites increases dramatically which means that there are more active sites in the MoS2catalyst upon using a carbon-containing molybdate precursor. Finally, MoS2catalyst derived from organic molybdate precursor has good performance in 4,6-DMDBT HDS reaction because of its high sulfidation degree and the existence of more active sites.
The activation condition for each molybdate precursor differs a lot. For the organic molybdate precursors, a temperature of 473 K—573 K might be the right temperature range for their total sulfidation. While for the inorganic molybdate precursors, a temperature of 573 K—623 K may be suitable for its total sulfidation. So different molybdate precursors should have their own suitable sulfidation temperature. At the suitable sulfidation temperature, the sulfidation degree of MoS2is high and there is a highest density of small MoS2slabs which also means the highest active sites. However, with activation conducted at a temperature higher than their suitable temperature range, the density of MoS2slabs will decrease and the size of slabs becomes bigger. So the active sites would decrease in that case, although their sulfidation degree is still high. Apparently, the activity of MoS-4 and MoS-5 catalysts for HDS of 4,6-DMDBT might not be their best performance in this reaction and could be optimized after adjusting their activation temperature. A more suitable sulfidation condition can be investigated for obtaining higher activity of MoS2catalyst in the future research work.
5 Conclusions
The activation law and morphology of active sites of MoS2catalysts prepared from various molybdate precursors were studied by XPS and TEM. The nature of the molybdate precursor has an significant effect on the sulfidation situation, active phase structure and HDS activity of MoS2catalyst. The MoS2catalyst originated from organic molybdate precursor (with C) has higher sulfidation degree, more active sites and better HDS performance compared with the MoS2catalyst originated from inorganic molybdate precursor. With the increase of carbon content in molybdate precursor, more MoS2active sites will be formed in the MoS2catalyst and its activity in 4,6-DMDBT HDS reaction will also improve.
Acknowledgments:The authors gratefully acknowledge the financial support by the National Key Basic Research Development Program “973” Project (2012CB224800) of China.
[1] Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catal Today, 2003, 86: 211-263
[2] Alvarez L, Espino J, Ornelas C, et al. Comparative study of MoS2and Co/MoS2catalysts prepared by ex situ/in situ activation of ammonium and tetraalkylammonium thiomolybdates[J]. J Mol Catal A, 2004, 210(1/2): 105-117
[3] Alonso G, Berhault G, Aguilar A, et al. Characterization and HDS activity of mesoporous MoS2catalysts prepared by in situ activation of tetraalkylammonium thiomolybdates[J]. J Catal, 2002, 208: 359-369
[4] Alonso G, Berhault G, Chianelli R R. Synthesis and characterization of tetraalkylammonium thiomolybdates and thiotungstates in aqueous solution[J]. Inorg Chim Acta, 2001, 316: 105-109
[5] Alonso G, Yang J, Siadati M H, et al. Synthesis of tetraalkylammonium thiometallates in aqueous solution[J]. Inorg Chim Acta, 2001, 325: 193-197
[6] Alvarez L, Espino J, Ornelas C, et al. Comparative study of MoS2and Co/MoS2catalysts prepared by ex situ/in situ activation of ammonium and tetraalkylammonium thiomolybdates[J]. J Mol Catal A, 2004, 210(1/2): 105-117
[7] Navaa H, Pedrazab F, Alonso F. Nickel-molybdenumtungsten sulphide catalysts prepared by in situ activation of tri-metallic (Ni-Mo-W) alkylthiomolybdotungstates[J]. Catalysis Letters, 2005, 99(1/2): 65-71
[8] Okamoto Y, Imanaka T, Teranishi S. Surface structure of CoO-MoO3/Al2O3catalysts studied by X-ray photoelectron spectroscopy[J]. J Catal, 1980, 65: 448-460
[9] Gajardo P, Mathieux A, Grange P, et al. Structure and catalytic activity of CoMo/γ-Al2O3and CoMo/SiO2hydrodesulphurization catalysts: An XPS and ESR characterization of sulfided used catalysts[J]. Appl Catal, 1982, 3: 347-376
[10] Li C P, Hercules D M. A surface spectroscopic study of sulfided molybdena-alumina catalysts[J]. J Phys Chem, 1984, 88(3): 456-464
[11] Sanders J V. Chapter 2: The electron microscopy of catalysts[M]// Anderson J R, Boudart M. Catalysis Science and Technology: vol. 7. Berlin: Springer-Verlag, 1985: 51-157
[12] Zaikovskii V I, Playasova L M, Burmistrov V A, et al. Sulphide catalysts on silica as a support. ii. High resolution electron microscopy data[J]. Appl Catal, 1984, 11: 15-27
[13] Payen E, Hubaut R, Kasztelan S, et al. Morphology study of MoS2-based and WS2-based hydrotreating catalysts by high-resolution electron-microscopy[J]. J Catal, 1994, 147(1): 123-132
[14] Bataille F, Lemberton J L, Perot G, et al. Sulfided Mo and CoMo supported on zeolite as hydrodesulfurization catalysts: Transformation of dibenzothiophene and 4,6-dimethyldibenzothio-phene[J]. Appl Catal A, 2001, 220 (1/2): 191-205
Recieved date: 2013-11-08; Accepted date: 2014-2-12.
Dr. Zhang Le, Telephone: +86-10-82368255; E-mail: zhangle.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Experimental Study on Liquid-Liquid Equilibria of Alcohol- Ester-Water-CaCl2System
- Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
- Friction Characteristics of Space Lubricating Oil No. 4129 in Rolling and Sliding Contact
- Research on New Silica Sol Matrix Used in Fluid Catalytic Cracking Reaction
- Corresponding Factors Influencing Crude Oils Assay Using Low-field Nuclear Magnetic Resonance
- Study on Relationship between Microstructure of Active Phase and HDS Performance of Sulfided Ni-Mo Catalysts: Effect of Metal Loading

