X衍射精细结构和晶体旋光角研究D-,L-,DL-缬氨酸晶格分子间N+H…O−氢键电子库珀对的自旋流超导相变
王文清 张玉凤 龚 ,*
(1北京大学化学与分子工程学院应用化学系,北京分子科学国家实验室,北京100871;2北京服装学院材料科学与工程学院,北京100029)
1 Introduction
The question of the origin of biochirality in nature has intrigued chemists and physicists for a century,and has been the impetus for both extensive speculation and varied experimentation.Starting fromZ0interaction and Bardeen-Cooper-Schrieffer(BCS)theory,Salam1-4proposed that chirality among the twenty amino acids which make up the proteins may be the analogy of“superfluidity”in superconductors.Figureauet al.5,6conducted a series of experiments to observe the optical rotation after exposing both racemic DL-cystine and L-cystine three days at 77,4 K,and four days at 0.6 K then dissolved at room temperature in the 2 mol·L-1HCl solution,and placed in transparent cells then illuminated with a He-Ne laser at 632.8 nm.They observed no changes in optical rotation,thus failing to validate Salam's predicted phase transitions.7
Based on Figureau lesson,first,the direct way to test for the hypothesis is to lower the temperature while measuring the optical rotatory anglein situwhen polarized light is shined upon D-valine,L-valine,and DL-valine crystals,respectively.If the polarization vector gets rotated,we can be sure that the appropriate phase transition has taken place.Second,the fundamental puzzle of the origin of biochirality is how to generate the right and left helicities of the peptide chain.The peptide bond is the result of a head to tail condensation of the amino group of one amino acid and the carboxyl group of another.Formation of this bond results in the release of 1 mol of water per mol of peptide bond formed(Fig.1).In aqueous solution,the reaction does not happen without the input of energy.This is because the equilibrium in aqueous solution lies far in favor of hydrolysis.Third,the transition mechanism of“superfludity in superconductors”is currently great interest in the study of the role of the electron spin degree of freedom in transport onto crystal lattices.
Valine is an essential amino acid and can be derived from alanine by the introduction of two methyl groups in place of two H-atoms present onα-carbon atom.Valine is hydrophobic,and is usually found in the interior of proteins.L-valine crystal structure is monoclinic,from space groupP21and there are four molecules in the unit cell.In the case of L-valine the carboxyl groups are ionized and each molecule exists as a zwitterion.There exist in the crystal two molecules taking thetransand two taking thegaucheI conformations which has been determined with X-ray diffraction by Torii8and Dalhus9et al.measurement reported at 120 K.On the other hand,the unit cell of the DL-valine crystal contains molecules with only onetrans-conformation.10-12Atomic force microscopy(AFM)images showed an ordered molecular morphology of monoclinic D-,L-,and DL-valine unit cells,13,14which are in fair agreement with the X-ray diffraction.This article focuses on the transition mechanism of D-,L-,and DL-valine crystals.The optical rotatory angle of monoclinic valine crystal is measuredin situafter exposing from 240 to 290 K by projecting He-Ne laser light along the direction ofb-axis defines as principal optic axis.15
2 Experimental
2.1 Sample recrystallization
The polycrystalline powders of D-and L-valine(Sigma Chemical Co.,98%)were thrice recrystallized from sterilization aqueous solution at 277 K by slow evaporation for 3 weeks,producing thin flakes elongated along thebaxis with well developed{001}faces,washed with dropping absolute alcohol,evacuated and kept in a desiccator.The crystal puritiesof D-and L-valine were identified by the following procedure.The positive-ion electrospray mass spectra(EI-MS)were recorded using a Bruker APEX IV 7.0 T Fourier transform ion cyclotron resonance(FT-ICR)mass spectrometer(Bruker Daltonics,Billerica,MA USA)equipped with an external ESI source(Analytica,Branford Inc.USA),which produced detection limits of 2 fmol for organic impurities.The purities of D-and L-valine single crystals were identified by gas chromatography mass spectrometry(GC-MS)of theirN-trifluoroacetyl(TFA)isopropyl ester on Chirasil-Val capillary column.16
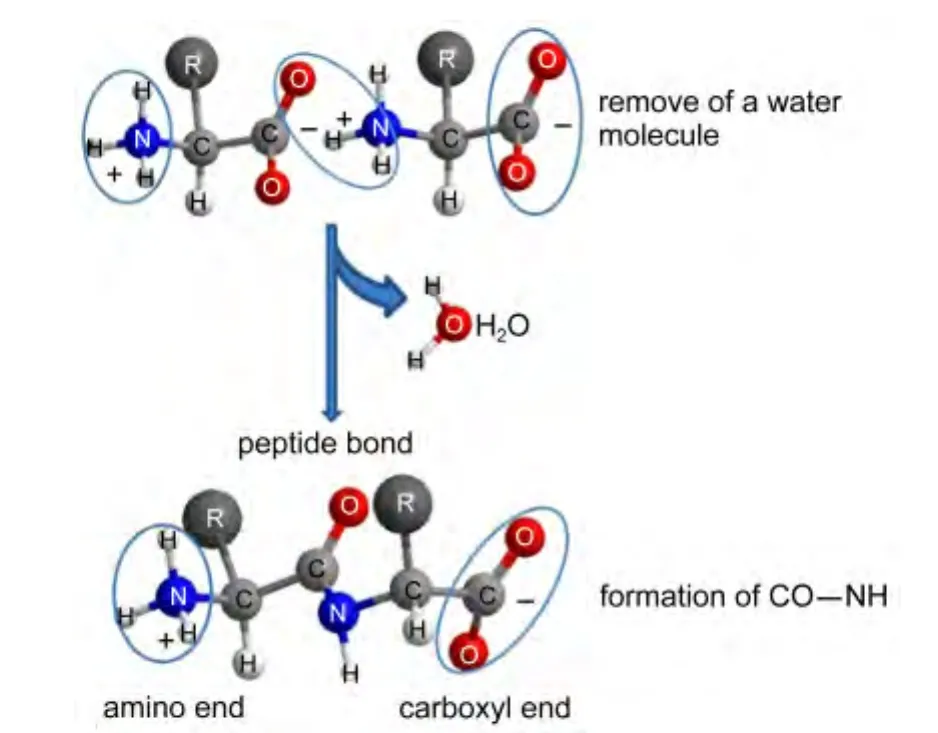
Fig.1 Formation of the peptide bond
2.2 Heat capacity measurements
In 2003,Sullivanet al.17studied the thermodynamic and magnetic properties of D-and L-valine and alanine in the range 220-340 K to examine the results reported by Wanget al.18and presented arguments against the Salam hypothesis.We accept the challenge to perform the heat capacity measurements of D-and L-valine with advanced differential scanning calorimetry(Pyris diamond DSC,Perkin Elmer Corp,USA),which is of the heat-flux type.19D-valine single crystal(12.80 mg)with Sigma product(98%,10.99 mg)and L-valine single crystal(14.01 mg)with Sigma product(98%,12.78 mg)were measured respectively,from 250 to 320 K at the scan rate of 10 K·min-1by triple curve method.Endothermic peaks were observed at transition temperatureTC=273.59 K,ΔH=1.75 J·mol-1andTC=273.76 K,ΔH=1.57 J·mol-1for D-and L-valine single crystals but vanished in the ground powder of pre-measured crystals and Sigma polycrystalline products.Temperaturedependent heat capacities of D-and L-valine were fitted coincidently with the Hutchens curve.However,there were no transition peaks shown from the polycrystalline L-valine(44.380 g)of Hutchenset al.,20,21and L-valine(4.3244 g),DL-valine(2.2046 g,Fluka,Biochemica)of Paukovet al.22around 270 K which demonstrated that this phase transition is observed only on the single crystal lattice.We have found spinphonon coupling and the intrinsically different spin properties between D-and L-alanine crystals near the transition temperature that resulted in a discrepancy of ultrasonic velocity and attenuation behaviors between alanine enantiomers.23
2.3 X-ray diffraction
The X-ray diffraction data of D-and L-valine were collected on a Nonius Kappa CCD diffractometer with graphite monochromated Mo-Kαradiation.A liquid nitrogen-cooled chargecoupled-device system was in control of the certain temperature for 10 min,and then recorded the diffraction data.Cell parameters were obtained by the global refinement of the positions of all collected reflection data.At 293 K,a total of 18661 reflections were collected,of which 2744(I>2σ(I))are unique;equivalent reflections were averaged.The integration was performed by program DENZO-SMN,and data correction was carried out by Lorentz-polarization effects and for absorption by using the program SCALE-PACK.The solution was obtained by the direct methods and followed by the subsequent Fourierdifference syntheses(SHELXL97).All of the non-hydrogen atoms were refined anisotropically,and the hydrogen atoms isotropically.The structures of D-/L-valine were refined by a fullmatrix least-squares technique to obtainR1=0.0325/0.0359 andwR1=0.0695/0.0787 for 2744/3526 reflections andR2=0.0441/0.0496 andwR2=0.0741/0.0840 for all data at 293 K.The weighting scheme was based on counting statistics,including a factor(p=0.050)to down-weight the intense reflections.All of the calculations were performed with the maXus computer program.The parameters and refinement data for D-and L-valine crystal at 293,270,223,and 173 K are summarized in Table 1 and Table 2.
2.4 Measurement of optical rotatory angle of chiral crystals
Chiral crystals are characterized by a natural optical activity.The difficulty of the experiment was how to measure the optical rotatory angles of monoclinic crystals of D-,L-valine and triclinic crystal of DL-valine,to eliminate the effect of ellipticity induced by the crystal anisotropy.We measured the optical rotatory angle in biaxial crystal of monoclinic valine by projecting He-Ne laser light along one of its optic axes(b axis).The other two principal axes of refractive index ellipsoid,aandcaxes were determined with Photo Elastic Modulator systems(PEM-90TM).24The temperature dependence of the optical rotatory angle of D-valine,L-valine,and DL-valine crystals can thus be determined.
3 Results and discussion
3.1 Crystal structures and hydrogen bonding
In D-and L-valine crystals,the carboxyl groups are ionized and the molecules exist as a zwitterion.The structure is made up of double layers with alternating hydrophobic and hydrophilic layers parallel to the(001)plane.Within the layers,the molecules are held together through the N―H…O hydrogen bonds.The hydrophobic layers contain hydrocarbon side chains,while the hydrophilic ones are composed of the charged carboxylate and amino groups.Fig.2(D-valine)and Fig.3(L-valine)show the two crystallographically independent molecules A(transform)and B(gaucheI form)in the asymmetric unit,which are rotational isomers with two kinds of conformations.The carboxylate groups of D-/L-valine in molecular A(transform)are asymmetric with C(2)―O(2)of 0.1238(2)/0.12375(17)nm and C(2)―O(1)of 0.12605(19)/0.12611(15)nm at 270 K resulting from the different strengths of hydrogen bonds to the different oxygen atoms.Yates25and Yamada26et al.pointed out that the length of the carbonyl bond was an indicator for quantifying the strength of a hydrogen bond,i.e.,the stronger hydrogen bonding,the longer C=O bond length.The third hydrogen atom on amino group linked mainly by electrostatic force to the NH3+group forms hydrogen bonds with the carboxylate group.From the backside observation of C(1)―H(4)bond,the interior group CO2→NH3is clockwise and counterclockwise rotation in D-and L-valine crystals,respectively.
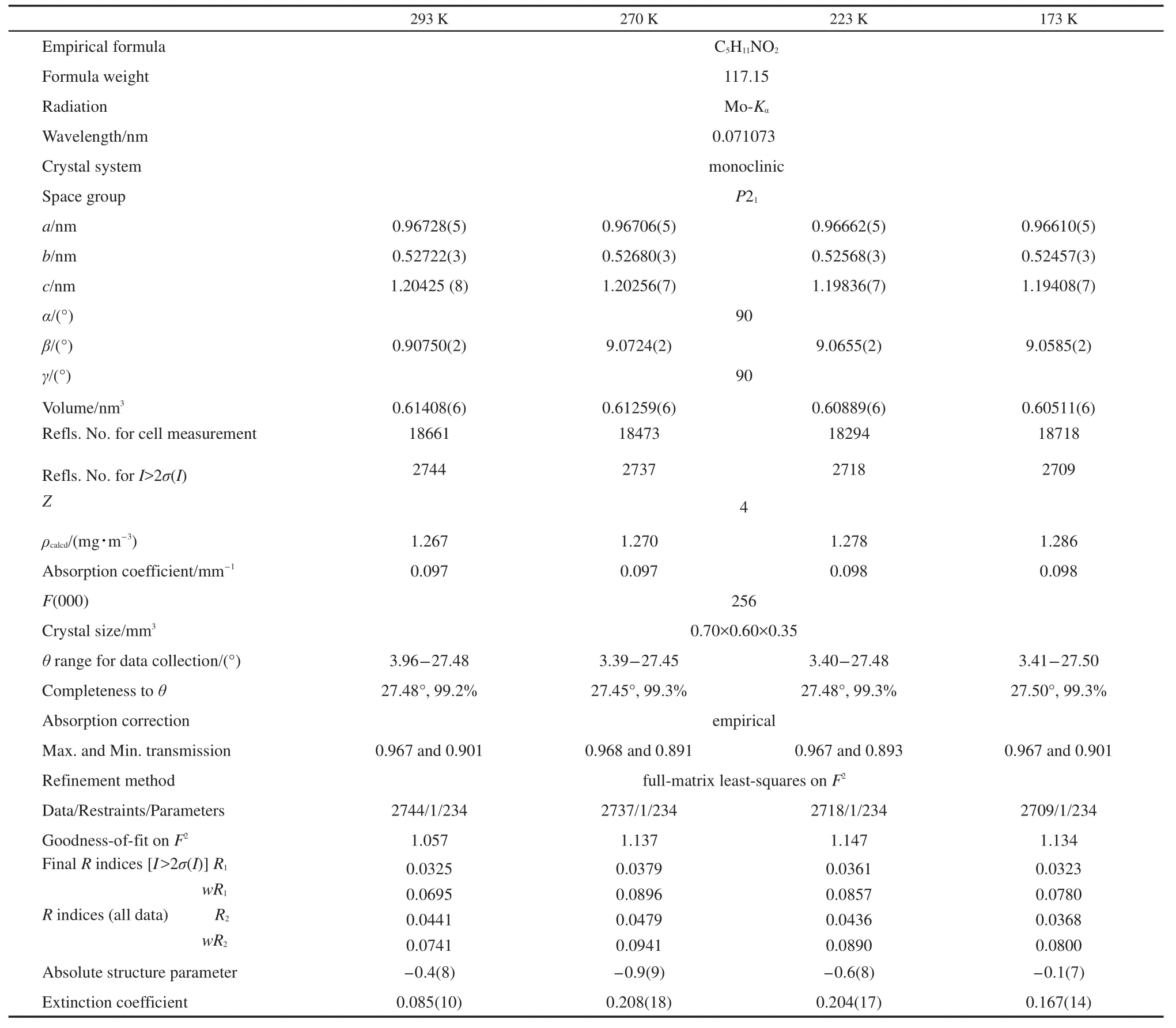
Table 1 Unit-cell dimensions of D-valine crystal at 293,270,223,and 173 K

Table 2 Unit-cell dimensions of L-valine crystal at 293,270,223,and 173 K

continued Table 2
3.2 Temperature dependence of the molecular structure
The temperature dependences of cell constants of D-and L-valine are shown in Tables 1 and 2.Atomic coordinates and equivalent isotropic displacement parameters at 293,270,223,and 173 K,the temperature dependence of the bond lengths and bond angles,the geometries of hydrogen bonds are listed in Tables 3-10,respectively.
X-ray diffraction showed the intramolecular N―H…O bond distances at 293,270,223,and 173 K of D-and L-valine crystals.Comparison of Tables 6 and 10,the bond lengths of N(1)―H(4),H(4)…(O)and the bond angle∠N―H…A are fluctuated in D-valine at 270 K,however,in L-valine are stable and measurable.The intramolecular N(1)―H(4)…O(4)bond distance is shown a little change(10-8cm)as a function of temperature in D-and L-valine crystals.There is no evidence for the configuration transformation from D-to L-valine at 270 K.In fermionic superconductivity and superfluidity,a central con-cept is electron Cooper pairing.Based on BCS theory,the Cooper pairs are rather large objects(10-4cm)which might be occurred by intermolecular N―H…O hydrogen bond onto the crystal lattices of D-,L-,and DL-valine,respectively.
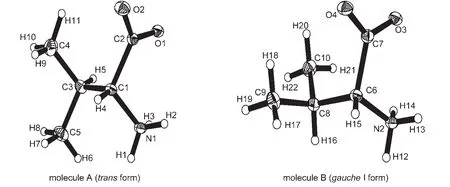
Fig.2 Structures of two crystallographically independent moleculesAand B in D-valine
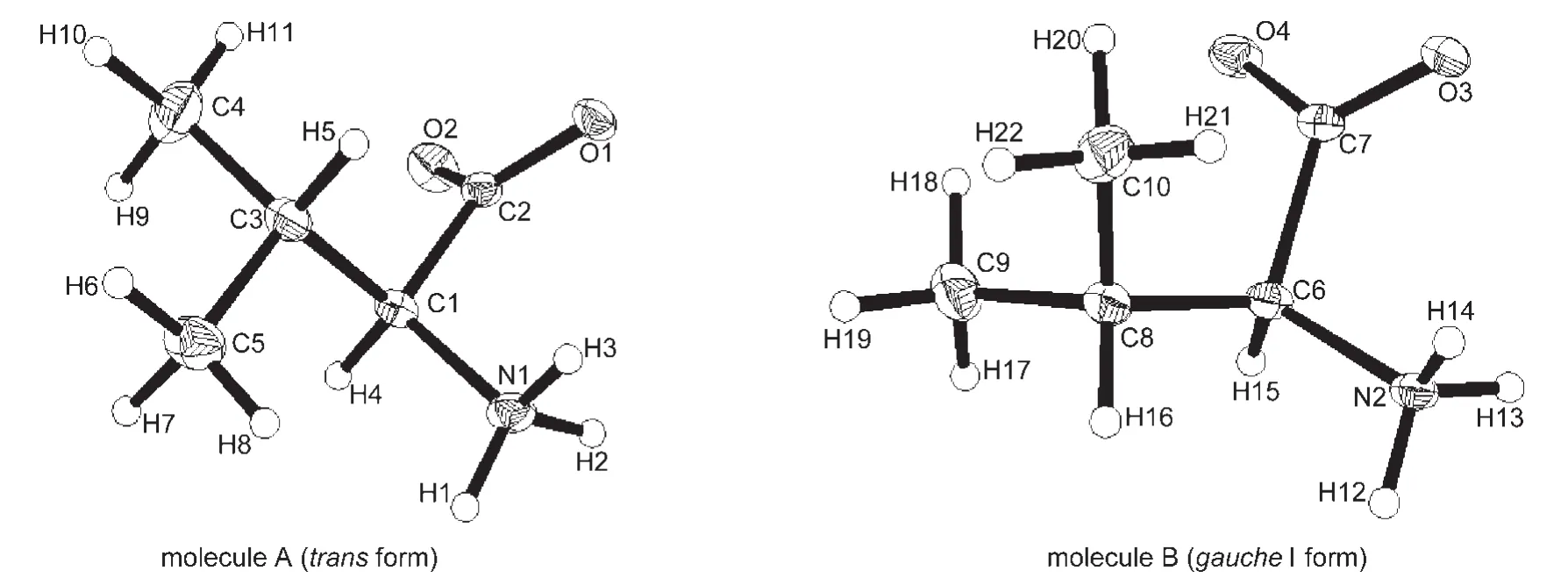
Fig.3 Structures of two crystallographically independent moleculesAand B in L-valine

Table 3 Atomic coordinates and equivalent isotropic displacement parameters(nm)of D-valine at 293,270,223,173 K

continued Table 3

Table 4 Temperature dependence of bond distances(nm)of D-valine
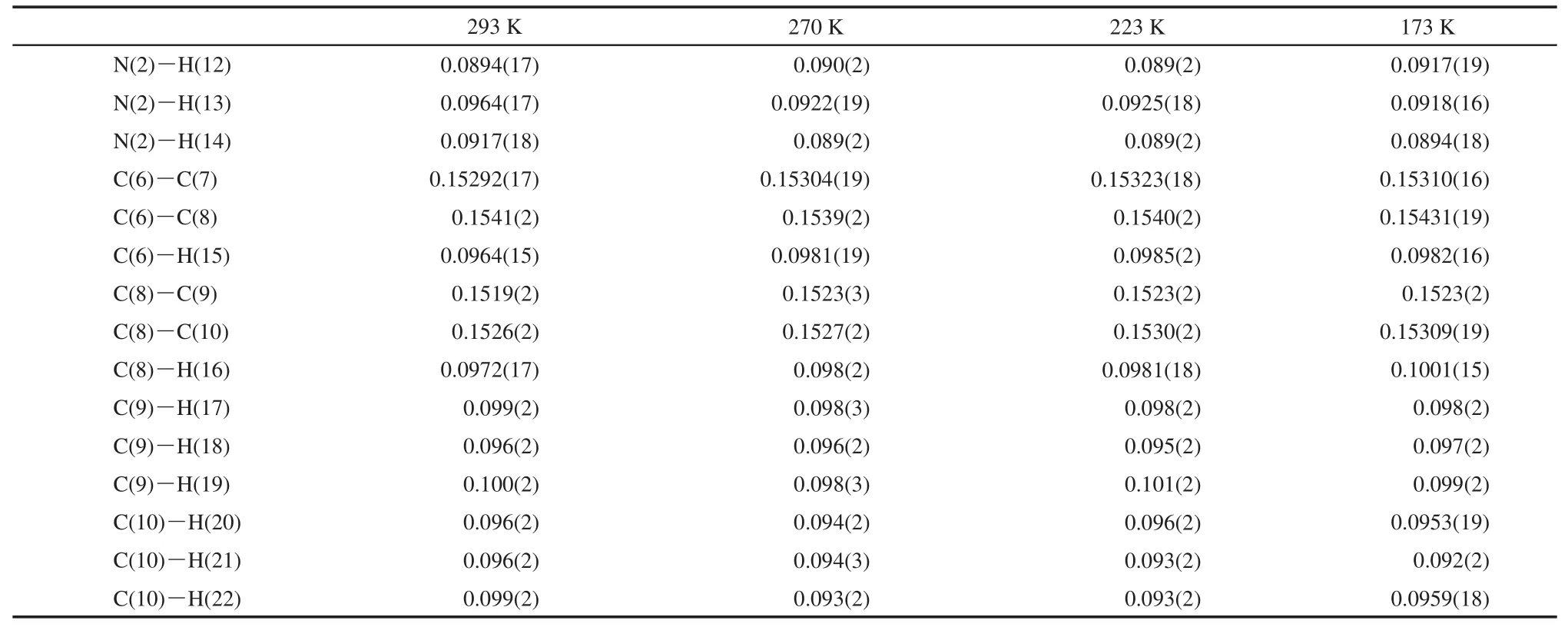
continued Table 4

Table 5 Temperature dependence of bond angles(°)of D-valine

continued Table 5

Table 6 N−H…O bond distances(nm)and bond angles(°)of D-valine at 293,270,223,173 K

Table 7 Atomic coordinates and equivalent isotropic displacement parameters(nm)of L-valine at 293,270,223,173 K
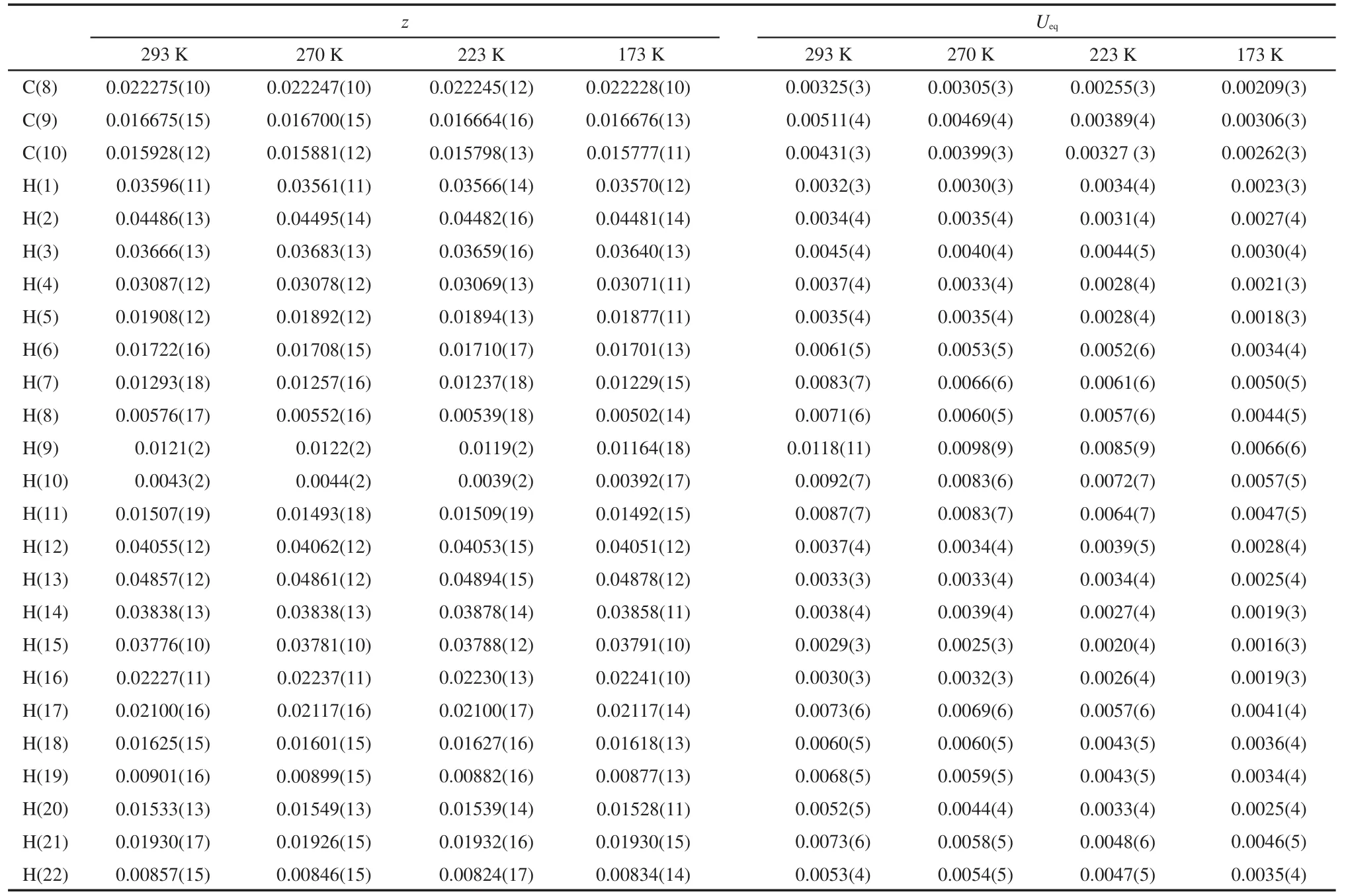
continued Table 7

Table 8 Temperature dependence of bond distances(nm)of L-valine

continued Table 8

Table 9 Temperature dependence of bond angles(°)of L-valine

continued Table 9
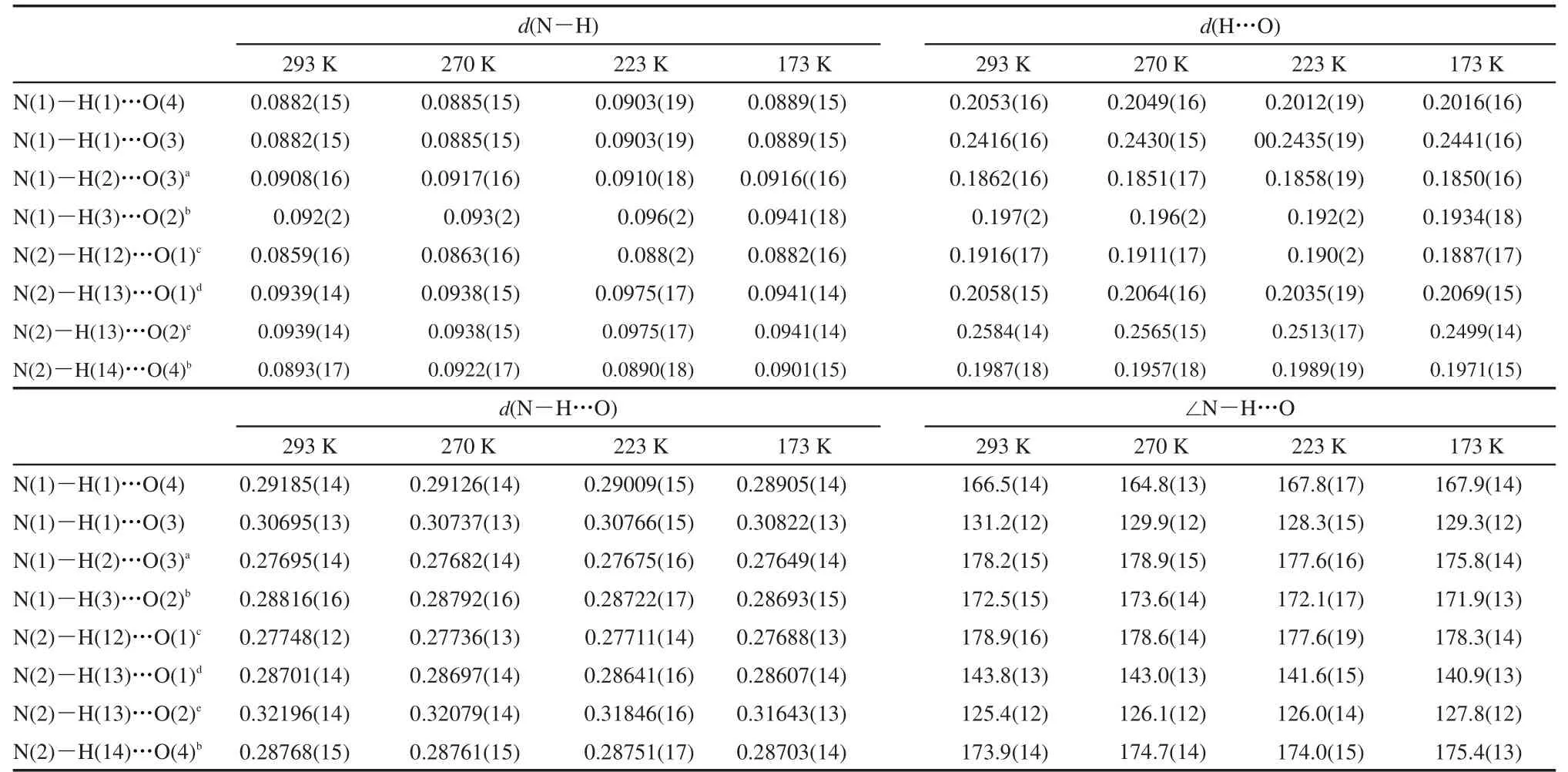
Table 10 N−H…O bond distances(nm)and bond angles(°)of L-valine at 293,270,223,173 K
3.3 Optical rotatory angles of D-,L-,and DL-valine crystals
In the measurement of optical rotatory angle,the probe diameter was 8 mm,and the diameter of laser spot was 1.5 mm.Thethickness of crystals was as follows:D-valine,0.12 mg·mm-2;L-valine,0.14 mg·mm-2;and DL-valine,0.23 mg·mm-2.Each sample was measured by cooling/heating and obverse/reverse side to eliminate the possible birefringent effect.The relationship between the optical rotatory angle(φ)of D-valine and temperature(T)was shown in Fig.4.The jump of rotatory angle was appeared at 270 K,then varied to-1.3°at 240 K with temperature decreasing.When temperature was increased from 270 to 290 K,the rotatory angle was kept close to-1.85°.

Fig.4 Temperature-dependent optical rotatory angle of D-valine crystal
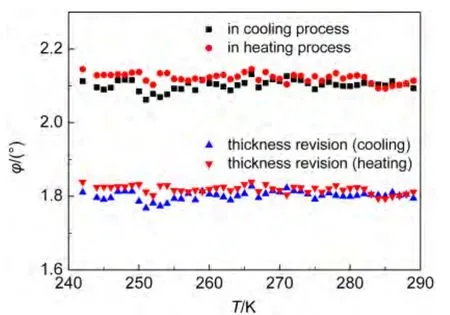
Fig.5 Temperature-dependent of optical rotatory angle of L-valine crystal
The optical rotatory angle of L-valine with temperature was shown in Fig.5,which was stabilized at+2.1°from 240 to 290 K.For comparison,φis multiplied by the revision of crystal thickness:φ=+2.1°×0.12/0.14=+1.8°,which coincides with the average value of optical rotatory angle of D-valine(φ=-1.85°).It is a significant characterization of the existence of macroscopic spin current in the ground state of chiral superconductor in the absence of applied external fields.27Because the dipole of N+H…O−in L-valine is originally aligned antiparallel to the internal field,it cannot turn to align itself parallel to the field unless it can get rid of the same amount of energy.28
The relationship between rotatory angle of DL-valine and temperature was shown in Fig.6.It is obvious when temperature is higher than transition temperature(270 to 290 K),the rotatory angle is kept at 0.05°(close to 0°).There was a jump of rotatory angle when the temperature was decreased from 270 to 240 K,the rotatory angle was increased to 1.6°at 240 K.Note that a single Cooper pair(k↑,-k↓)carries a spin current not a charge current,Fig.6 showed the characteristic of the metastable superfluidity in optical lattices.The spin superfluidity exhibited in racemic DL-valine crystal.

Fig.6 Temperature dependent optical rotatory angle of DL-valine crystal
4 Conclusions and prospects
Heat capacity confirmed the asymmetry of electron spin-flip transition of N+H…O-bond in D-and L-valine single crystals around 270 K.Temperature dependence of crystal fine structures showed the transition in D-valine but excluded the configuration transformation from D-to L-valine.Measurement of the optical rotatory angle in polar crystals observed the slow motion of electron at low temperature29and proved the“superfluidity”of spin currents onto D-,L-,and DL-valine optical lattices from 270 to 290 K.In summary,the significance of this article provides evidence for the existence of macroscopic spin currents in superconductors in the absence of applied external field.The spin current arises through an intrinsic spin Hall effect upon the transition to the superconducting state.30-32
(1) Salam,A.J.Mol.Evol.1991,33,105.doi:10.1007/BF02193624
(2) Salam,A.Phys.Lett.1992,B288,153.
(3) Salam,A.The Origin of Chirality,the Role of Phase Transitions and Their Induction inAminoAcids.InChemical Evolution:Origin of Life;Ponnamperuma,C.,Chela-Flores,J.Eds.;A.Deepak Publishing:Hampton,Virginia,U.S.A.,1993;pp 101-117.
(4) Salam,A.Biological Macromolecules and the Phase TransitionsThey BringAbout.Proceeding of the Second International Symposium on Conceptual Tools for Understanding Nature;Costa,G.,Claucci,G.,Giorgi,M.Eds.;World Scientific:Singapore,Trieste,Sept 23-25,1992;pp 209-220.
(5) Figureau,A.;Duval,E.;Boukenter,A.Search for Phase Transitions Changing Molecular Chirality.Proceeding of the Trieste Conference on Chemical Evolution and the Origin of Life;Ponnamperuma,C.,Chela-Flores,J.Eds.;A.Deepak Publishing:Hampton,Virginia,U.S.A.,1993;pp 157-164.
(6) Figureau,A.;Duval,E.;Boukenter,A.Origin of Life and Evolution of the Biosphere1995,25,211.doi:10.1007/BF01581584
(7) Bonner,W.A.Chirality2000,12,114.
(8) Torri,K.;Iitaka,Y.Acta Cryst.B1970,26,1317.doi:10.1107/S0567740870004065
(9) Dalhus,B.;Görbitz,C.H.Acta Chem.Scand.1996,50,544.doi:10.3891/acta.chem.scand.50-0544
(10) Mallikarjunan,M.;Rao,S.T.Acta Cryst.B1969,25,296.
(11) Pawlukojć,A.;Bobrowicz,L.;Natkaniec,I.;Leciejewicz,J.Spectrochimica Acta A1995,51,303.
(12)Gong,Y.;Wang,W.Q.;Liu,H.W.;Du,S.X.;Guo,H.M.;Wang,Y.L.;Gao,H.J.Acta Phys.-Chim.Sin.2005,21,867.[龚,王文清,刘虹雯,杜世萱,郭海明,王业亮,高鸿钧.物理化学学报,2005,21,867.]doi:10.3866/PKU.WHXB20050809
(13)Guo,H.M.;Liu,H.W.;Wang,Y.L.;Gao,H.J.;Gong,Y.;Jiang,H.Y.;Wang,W.Q.Surface Science2004,552,70.
(14)Wang,W.Q.;Gong,Y.;Liang,Z.;Sun,F.L.;Shi,D.X.;Gao,H.J.Surface Science2002,512,L379.
(15) Carlin,R.L.Magnetochemistry;Springer-Verlag:Berlin,Heidelberg,1986;p 309.
(16) Cronin,J.R.;Pizzarello,S.Science1997,275,951.doi:10.1126/science.275.5302.951
(17) Sullivan,R.;Pyda,M.;Pak,J.;Wunderlich,B.;Thompson,J.R.;Pagni,R.;Pan,H.;Barnes,C.;Schwerdtfeger,P.;Compton,R.J.Phys.Chem.A2003,107,6674.doi:10.1021/jp0225673
(18)Wang,W.Q.;Yi,F.;Ni,Y.M.;Zhao,Z.X.;Jin,X.L.;Tang,Y.Q.J.Biol.Phys.2000,26,51.doi:10.1023/A:1005187416704
(19)Wang,W.Q.;Shen,X.C.;Wu,J.L.;Gong,Y.;Shen,G.H.;Zhao,H.K.Acta Phys.-Chim.Sin.2012,28,773.[王文清,沈新春,吴季兰,龚,申国华,赵洪凯.物理化学学报,2012,28,773.]doi:10.3866/PKU.WHXB201202132
(20) (a)Hutchens,J.O.;Cole,A.G.;Stout,J.W.J.Am.Chem.Soc.1960,82,4813.doi:10.1021/ja01503a014(b)Handbook of Biochemistry and Molecular Biology,Physical and Chemical Data;CRC Press:Cleveland,1976;Vol.4.
(21) Hutchens,J.O.;Cole,A.G.;Stout,J.W.J.Phys.Chem.1963,67(5),1128.doi:10.1021/j100799a047
(22) Paukov,I.E.;Kovalevskaya,Y.A.;Boldyreva,E.V.J.Therm.Anal.Calorim.2013,111,905.
(23)Wang,W.Q.;Min,W.;Zhu,C.F.;Yi,F.Phys.Chem.Chem.Phys.2003,5,4000.doi:10.1007/s10973-012-2286-6
(24)Chen,Y.;Wang,W.Q.;Du,W.M.Acta Phys.-Chim.Sin.2004,20,540.[陈 渝,王文清,杜为民.物理化学学报,2004,20,540.]doi:10.3866/PKU.WHXB20040519
(25) Yates,J.R.;Pickard,C.J.;Payne,M.C.;Dupree,R.;Profeta,M.;Mauri,F.J.Phys.Chem.A2004,108,6032.doi:10.1021/jp049362+
(26)Yamada,K.;Nemoto,T.;Asanuma,M.;Honda,H.;Yamazaki,T.;Hirota,H.Solid State Nuclear Magnetic Resonance2006,30,182.doi:10.1016/j.ssnmr.2006.09.003
(27) Yang,S.J.;Feng,S.P.New Journal of Physics2010,12,1.
(28) Eisberg,R.;Eesnick,R.Quantum Physics of Atoms,Molecules,Solids,Nuclei,and Particles;John Wiley&Sons:New York,1985;p 266.
(29) (a)Lee,T.D.;Low,F.E.;Pines,D.Phys.Rev.1953,90,297.doi:10.1103/PhysRev.90.297(b)Lee,T.D.Lee,T.D.Scientific Papers Selected,Vol.1;China Center ofAdvanced Science and Technology,Ed.;Shanghai Science and Technology Press:Shanghai,2006;pp 64-66.[李政道.李政道科学论文选(上册).中国高等科学技术中心编.上海:上海科学技术出版社,2006:64-66.]
(30) Hirsch,J.E.Physical Review B2005,71(18),4521.
(31)Wang,W.Q.;Gong,Y.;Shen,X.C.;Zhang,Y.F.Acta Phys.-Chim.Sin.2013,29,473.[王文清,龚,沈新春,张玉凤.物理化学学报,2013,29,473.]doi:10.3866/PKU.WHXB201212273
(32)Wang,W.Q.;Shen,X.C.;Zhang,Y.F.;Gong,Y.Acta Phys.-Chim.Sin.2013,29,1396.[王文清,沈新春,张玉凤,龚.物理化学学报,2013,29,1396.]doi:10.3866/PKU.WHXB201304163

