废糟液全循环条件下絮凝酵母乙醇连续发酵
孜力汗,刘晨光,白凤武大连理工大学生命科学与技术学院,辽宁 大连 116024
废糟液全循环条件下絮凝酵母乙醇连续发酵
孜力汗,刘晨光,白凤武
大连理工大学生命科学与技术学院,辽宁 大连 116024
丙酸是以玉米为原料自絮凝酵母乙醇连续发酵系统废糟液全循环过程中积累的主要抑制物。基于丙酸对酵母细胞抑制机理,开发了3种废糟液全循环条件下乙醇连续发酵工艺策略。首先根据高温导致丙酸生成的现象,去除了物料灭菌环节,使发酵液丙酸浓度显著降低,生物量和乙醇浓度分别提高了59.3%和7.4%。其次,以丙酸浓度达到半数抑制浓度(IC50)40 mmol/L为目标,通过拟合丙酸积累数据预测废糟液全循环的最长运行时间,发酵装置运行应控制在此时间范围内。再次,较低的环境pH值提高了丙酸毒性,而实验证明发酵液pH为5.5时,丙酸对细胞生长的抑制影响最小,因此控制发酵过程中的pH有利于弱化丙酸毒性。
自絮凝酵母,乙醇连续发酵,废糟液全循环,丙酸抑制
我国“十五”期间开始发展燃料乙醇,先后在黑、吉、皖、豫和桂五省区建设了大型生产装置,但自投产以来都依赖国家财政补贴运行。截止到2012年底国家的直接财政补贴已经超过100亿。目前糖质和淀粉质原料生产的燃料乙醇占市场总量的90%以上,生产成本主要来自原料和能耗[1-2],其中能耗主要来自发酵液精馏和废糟液处理过程,特别是常用的废糟液离心后上清液多效蒸发浓缩技术[3]。因此,废糟液循环利用是降低其处理能耗最有效的策略。
自絮凝酵母乙醇发酵可以在发酵结束后通过沉降的方式将酵母从发酵液中分离,显著降低精馏过程产生废糟液的COD,为大比例乃至全循环废糟液创造了良好条件[4]。然而在废糟糟液全循环过程中,高沸点副产物会在系统中积累,对酵母细胞生长和乙醇发酵产生影响[5-6]。丙酸被认为是主要的抑制物[7],主要来自于原料液化、培养基灭菌和发酵液蒸馏等高温环节[8-9]。
丙酸对酵母生长的影响与其他有机酸类似。在pH值较低的条件下,多以未解离形态存在,较容易透过脂质膜进入细胞内部。在胞内pH接近中性环境下,有机酸解离释放出质子,造成胞质酸化而使细胞死亡[10-11]。因此浓度和环境pH值是影响丙酸对酵母细胞毒性的关键因素。本文针对丙酸的抑制机理,通过实验研究开发了3种废糟液全循环絮凝酵母乙醇发酵策略,为该工艺的工业化生产和稳定运行提供技术支持。
1 材料与方法
1.1 菌株和原料
自絮凝酿酒酵母(SPSC01)由中国微生物保藏中心保存,菌株号为0587[12]。玉米粉由中粮生化能源 (肇东) 有限公司赠送;液化酶和糖化酶由诺维信 (中国) 公司赠送。
1.2 培养基与发酵
种子培养基:葡萄糖30 g/L,酵母粉4 g/L,蛋白胨3 g/L,121 ℃灭菌20 min。
发酵培养基:玉米糖化液还原糖浓度约为220 g/L,(NH4)2HPO42.5 g/L,KH2PO41.0 g/L,121 ℃灭菌20 min。玉米糖化液的制备采用双酶法水解玉米粉[13]。
将种子培养液静置后弃去上清液,加入100 mL 灭好菌的100 mmol/L 柠檬酸钠溶液使絮凝酵母解絮以控制接种量,将解絮后的酵母种子按照5% (V/V)的接种量接入发酵培养基中。
1.3 丙酸影响实验
以无添加为对照,将丙酸分别按照10、20、30、40、50和60 mmol/L的浓度添加到发酵培养基中,调节发酵培养基初始pH值为4.5,温度30 ℃,搅拌速率150 r/min,发酵36 h后检测乙醇、残糖、生物量浓度。
通过丙酸对酵母生长的抑制率 (y) 来评价丙酸对酵母乙醇发酵的影响,计算公式如下:
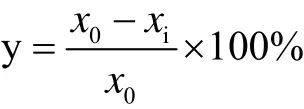
其中x0和xi分别为对照组和丙酸添加组对应的菌体生物量,g (DCW)/L。
1.4 废糟液全循环条件下絮凝酵母乙醇连续发酵
在有效体积为1.5 L的搅拌式反应器中,种子扩大培养结束后以稀释速率0.04 h-1流加发酵培养基进行连续发酵,温度30 ℃,pH 4.2–4.5,搅拌速率150 r/min,通气量0.05 vvm。每隔3天将收集到的发酵液进行蒸馏,得到的废糟液全部用于玉米粉调浆制备糖化液。玉米糖化液制备好后进行过滤,清液稀释至发酵所需还原糖浓度,并加入相应浓度营养盐配制成发酵培养基[14]。
1.5 分析方法
乙醇和甘油浓度用Waters高效液相色谱测定[15]。还原糖采用二硝基水杨酸法测定[16]。生物量测量采用干重法[17]。丙酸和乳酸采用Dionex ICS-2500离子色谱系统分析[18]。
2 结果与分析
2.1 丙酸的来源及毒性
前期工作已经证明,丙酸是废糟液全循环乙醇连续发酵过程积累的主要抑制物,主要来源于原料高温处理过程。玉米粉中主要来自籽皮的半纤维素组分水解产生的戊糖及其衍生物在高温作用下分解产生丙酸[19]。因此,高温灭菌和蒸馏环节是丙酸的主要来源[7]。
随着丙酸浓度的增加,对酵母生长的抑制逐渐明显 (图1)。丙酸浓度为40 mmol/L时对酵母生长的抑制达到约50%,因此其半数抑制浓度(IC50) 约为40 mmol/L。降低丙酸浓度可以有效弱化其对酵母细胞的抑制作用。

图1 丙酸对絮凝酵母生长的影响Fig. 1 Effect of propionic acid on the growth of the flocculating yeast.
2.2 策略1:减少高温处理环节
高温有利于副产丙酸[7,20],因此避免过程高温操作环节可以减少丙酸生成。在乙醇发酵过程中,原料液化、糖化、灭菌和发酵液蒸馏都需要较高温度,而灭菌又是温度最高的环节。在满足生产要求的前提下,去除发酵培养基的灭菌环节可以减少丙酸生成,改善乙醇发酵过程。
发酵培养基不经高温灭菌直接进行乙醇连续发酵,使得废糟液循环条件下丙酸积累量显著降低 (图2),生物量积累升高了59.3%,乙醇浓度升高了7.4%,残糖浓度降低了40.1% (表1)。测定乳酸浓度来评价发酵过程杂菌污染情况[21],培养基不灭菌条件下发酵液乳酸含量有所增高,但总量依然较低,且经过显微镜镜检未发现大量杂菌。虽然糖醇转化率稍有降低,但仍然可以满足发酵需求。更重要的是,由于降低了主要抑制物丙酸的影响,发酵过程平均乙醇浓度是增高的。
乙醇工业生产过程中,醪液经高温液化后进行预糖化,然后直接进入发酵罐,进行同步糖化发酵。液化温度对大多数微生物都有杀灭作用,而酵母作为优势菌种、发酵罐中的低pH环境以及生成的乙醇可以有效抑制细菌繁殖,因此醪液不灭菌处理在乙醇发酵工业化生产中普遍使用。
2.3 策略2:控制装置运行时间
虽然去除了灭菌环节使得丙酸生成量显著降低,但由于酵母不代谢丙酸且丙酸沸点高不易挥发,随着废糟液全循环乙醇连续发酵长时间运行得到积累。了解丙酸积累规律,并控制废糟液循环时间,可以使丙酸积累对酵母细胞的影响维持在较低水平。

图2 废糟液全循环乙醇连续发酵过程丙酸积累Fig. 2 Propionic acid accumulation during ethanol fermentation by the flocculating yeast under the stillage backset condition.

表1 培养基高温灭菌和不经高温灭菌条件下废糟液全循环乙醇连续发酵过程主要工艺技术指标Table 1 Biomass, ethanol, residual sugars and lactic acid detected during continuous ethanol fermentation by the flocculating yeast under the stillage backset and medium with/without sterilization conditions
如图2可以看出,培养基灭菌条件下丙酸积累较明显。通过拟合并获得了丙酸随废糟液循环时间积累的方程为:

Y为丙酸浓度 (mg/L),X为发酵装置运行时间 (h)。设定丙酸浓度不超过IC50(40 mmol/L),按上述公式计算丙酸达到IC50时发酵装置运行时间分别为28 d (675.8 h) 和38 d (908.7 h)。因此减少高温灭菌环节使得发酵装置运行时间显著延长。
2.4 策略3:控制发酵过程pH值
丙酸作为一种有机酸,其对酵母细胞的毒性与环境pH值密切相关。图3所示为环境pH值对酵母生长的影响。
发酵培养基初始pH分别调节至3.0、4.0、4.5和5.5。在相同pH值条件下,丙酸浓度的增加对酵母生长的抑制增强。当丙酸浓度小于30 mmol/L时,不同pH值对酵母生长的影响几乎无差异。当丙酸浓度大于IC50时,pH值减小,增加了丙酸的抑制效果。由于丙酸的pKa为4.87,当环境pH低于该值时,丙酸多以未解离形式存在并较容易进入细胞对酵母产生毒性。培养基pH 5.5大于丙酸pKa值,使得未解离形式丙酸量大大降低,因此控制发酵液pH在5.5比较合适。

图3 pH值和丙酸浓度对絮凝酵母生长的影响Fig. 3 Effect of propionic acid and pH on yeast growth.
3 结论与展望
基于丙酸对酵母细胞抑制机理,提出了3种废糟液全循环条件下乙醇连续发酵的工艺策略。首先在满足乙醇生产要求的前提下,通过去除原料灭菌环节使丙酸的生成显著降低;其次通过控制发酵装置运行时间避免丙酸浓度超过IC50;再次,环境pH对丙酸的细胞毒性有重要作用,控制发酵液pH值5.5显著弱化丙酸对酵母细胞的毒性。以上3种方法可以单独使用,也可以同时应用以达到更好效果。
REFERENCES
[1] Bai FW, Anderson WA, Moo-Young M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv, 2008, 26: 89–105.
[2] Li F. Consecutive batch fermentation for very high gravity ethanol production using flocculating yeast [D]. Dalian: Dalian University of Technology, 2010 (in Chinese).李凡. 絮凝酵母重复批次高浓度乙醇发酵的研究[D].大连: 大连理工大学, 2010.
[3] Hill J, Nelson E, Tilman D, et al. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Nat Acad Sci USA, 2006, 103: 11206–11210.
[4] Li DX, Bai FW, Song Q, et al. Effect of distilled effluent recycle on the continuous ethanol fermentation usingself-flocculating yeast cells. Chin J Appl Environ Biol, 1999, 5(5): 533–536 (in Chinese).李东侠, 白凤武, 宋琪, 等. 自絮凝颗粒酵母酒精连续发酵过程精馏废液循环回用工艺的研究. 应用与环境生物学报, 1999, 5(5): 533–536.
[5] Egg RP, Sweeten JM, Coble CG. Grain sorghum stillage recycling: effect on ethanol yield and stillage quality. Biotechnol Bioeng, 1985, 27: 1735–1738.
[6] Maiorella BL, Blanch HW, Wilke CR. By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng, 1983, 24: 103–121.
[7] Zi LH, Liu CG, Xin CB, et al. Stillage backset and its impact on ethanol fermentation by the flocculating yeast. Process Biochem, 2013, 48(5/6): 753–758.
[8] Girisuta B, Danon B, Manurung R, et al. Experimental and kinetic modeling studies on the acid-catalysed hydrolysis of the water hyacinth plant to levulinic acid. Bioresource Technol, 2008, 99(17): 8367–8375.
[9] Shen DK, Gu S, Bridgwater AV. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J Anal Appl Pyrol, 2010, 87(2): 199–206.
[10] Lambert RJ, Stratford M. Weak-acid preservatives: modelling microbial inhibition and response. J Appl Microbiol 1999, 86: 157–164.
[11] Piper P, Calderon CO, Hatzixanthis K, et al. Weak acid adaptation: the stress response that confers yeasts resistance to organic acid food preservatives. Microbiology, 2001, 147: 2635–2642.
[12] Xue C, Zhao XQ, Bai FW. Effect of the size of yeast flocs and zinc supplementation on continuous ethanol fermentation performance and metabolic flux distribution under very high concentration conditions. Biotechnol Bioeng, 2010, 105(5): 935–944.
[13] Xu TJ, Zhao XQ, Bai FW. Continuous ethanol production using self-flocculating yeast in a cascade of fermentors. Enzyme Microb Technol, 2005, 37: 634–640.
[14] Yan Z, Zi LH, Li N, et al. Continuous ethanol fermentation using Self-flocculating yeast in multi-stage suspended bioreactors coupled with directly recycling of waste distillage. Chin J Biotech, 2005, 21(4): 628–632 (in Chinese).严正, 孜力汗, 李宁, 等. 多级串联悬浮床反应器系统中自絮凝颗粒酵母乙醇连续发酵耦合废糟液直接全循环使用的研究. 生物工程学报, 2005, 21(4): 628–632.
[15] Shen Y, Zhao XQ, Ge XM, et al. Metabolic flux and cell cycle analysis indicating new mechanism underlying process oscillation in continuous ethanol fermentation with Saccharomyces cerevisiae under VHG conditions. Biotechnol Adv, 2009, 27: 1118–1123.
[16] Chen YQ. Biochemistry Experiment Method and Technology. Beijing: Beijing Science and Technology Press, 2002: 90–94 (in Chinese).陈毓荃. 生物化学实验方法和技术, 北京: 科学出版社, 2002, 90–94.
[17] Bai FW, Chen LJ, Anderson WA, et al. Parameter oscillations in high gravity medium continuous ethanol fermentation and their attenuation on multi-stage packed column bioreactor system. Biotechnol Bioeng, 2004, 88: 558–566.
[18] Shen Y. Exploration for oscillation in continuous VHG ethanol fermentation with Saccharomyces cerevisiae [D]. Dalian: Dalian University of Technology, 2009 (in Chinese).申渝. 酵母细胞超高浓度乙醇连续发酵振荡行为的研究[D]. 大连: 大连理工大学, 2009.
[19] Shen DK, Gu S, Bridgwater AV. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J Anal Appl Pyrol, 2010, 87(2): 199–206.
[20] Palma CF. Modelling of tar formation and evolution for biomass gasification: a review. Appl Energ, 2013, 111: 129–141.
[21] Narendranath NV, Hynes SH, Thomas KC, et al. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl Environ Microbiol, 1997, 63: 4158–4163.
(本文责编 郝丽芳)
Process development for continuous ethanol fermentation by the flocculating yeast under stillage backset conditions
Lihan Zi, Chenguang Liu, and Fengwu Bai
School of Life Science and Biotechnology, Dalian University of Technology, Dalian 116024, Liaoning, China
Propionic acid, a major inhibitor to yeast cells, was accumulated during continuous ethanol fermentation from corn meal hydrolysate by the flocculating yeast under stillage backset conditions. Based on its inhibition mechanism in yeast cells, strategies were developed for alleviating this effect. Firstly, high temperature processes such as medium sterilization generated more propionic acid, which should be avoided. Propionic acid was reduced significantly during ethanol fermentation without medium sterilization, and concentrations of biomass and ethanol increased by 59.3% and 7.4%, respectively. Secondly, the running time of stillage backset should be controlled so that propionic acid accumulated would be lower than its half inhibition concentration IC50(40 mmol/L). Finally, because low pH augmented propionic acid inhibition in yeast cells, a higher pH of 5.5 was validated to be suitable for ethanol fermentation under the stillage backset condition.
self-flocculating yeast, continuous ethanol fermentation, stillage backset, propionic acid inhibition
May 14, 2013; Accepted: July 1, 2013
Fengwu Bai. Tel: +86-411-84706308; Fax: +86- 411-84706329; E-mail: fwbai@dlut.edu.cn
孜力汗, 刘晨光, 白凤武. 废糟液全循环条件下絮凝酵母乙醇连续发酵. 生物工程学报, 2014, 30(2): 310-314.
Zi LH, Liu CG, Bai FW. Process development for continuous ethanol fermentation by the flocculating yeast under stillage backset conditions. Chin J Biotech, 2014, 30(2): 310-314.
Supported by: National High Technology Research and Development Program of China (863 Program) (No. 2012AA021205), International S&T Cooperation Program of China (No. 2013DFA60470).
国家高科技研究发展计划 (863计划) (No. 2012AA021205),国家国际科技合作专项 (No. 2013DFA60470) 资助。

