3,5-二氨基苯甲酸构筑的Zn(Ⅱ)、Cd(Ⅱ)两种配合物的合成及晶体结构
张美丽 任宜霞 陈小利 闫洪涛
(1西北大学化学与材料科学学院,西安 710069)(2延安大学化学与化工学院,陕西省化学反应工程重点实验室,延安 716000)
Recently,the chemistry of Zn(Ⅱ)and Cd(Ⅱ)complexes has attracted interest for a number of reasons.The Zn(Ⅱ) and Cd(Ⅱ) are d10electronic configuration and can adopt different coordination numbers from 4 to 6.Moreover,the Zn and Cd complexes offer not only the fascinating structure,but only a wide range of potential application in many aspects,such as optical,electricalconductivity,catalysis and even photoluminescent materials[1-4].Up to now,a large numbers of complexes formed by Zn(Ⅱ),Cd(Ⅱ) and various N-donor,aromatic/heterocyclic multicarboxylate ligands have been successfully synthesized and characterized[5-10]. However, the complex based on 3,5-diaminobenzoic acid with Zn(Ⅱ),Cd(Ⅱ) have never been reported before.
Inspired by our previous works[11],we employed 3,5-diaminobenzoic acid and Zn(Ⅱ) ,Cd(Ⅱ) to synthesize two novel complexes,namely,[Cd(diaba)(phen)2]NO3·H2O(1)and [Zn(diaba)(2,2′-bipy)2](2)(diaba=3,5-diaminobenzoic acid;phen=1,10-phenanthroline,2,2′-bipy=2,2′-bipyridine),which provides the first example of complex based on 3,5-diaminobenzoic acid-Zn(Ⅱ) ,Cd(Ⅱ).
1 Experimental
1.1 Materials and methods
The reagents and solvents employed were commercially available and used as received without further purification.Elemental analyses for carbon,hydrogen,and nitrogen were performed with a Vario EL III elemental analyzer.The FTIR spectra were recorded from KBr pellets in the range 4 000~400cm-1on a Bruker EQUINOX-55 spectrometer.Fluorescence spectra were performed on a Hitachi F-4500 fluorescence spectrophotometer at room temperature.Thermogravimetric analyses (TGA)were performed under nitrogen with a heating rate of 10 ℃·min-1usingaNETZSCH STA 449C thermogravimetric analyzer.
1.2 Syntheses of title complex
Single-crystal samples of complex 2 were obtained by the similar method as described for 1.
[Cd(diaba)(phen)2]NO3·H2O(1).1 was synthesized hydrothermally in a 23-mL teonlined autoclave by heating a mixture of Cd(NO3)2·6H2O(1.0 mmol),daba(1.0 mmol),phen (0.5 mmol),and H2O (8 mL)at 160℃for 4 days.After the reactive mixture was slowly cooled to room temperature,colorless block crystals of 1 were obtained (Yield:41%,based on Cd).Anal.Calcd.for C31H27O6N7Cd(%):C,52.74;H,3.85;N,13.89.Found(%):C,52.45;H,3.53;N,13.31.IR(cm-1):3 327(br),1 618(w),1 558(s),1 411(s),1 375(w),1 184(w),999(w),854(s),777(s).
[Zn(diaba)(2,2′-bipy)2](2).Yield:38%(based on Zn).Anal.Calcd.for C27H23N6O2Zn(%):C,61.32;H,4.38;N,15.89.Found(%):C,61.26;H,4.54;N,15.96.IR(cm-1):3 464(br),1 602(s),1 537(s),1 438(s),1 354(s),1 195(w),1 026(s),862(w),788(m).
1.3 Crystal structure determination
Diffraction intensities for the complexes 1 and 2 were collected at 293 K on a Bruker SMART 1000 CCD diffractometer employing graphite-monochromated Mo Kα radiation (λ=0.071 073nm).A semiempirical absorption correction was applied using the SADABS program[12].The structure was solved by direct methods and refined by full-matrix least-squares on F2using the SHELXS 97 and SHELXL 97 programs,respectively[13-14].Non-hydrogen atoms were refined anisotropically and hydrogen atoms were placed in geometrically calculated positions.The crystallographic data for complex 1 are listed in Table1,and selected bond lengths and angles are listed in Table2.
CCDC:936420,1;936421,2.

Table1 Crystallographic data for compounds 1 and 2

Continued Table1
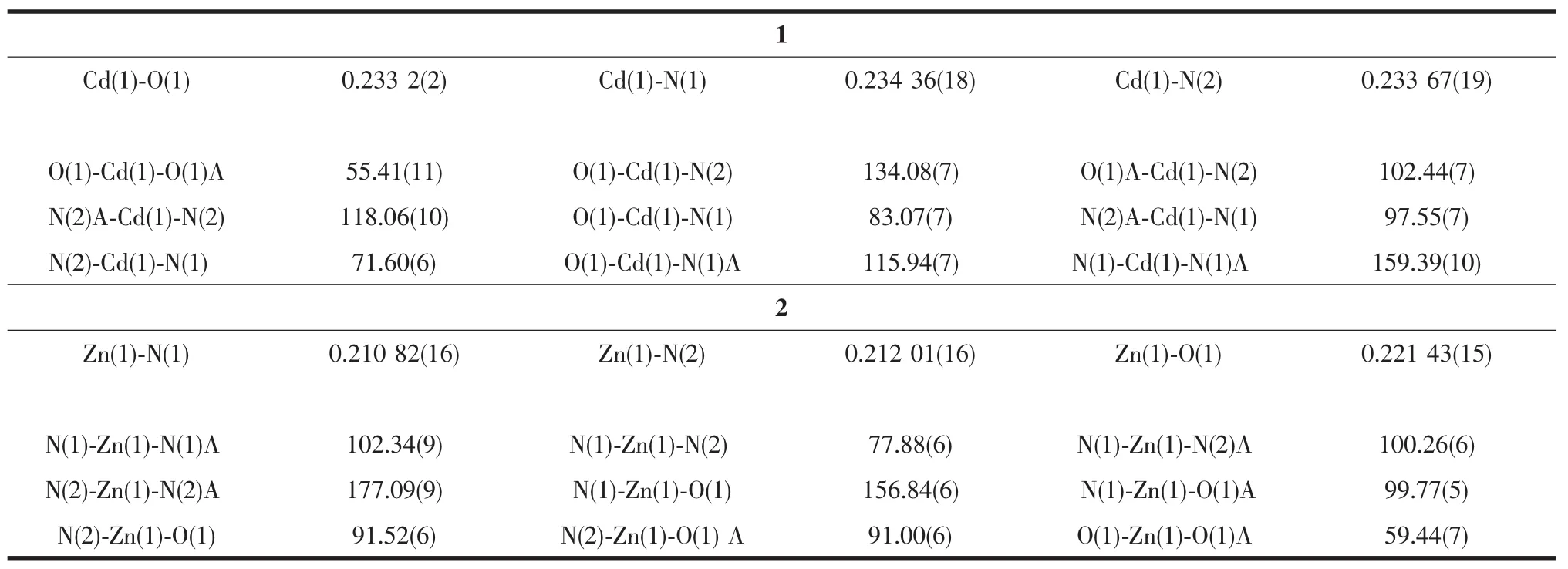
Table2 Selected bond length(nm)and angles(°)for compounds 1 and 2
2 Result and discussion
2.1 Crystal structure of[Cd(diaba)(phen)2]NO3·H2O(1)
The structures ofthe two complexes were determined by single-crystal X-ray diffraction analyses.Complex 1 belongs to orthorhombic,space group Fddd.While complex 2 belongs to monoclinic with C2/c space group.
The crystalstructure of1 consists ofone crystallographic independent CdIIcation,one diaba ligand and two phen ligands.Each CdIIcation is surrounded by four nitrogen atoms from two 1,10-phen ligands,and two oxygen atoms from one diaba ligand,composing a distorted octahedron pyramidal geometry(Fig.1).The Cd-N bond lengths are 0.233 67(19),0.234 36(18)nm,respectively,and the Cd-O bond lengths are 0.233 2(2)nm,which drop into the normal scope of Cd-N and Cd-O bond lengths[15].The details are depicted in Table2.The deproton NO3-ions do not coordinate with the central atoms,acting as free ions in the crystal lattice to charge balance.At last the Cd octahedrons,crystal waters and NO3-ions construct the new 3D supramolecular network by intricate hydrogen bonds and weak π … π stacting interaction.
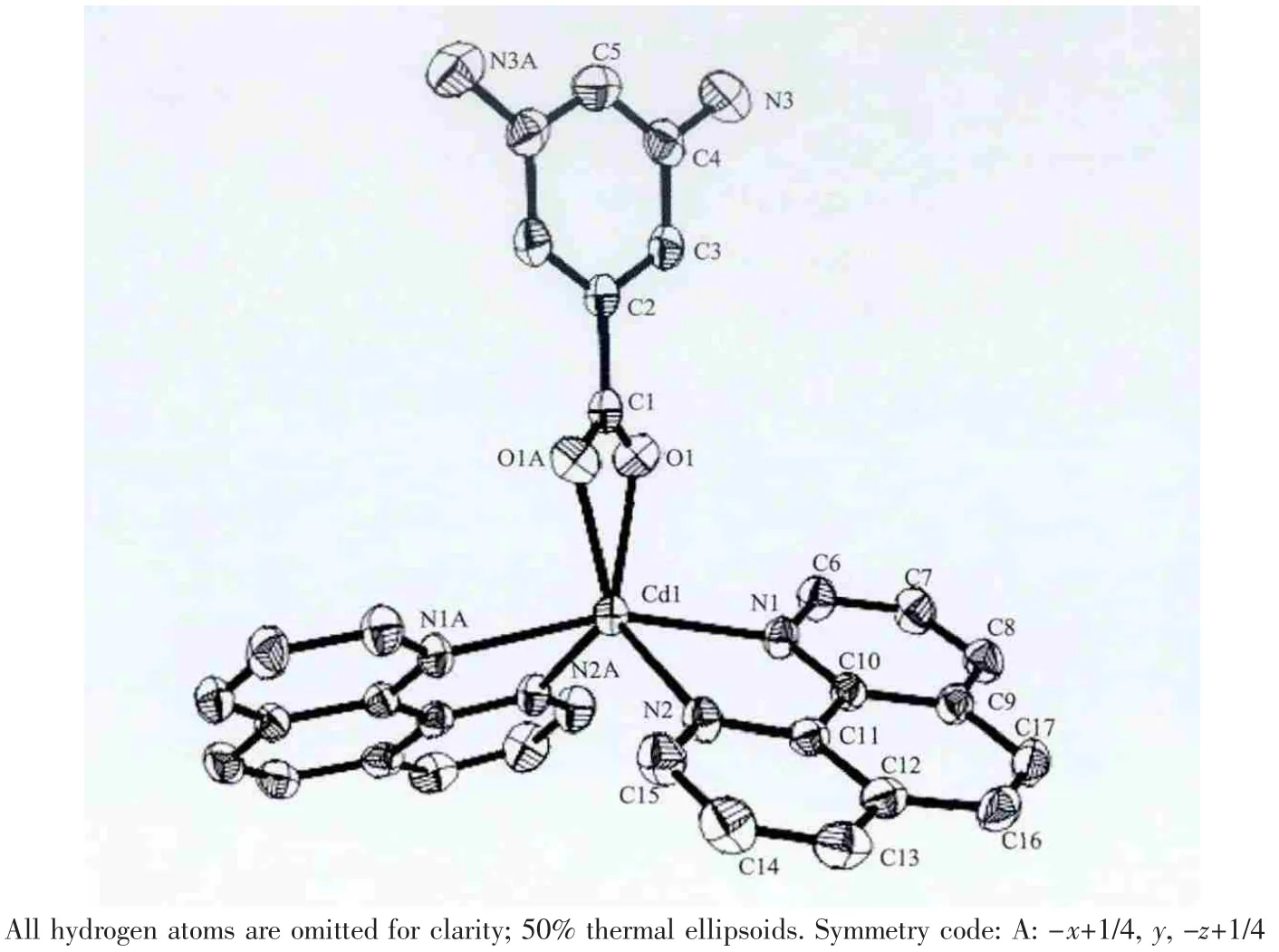
Fig.1 Coordination environments of Cd atom in complex 1
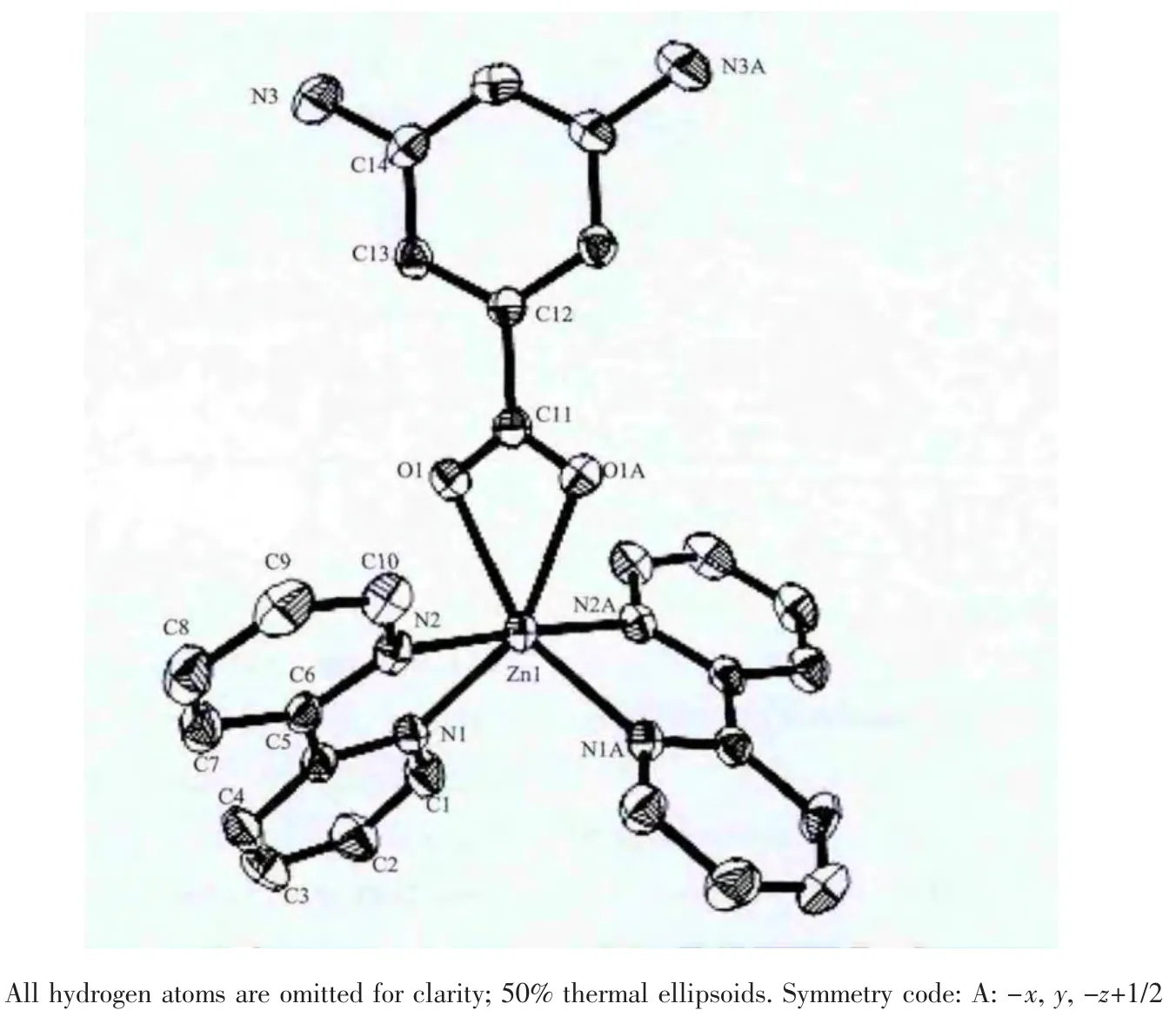
Fig.2 Coordination environments of Zn atom in complex 2
The structure of complex 2 is the same as complex 1,they are mononuclear structure.Each ZnIIion is six-coordinated to four nitrogen atoms of two 2,2′-bipy ligands and two oxygen atoms of one diaba ligand,fabricating a distorted octahedron pyramidal geometry (Fig.2).The Zn-N bond lengths are 0.210 82(16),0.212 01(16)nm,respectively,and the Zn-O bond lengths are 0.221 43(15)nm,which drop into the normal scope of Zn-N and Zn-O bond lengths[16].The 3D supramolecular network is also constructed via intricate hydrogen bonds and weak π…π stacting interaction.
2.2 Thermogravimetric analyses
Thermogravimetric analyses have been performed in air for 1 and 2 between 20 and 800℃(Fig.3,Fig.4).TGA curve of complex 1 illustrates that weight loss was observed for lattice water and NO3-ion up to 300℃;after this,significant weight loss occurred and endedatca.700℃,indicatingthecomplete decomposition of the complex to form CdO.This conclusion has been also supported by the percentage of the residues(18.03%),which is in accordance with the expected value (18.13%).For 2,there are not lattice and coordination water molecules and thus decomposition of the organic components occurs at 430℃,indicating the complete decomposition of the complex to form ZnO(Obsd:14.7%and Calcd:15.3%).
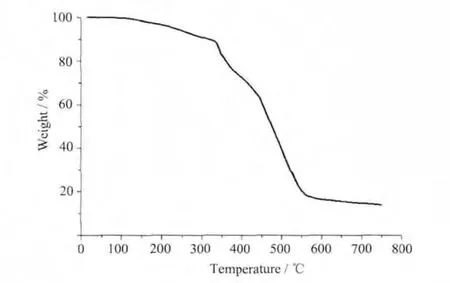
Fig.3 TGA curve for complex 1
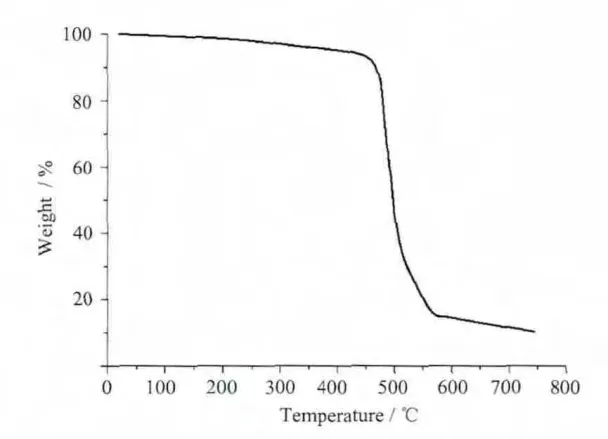
Fig.4 TGA curve for complex 2
2.3 Fluorescent properties
Solid-state Luminescentemission spectra of complexes 1 and 2 are depicted in Fig.5.The intense broad photoluminescence emissions are found at 399 nm(λex=335 nm)for 1,at 395 nm(λex=325 nm)for 2.In order to understand the nature of the emission band,the photoluminescence property of free diaba ligand was measured with the observation of one weak emission at 367 nm (λex=300 nm).In comparison to the free ligand,most of the emission maxima of complexes 1 and 2 are changed,which are neither metal-to-ligand charge transfer(MLCT)nor ligand-tometal transfer (LMCT)in nature,since the ZnIIand CdIIions are difficult to oxidize or reduce.Thus,they may be assigned to intraligand (π-π*)fluorescent emission[17].Many aromatic ligands that are not strongly emissive themselves display significant luminescence when coordinated to ZnIIor CdII[18].The enhancement of luminescence is perhaps a result of the coordination effect of those ligands to the metal center,which effectively increases the rigidity of ligands,thereby reducing the non-radiative decay of the intraligand(π-π*)excited state[19].
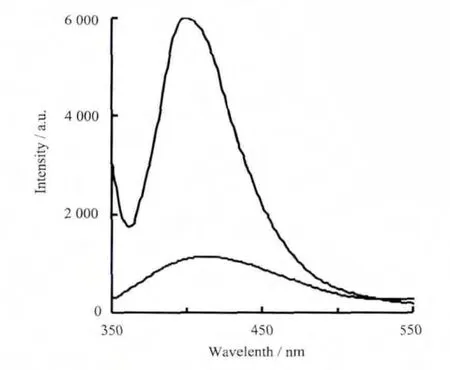
Fig.5 Solid-state emission spectra of 1(low curve),2(tall curve)at room temperature
[1]Allendorf M D,Bauer C A,Bhaktaa R K,et al.Chem.Soc.Rev.,2009,38:1330-1352
[2]Rocha J,Carlos L D,Paza F A A,et al.Chem.Soc.Rev.,2011,40:926-940
[3]Karabach Y Y,Silva M F C G,Kopylovich M N,et al.Inorg.Chem.,2010,49:11096-11105
[4]XUE Ming(薛铭),CHEN Si-Ru(陈思如),GUO Li-Jia(郭莉佳),et al.Chem.J.Chinese Universities(高等学校化学学报),2012,33(9):1889-1894
[5]Ma L F,Wang L Y,Wang Y Y,et al.Inorg.Chem.,2009,48(3):915-924
[6]Ma L M,Li B,Sun X Y,et al.Anorg.Allg.Chem.,2010,636:1606-1611
[7]Xu C Y,Li L K,Wang Y P,et al.Cryst.Growth Des.,2011,5:1869-1879
[8]Sun D,Yan Z H,Blatov V A,et al.Cryst.Growth Des.,2013,13(3):1277-1289
[9]Zhang Z H,Chen S C,He M Y,et al.Cryst.Growth Des.,2013,13(3):996-1001
[10]HU Jing-Song(胡劲松),LIU Xi-Hui(刘希慧),SHI Jian-Jun(石建军),et al.Chinese J.Inorg.Chem.(无机化学学报),2013,29(3):444-448
[11]Zhang M L,Li D S,Wang J J,et al.Dalton Trans.,2009,11:5355-5364
[12]Sheldrick G M.SADABS,A Program for Empirical Absorption Correction of Area detector Data,University of Gttingen,Germany,1997.
[13]Sheldrick G M.SHELXS 97,Program for Crystal Structure Solution,University of Gttingen,Germany,1997.
[14]Sheldrick G M.SHELXL 97,Program for Crystal Structure Refinement,University of Gttingen,Germany,1997
[15]Tian D,Pang Y,Zhou Y H,et al.CrystEngComm,2011,13:957-966
[16]Liu B,Yang G P,Wang Y Y,et al.Inorg.Chim.Acta,2011,367:127-134
[17]Wen L L,Dang D B,Duan C Y,et al.Inorg.Chem.,2005,44:7161-7170
[18]Bai H Y,Ma J F,Yang J,et al.Cryst.Growth Des.,2010,10:1946-1959
[19]Chang Z,Zhang A S,Hu T L,et al.Cryst.Growth Des.2009,9:4840-4846

