基于对叔丁基杯[8]芳烃的3d-5f核簇化合物(MxThy,M=Co、Ni、Zn)的合成、结构与磁性研究
杜尚超 谭华桥 毕研峰 于 洋 廖伍平*,
(1中国科学院长春应用化学研究所稀土资源利用国家重点实验室,稀土及钍清洁分离工程技术中心,长春 130022)
(2中国科学院大学,北京 100049)
The chemistry of the actinide elements is of critical importance to the nuclear energy industry[1].And the development of nuclear reprocessing and the study on the environmental impact of the radioactive actinides have driven many advances in fundamental actinide chemistry[2-3].In the recentyears,the heterometallic compounds with transition metals and actinides have drawn increasing attention due to their promising applications in magnetism,luminescence,photochemistry and catalysis[4-6].And most of them are studied with transition metals and uranium.The compounds of thorium were studied less[7].
Calixarenes,one type of macrocyclic ligands comprised ofseveralphenolunits,have been documented to be good multidentate ligands in the construction of polynuclear compounds[8-10].A series of 3d-4f heterometallic compounds based on p-tert-butyl(thia)calix[4]-arene and its derivatives have been synthesized[11-17].However,the compounds based on calix[8]arene were less reported due to higher charge,more flexible framework and a bigger cavity size of C8A[18-19].Actually,more coordination points and larger coordination region of calix[8]arene would benefit the construction of polynuclear compounds including the heterometallic complexes.Recently,we obtained a series of Co-Ln(Ln=Sm,Gdand Dy)compounds based on the p-tert-butylcalix[8]arene (H8C8A,scheme 1)under the solvothermal conditions[20].As continuation,this work presents three heterometallic compounds of 3d transition metal (Co,Ni and Zn)and thorium,namely,[Co2Th4(HC8A)2O2(OH)2(DMF)6](1),[Ni2Th5(H2C8A)(C8A)O4(OH)2(DMF)5(CH3OH)2](2)and[Zn2Th6(HC8A)(C8A)O5(CH3O)(C3H6NO2)2(DMF)5(CH3OH)] (3).These compounds give the first high nuclear clusters based on the related transition metals and thorium.
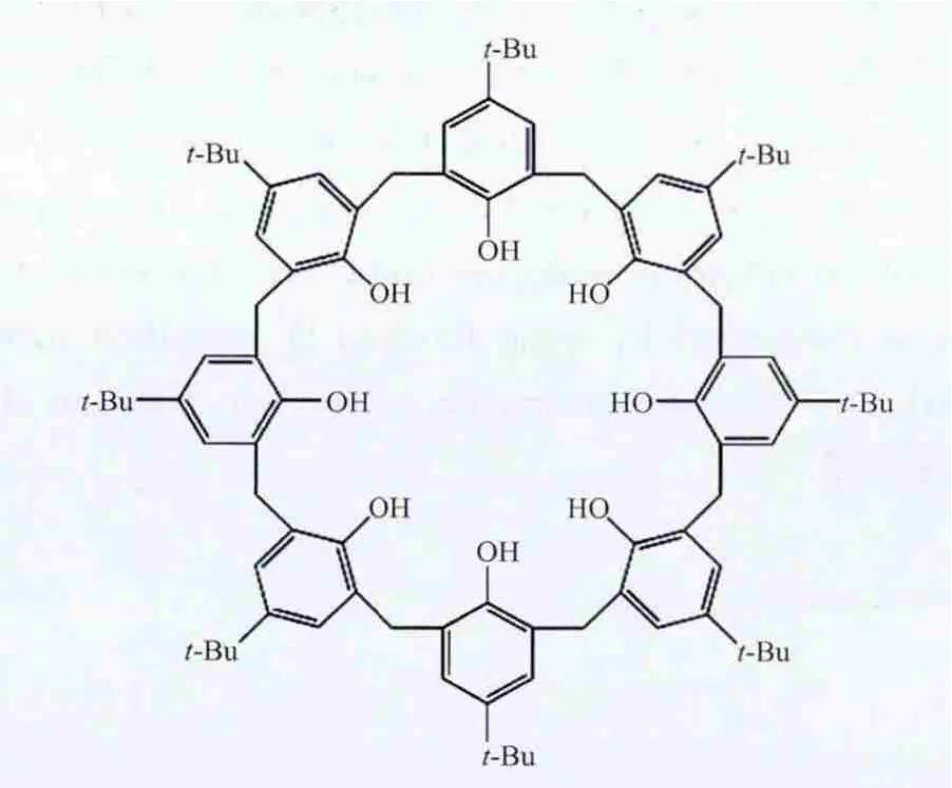
Scheme 1 Molecular structure of p-tert-butylcalix[8]arene(H8C8A)
1 Experimental
1.1 Materials and measurements
p-tert-Butylcalix[8]arene was synthesized by literature methods[21]and other reagents were purchased from commercial sources and used as received.Co/Ni/Zn and Th analyses were performed on a HITACHI S-4800 Scanning Electron Microscope equipped EDS.Elemental analyses for C,H and N were recorded on a VarioEL instrument.TGA measurementwas performed on a NETZSCH STA 449F3.FTIR(KBr pallets)spectra were recorded using a Bruker Vertex 70 spectrometer.Magnetic susceptibility measurement for compound 1 was performed on a Quantum Design MPMS XL-5 SQUID system with a 1000 Oe magnetic field in the range of2 ~300 K.Diamagnetic corrections for the sample and sample holder were applied to the data.
1.2 Synthesis of compounds
Purple single crystals of 1 were obtained by the reaction of a mixture of H8C8A (0.05 g,0.039 mmol),ThCl4·4H2O(0.05 g,0.11 mmol),CoSO4·7H2O(0.05 g,0.18 mmol),CH3OH (3.0 mL),DMF (3.0 mL)and triethylamine (0.65 mL)in a 20 ml Teflon-lined stainless steel autoclave which was kept at 130℃for 3 d and then slowly cooled to room temperature.The crystals were picked out for X-ray diffraction determination or washed with CH3OH and DMF for other measurements.The TG result of compound 1 was shown in Fig.1.Based on the TG results and SQUEEZE analysis,a suitable formula containing solvent molecules for 1 would be [Co2Th4(HC8A)2O2(OH)2(DMF)6]·6DMF·7CH3OH.The EDS analysis reveals that the molar ratio of nCo∶nTh=2.62 ∶5.08,comparable to the expected nCo:nThratio(1∶2).Element analysis (%):calculated for Co2Th4C219H324O39N12∶C,54.86,H,6.81,N,3.51;Found:C,53.86,H,6.65,N,3.46.FTIR(cm-1):3 636(w),3 442(w),3 046(w),2 949(s),2 865(m),1 682(s),1 653(s),1 481(s),1 391(m),1 360(m),1 303(m),1 254(m),1 209(m),1 124(m),1 094(m),913(m),866(m),821(m),748(m),682(m),530(m),490(m),438(m).
Green single crystals of 2 were obtained by the analogous method with CoSO4·7H2O replaced by NiSO4·6H2O (0.05 g,0.19 mmol)and the amount of DMF reducedto2.0 mL.TheTG analysisof compound 2 was shown in Fig.1.Based on the TG results and SQUEEZE analysis,a suitable formula containing solvent molecules for 2 would be[Ni2Th5(H2C8A)(C8A)O4(OH)2(DMF)5(CH3OH)2]·DMF.EDS result reveals that the molar ratio of nNi∶nTh=0.29∶0.71,comparable to the expected nNi∶nThratio(2∶5).Element analysis (%):calculated for Ni2Th5C196H262O30N6∶C,52.73,H,5.87,N,2.02;Found:C,49.61,H,5.92,N,2.22.FTIR(cm-1):3 548(w),3 404(w),2 952(s),2 867(m),1 647(s),1 478(s),1 392(m),1 360(m),1 299(m),1 260(m),1 206(m),1 120(m),908(m),866(m),820(m),743(m),681(m),484(m).
Colorless single crystals of 3 were obtained by replacing CoSO4·7H2O with ZnSO4·7H2O(0.05 g,0.17 mmol)and reducing the amount of DMF to 1.5 mL.TG result of 3 was shown in Fig.1.Based on the TG results and SQUEEZE analysis,a suitable formula containing solvent molecules for 3 would be[Zn2Th6(HC8A)(C8A)O5(CH3O)(C3H6NO2)2(DMF)5(CH3OH)]·DMF·3CH3OH.EDS analysis reveals that the molar ratio of nZn∶nTh=0.50∶1.45,comparable to the expected nZn:nThratio(2∶6).Element analysis(%):calculated for Zn2Th6C205H282O36N8:C,49.61,H,5.68,N,2.42;Found:C,47.57,H,5.70,N,2.50.FTIR (cm-1):3415(w),3047(m),2 955(s),2 861(m),1 649(s),1 540(m),1 478(s),1 392(s),1 361(m),1 300(s),1 206(s),1 124(m),908(m),864(m),822(m),748(m),679(m),617(m),490(s).
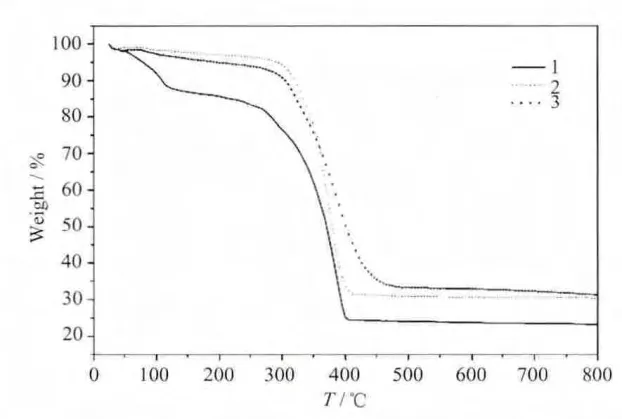
Fig.1 TG curves of compounds 1~3
1.3 Crystal structure determination
The X-ray intensity data for compounds 1~3 were collected on a Bruker APEX-II CCD diffractometer with graphite-monochromatized Mo Kα radiation (λ=0.071 073 nm)operated at 1.5 kW (50 kV,30 mA).The crystal structures were solved by means of direct methodsand refined employing full-matrix least squares on F2(SHELXTL-97)[22].Details for compound 1:All non-hydrogen atoms were refined anisotropically except for C83-C88 from butyl groups.Hydrogen atoms of the organic ligands were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors.
The carbon atoms of four butyl groups(C65-C67,C74-C76,C83-C88)were refined with disordered positions and the same displacement parameters.C89,C90 and C91 from one coordinated DMF molecules were also refined with disordered positions and the same displacement parameters.Details of compound 2:All non-hydrogen atoms were refined anisotropically except for N4,C100-C102,C200-C202.Hydrogen atoms of the organic ligands were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors.C71-C73,C101-C103 and N4 were refined with disordered positions and the same equal occupation of 0.25.Details for compound 3:All non-hydrogen atoms were refined anisotropically except for C196-C199 and N7 from coordinated DMF molecules.Hydrogen atoms of the organic ligands were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors.Th5,Zn1,C129-C131,C135-C137,C144-C152,C159-C161,C179,C184-C186,C196-C198 and N7 were refined with disordered positions and the same displacement parameters.The disordered C199 site was refined with an equal occupation of 0.5. Solvent molecules in these structurescan notbe properly modeled,whose contributions were subtracted by the “SQUEEZE”command as implemented in PLATON[23].The high R1and wR2factors of compounds 1~3 would be due to the weak high-angle diffractions and disorders.The crystallographic data for compounds 1~3 are listed in Table 1.
CCDC:968469,1;968470,2;968471,3.
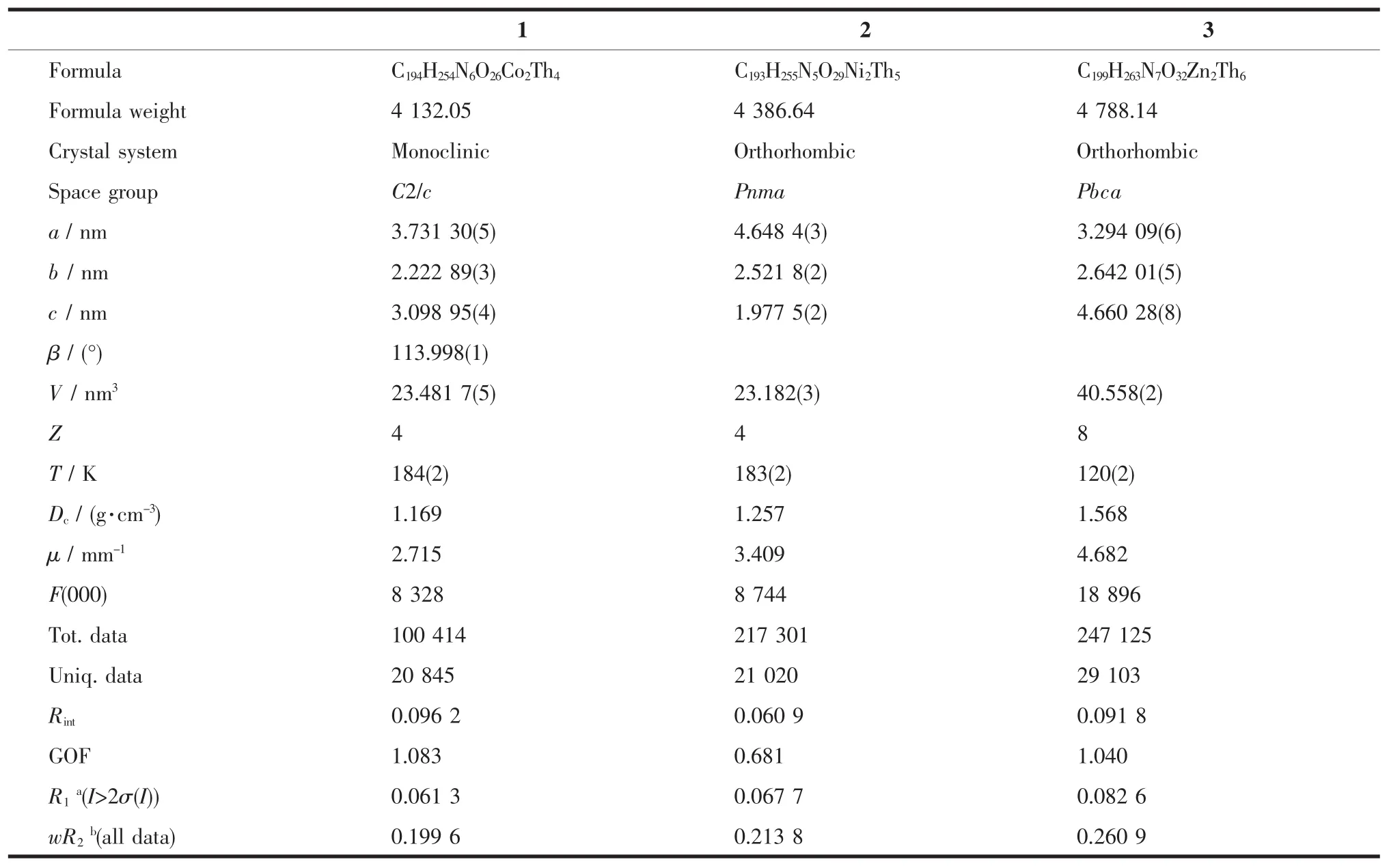
Table 1 Crystal data for compounds 1~3
2 Results and discussion
2.1 Synthesis
The reaction of p-tert-butylcalix[8]arene,MSO4(M=Co,Ni,Zn),ThCl4and triethylamine in a MeOH/DMF mixed solvent at 130℃results in the formation of compounds 1~3.It should be noted that single crystals of compound 1 could also be obtained when CoSO4·7H2O was replaced by CoCl2·6H2O while two others could not be obtained if the sulfates were substituted with the chlorides.In addition,another compound was obtained with ZnCl2·4H2O,whose structure can not be refined well due to its low crystal quality.It seems that the kind of transition metal salts would influence the final products.
2.2 Description of structures
In these three structures,all the calix[8]arene molecules adopt the double partial-cone conformation,in which each cone subunit bonds onethorium through four phenolic oxygen atoms at the lower rim and one coordinated DMF molecule occupies the cone cavity.Two tail-to-tail C8A molecules are bridged by four thorium cations through the Th-Ophenolicbonds into a sandwich-like tetranuclear conglomerate[Th4(HxC8A)(DMF)4O2](Fig.2)which is similar to the reported[Th4(HC8A)(H2C8A)(dmso)4(OH)3(OH2)][24].As shown in Fig.2,there are four vacant coordination sites 1~4.Sites 1 and 2 are located on the waist of one calixarene molecule while sites 3 and 4 on the waist of another.All these four sites could be further bonded by Co,Ni,Zn or Th with the assistance of some auxiliary ligands(such as OH-and solvent molecules).In these three compounds,the bond distances of Co-O,Ni-O,Zn-O and Th-O bonds are comparable with those in the reported literature[7,16-17,20,24].The metal oxidation states and the protonation of O2-and OH-groups are determined by bond valence sum(BVS)[7,25-26].In these three compounds,all transition metals are divalent and thorium being tetravalent.The deprotonation of calixarene molecules was decided based on both BVS of the phenolic oxygen atoms and charge balance.
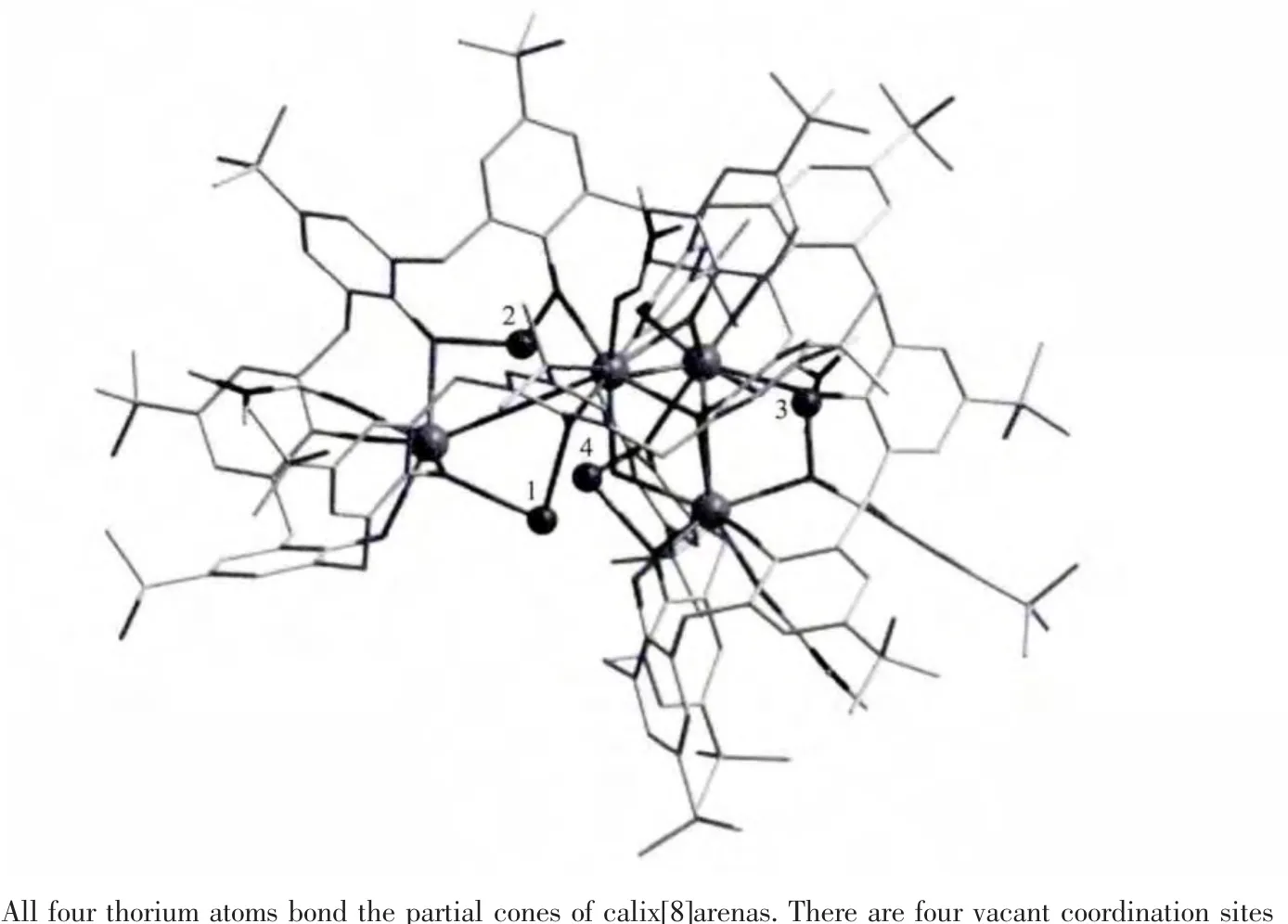
Fig.2 One tetranuclear conglomerate with two tail-to-tail calix[8]arene molecules
Single-crystal X-ray diffraction reveals that compound 1 crystallizes in a monoclinic system with space group C2/c.In an asymmetric unit,there are one whole p-tert-butylcalix[8]arene molecule, one cobalt(Co1)and two thorium atoms(Th1 and Th2).As shown in Fig.3,two cobalt atoms occupy sites 1 and 3 of the[Th4]conglomerate to form a[Co2Th4(HC8A)2O2(OH)2(DMF)6]unit.In this unit,the distances for two adjacent Th atoms is 0.382 nm while the trans Th …Th being of 0.375 or 0.651 nm.The Th…Co distances are in the range of 0.344~0.354 nm.According to the Ugozzoli-Andreetti convention[27],the actual φ and χ torsion angles values,which define the solid-state conformation of C8A,are-77.0,+81.4;-87.0,+76.1;-72.0,+86.5;+81.4,-75.2;-83.9,+78.1;-88.3,+74.4;-67.3,+91.9;+77.7,-84.4.The deprotonation of calix[8]arene molecule gives a negative heptavalent ligand which bonds three thorium and two cobalt cations.
Compound 2 crystallizes in an orthorhombic system with space group Pnma.In an asymmetric unit,there are two halves from different p-tert-butylcalix[8]arene molecules,one nickel (Ni1)and four thorium atoms (Th1,Th2,Th3.Th4).As shown in Fig.4,a[Ni2Th5(H2C8A)(C8A)O4(OH)2(DMF)5(CH3OH)2]unit was constructed by occupying sites 1 and 2 with two nickel atoms and site 4 with one thorium atom.The distances between two adjacent thorium atoms are in the range of 0.380~0.479 nm.The distances from Ni1 to Th1,Th2 and Th4 are of 0.314,0.317 and 0.336 nm,respectively.The actual φ and χ torsion angles values for two independent C8A molecules,are-81.0,+72.4;+88.7,-77.1;-79.6,+74.2;-82.8,+82.8;-74.2,+79.6;+77.1,-88.7;-72.4,+81.0;-81.9,+82.0 and-84.2,+73.8;-74.8,+97.4;-89.3,+72.9;+89.4,-89.4;-72.9,+89.3;-97.4,+74.8;-73.8,+84.2;+77.2,-77.2,respectively.Two calix[8]arene molecules deprotonated differently,one giving out eight protons and another giving out six ones.And one calixarene bonds six metal cations and the other bonding three metals.
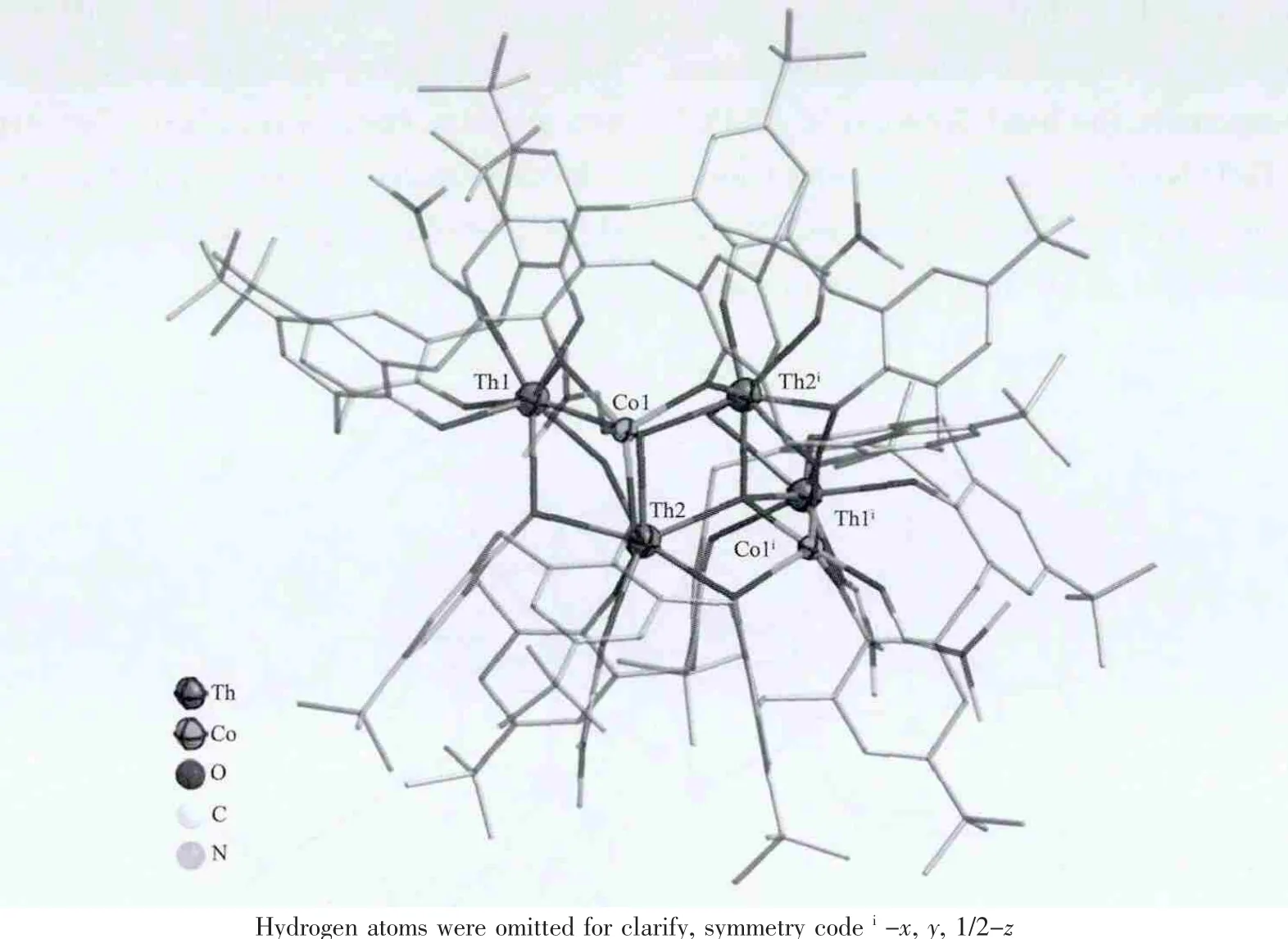
Fig.3 Molecular structure of compound 1
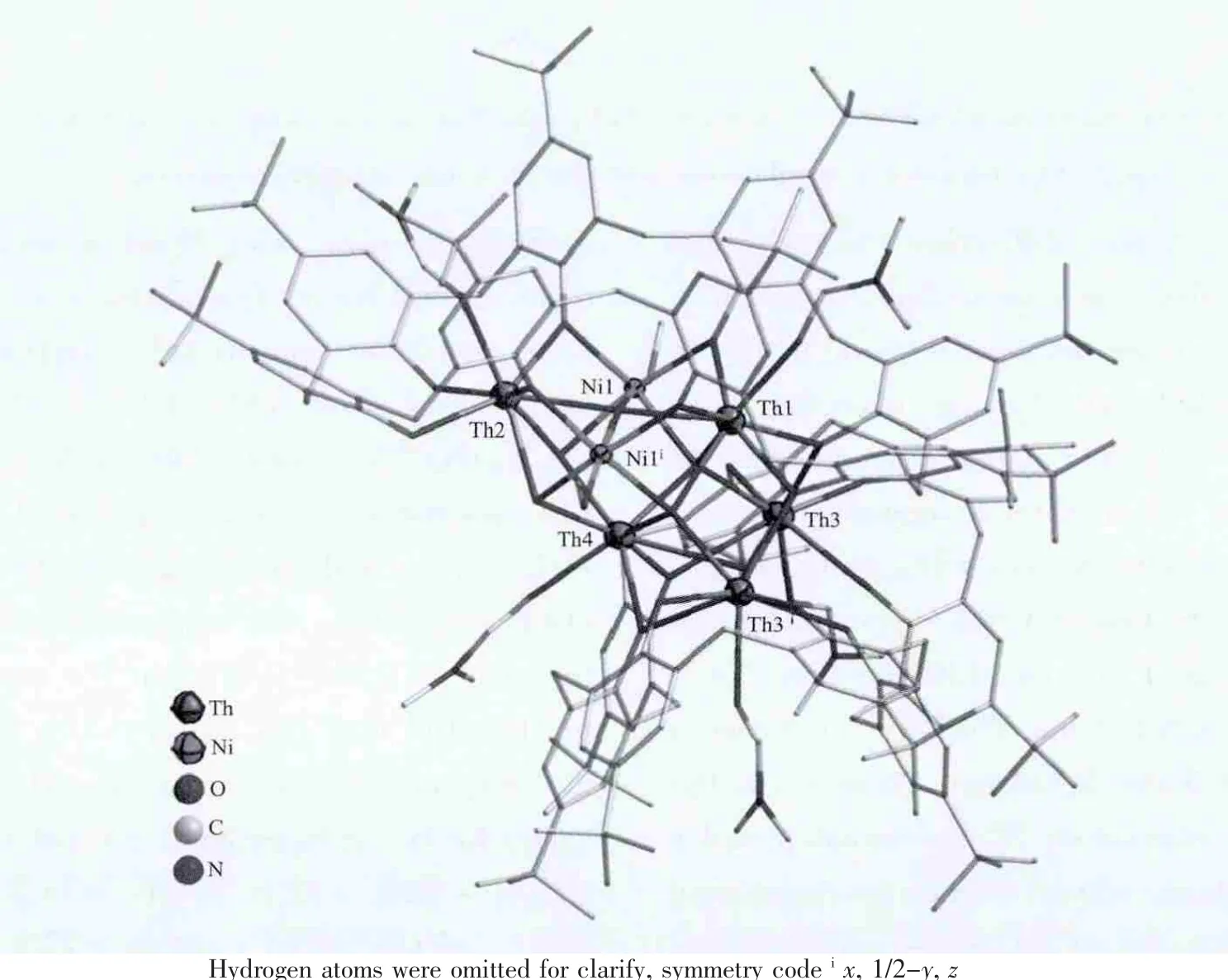
Fig.4 Molecular structure of compound 2
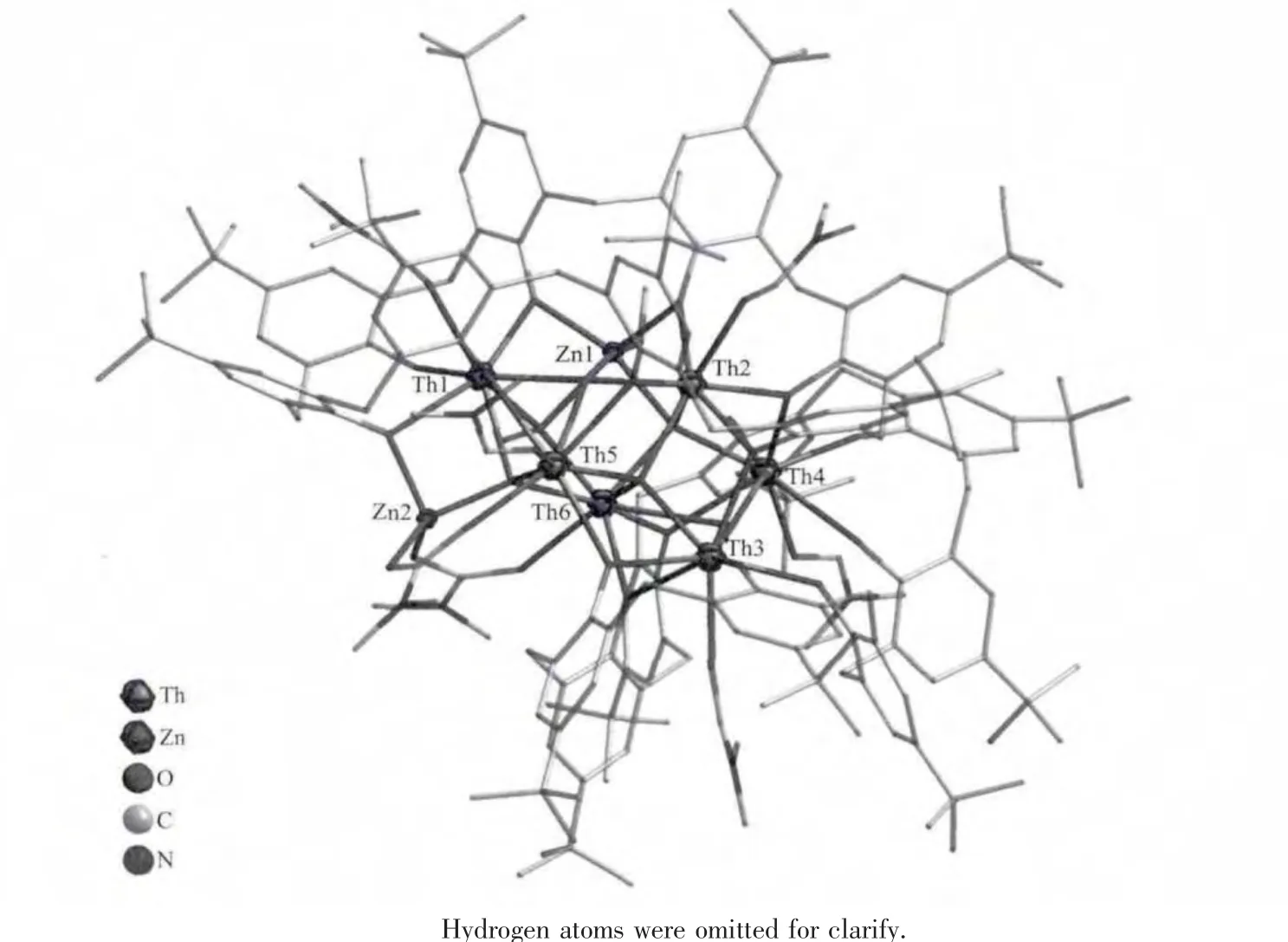
Fig.5 Structure of compound 3
Compound 3 crystallizes in an orthorhombic system with space group Pbca.In an asymmetric unit,there are two whole calix[8]arene molecules,two zinc and six thorium atoms(as shown in Fig.5).Sites 1 and 3 are occupied by two thorium atoms,and site 2 is occupied by one zinc atom.The left zinc atom(Zn2)is further bonded by one phenolic oxygen atom,two N,N-dimethylformate (DMFA)anions and one μ3-O2-.And then [Zn2Th6(HC8A)(C8A)O5(CH3O)(C3H6NO2)2(DMF)5(CH3OH)]unit formed.The DMFA anion is generated in situ from the oxadiation of DMF[28].The Th…Th distances for the adjacent atoms are in the range from 0.367 to 0.394 nm.The distances from the zinc atoms to the adjacent thorium atoms are in the range of 0.317~0.410 nm.The wide range for the Zn-Th distance would be due to the disorder of thorium and zinc atoms.The actual φ and χ torsion angles values for two independent C8A molecules,are-74.9,+91.5;-92.9,+76.3;-78.5,+84.0;+80.2,-81.9;-80.9,+82.2;-81.0,+79.4;-76.2,+82.9;+79.5,-86.1 and-74.8,+91.9;-98.1,+71.4;-78.3,+83.4;+80.3,-70.3;-85.4,+73.4;-71.9,+94.5;-89.6,+68.7;+92.9,-88.3.Two calix[8]arene molecules deprotonated differently,this is,one fully deprotonated and the other keeping one proton.
Comparing the polynuclearconglomeratesin compounds 1~3,one can find that the relative calix[8]-arene arrangements differ more.In compound 1,the angle between two calixarene molecules is of 45.5°(measured with that between two lines through the thorium atoms bonding the partial cones)while that being of 90.0°and 88.7°for compounds 2 and 3,respectively.This might be due to,on one hand,the flexibility of calix[8]arene,which allows it to coordinate with different metal centers,and on the other hand,the different radii and the coordination modes of the metal ions.All the extended structures of 1~3 are constructed by stacking these conglomerates via van der Waals interactions and the interstices between the polynuclear units are filled by the solvents.
2.3 Magnetic property of compound 1
Themagneticpropertyofcompound 1 was examined on the polycrystalline samples in 2~300 K(Fig.6).The χMT value at room temperature is 5.47 cm3·mol-1·K which is larger than two expected spinonly value of 3.75 cm3·mol-1·K for two non-interacting cobalt(Ⅱ) ions (S=3/2,g=2.0) (thorium (Ⅳ) being diamagnetic[7]).This may be due to the orbital contribution of Co ions.As the temperature decreased from 300 K to 100 K,the χMT values remained nearly unchanged,and then decreased slowly to 4.43 cm3·mol-1·K at 20 K.The decrease may due to the singleion property of Co ions[29].As the temperature decreased continued,the χMT value slightly increases to 4.68 cm3·mol-1·K at 10 K then declines quickly to 2.52 cm3·mol-1·K at 2 K.Due to the orbitally degenerate ground state of cobalt(Ⅱ)ion,it is difficult to explain the magnetic behavior[29].The increase may due to the weak ferromagnetic interactions between the metals.
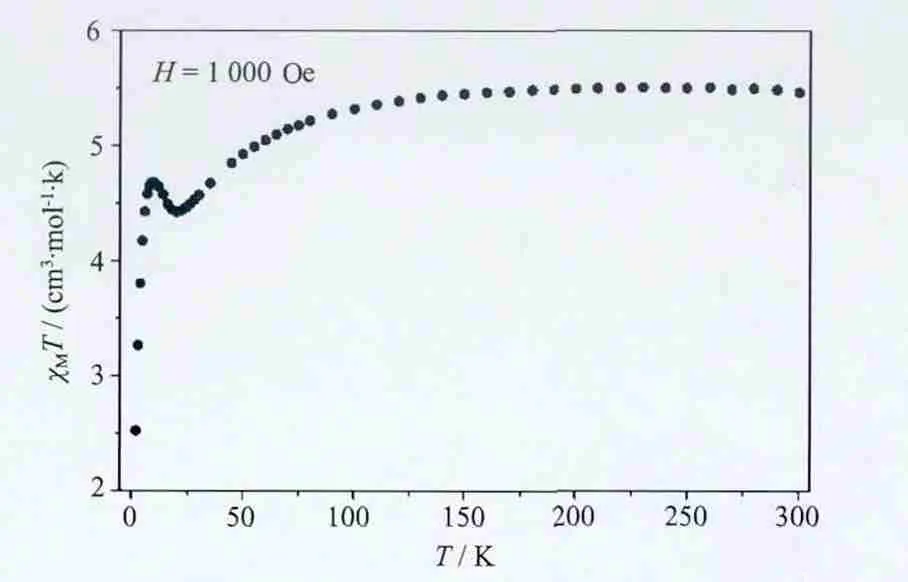
Fig.6 Plot of χMT vs.T for 1 in a 1 kOe applied DC field
3 Conclusion
In conclusion,we have synthesized three 3d-5f compounds,M-Th (M=Co,Ni,Zn)with the similar thorium conglomeratesskeleton,based on p-tertbutylcalix[8]arene under solvothermal conditions.In these three structures,all the cluster cores are capped by two tail-to-tail calix[8]arene molecules to form some sandwich-like units.All the calix[8]arenes adopt a double partial cone conformation.They exhibit different coordination modes to the metals and different relative arrangement in polynuclear motifs.Magnetic study on compound 1 indicates that the metal centers exhibit weak ferromagnetic interactions at 10~20 K.
[1]ZHANG Jia-Hua(张家骅),BAO Bo-Rong(包伯荣),XIA Yuan-Xian(夏源贤).Nucl.Technol.(核技术),1988,11(10):27-33
[2]SHEN Chao-Hong(沈朝洪),BAO Ya-Zhi(包亚之),BAO Bo-Rong(包伯荣),et al.J.Nucl.Radiochem.(核化学与放射化学),1992,14(2):94-100
[3]ZHANG Li-Qing(张丽清),WANG Zhi-Chang(王之昌),WANG Xiao-Huan(王 小 欢 ),et al.J.Nucl.Radiochem.(核化学与放射化学),2006,28(1):61-64
[4]Borgne T L,Rivière E,Marrot J,et al.Angew.Chem.Int.Ed.,2000,39:1647-1649
[5]Maynard B A,Sykora R E,Maguec J T,et al.Chem.Commun.,2010,46:4944-4946
[6]Vaughn A E,Bassil D B,Barnes C L,et al.J.Am.Chem.Soc.,2006,128:10656-10657
[7]Mishra A,Abboud K A,Christou G.Inorg.Chem.,2006,45:2364-2366
[8]YAN Bing(闫冰),ZHANG Hong-Jie(张洪杰),NI Jia-Zan(倪嘉缵).Chem.Res.Appl.(化学研究与应用),1998,10(2):111-117
[9]Harrowfield J,Koutsantonis G.Calixarenes in the Nanoworld.Dordrecht:Springer,2007:197
[10]Kajiwara T,Iki N,Yamashita M.Coord.Chem.Rev.,2007,251:1734-1746
[11]Bi Y F,Li Y L,Liao W P,et al.Inorg.Chem.,2008,47:9733-9735
[12]Bi Y F,Wang X T,Wang B W,et al.Dalton Trans.,2009,2250-2254
[13]Karotsis G,Evangelisti M,Dalgarno S J,et al.Angew.Chem.Int.Ed.,2009,48:9928-9931
[14]Karotsis G,Kennedy S,Teat S J,et al.J.Am.Chem.Soc.,2010,132:12983-12990
[15]Sanz S,Ferreira K,McIntosh R D,et al.Chem.Commun.,2011,47:9042-9044
[16]Xiong K C,Wang X Y,Jiang F L,et al.Chem.Commun.,2012,48:7456-7458
[17]Su K Z,Jiang F L,Qian J J,et al.Inorg.Chem.,2013,52:3780-3786
[18]Bergougnant R D,Robin A Y,Fromm K M.Tetrahedron,2007,63:10751-10757
[19]Redshaw C.Coord.Chem.Rev.,2003,244:45-70
[20]DU Shang-Chao(杜尚超),BI Yan-Feng(毕研峰),YU Yang(于洋),et al.Sci.Sin.:Chim.(中国科学:化学),2012,42(9):1356-1363
[21]Gutsche C D,Dhawan B,No K H,et al.J.Am.Chem.Soc.,1981,103:3782-3792
[22]Sheldrick G M.Acta Crystallogr.,Sect.A,2008,64:112-122
[23]Spek A L.J.Appl.Crystallogr.,2003,36:7-13
[24]Harrowfield J M,Ogden M I,White A H.J.Chem.Soc.Dalton Trans.,1991,2625-2632
[25]Brown I D,Altermatt D.Acta Crystallogr.,Sect.B,1985,41:244-247
[26]Liu W T,Thorp H H.Inorg.Chem.,1993,32:4102-4105
[27]Ugozzoli F,Andreetti G D.J.Inclusion Phenom.Mol.Recognit.Chem.,1992,13,337-348
[28]Yu J Y,Schreiner S,Vaska L.Inorg.Chim.Acta,1990,170:145-147
[29]Langley S,Helliwell M,Sessoli R,et al.Inorg.Chem.,2008,47:497-507
——李振声

